Structural Characteristics and Non-Linear Optical Behaviour of a 2-Hydroxynicotinate-Containing Zinc-Based Metal-Organic Framework
Abstract
:1. Introduction
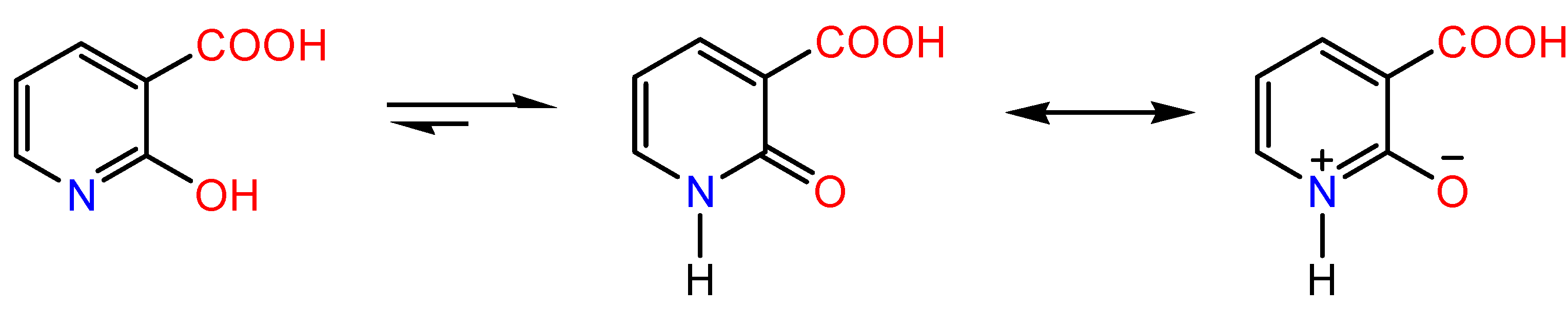
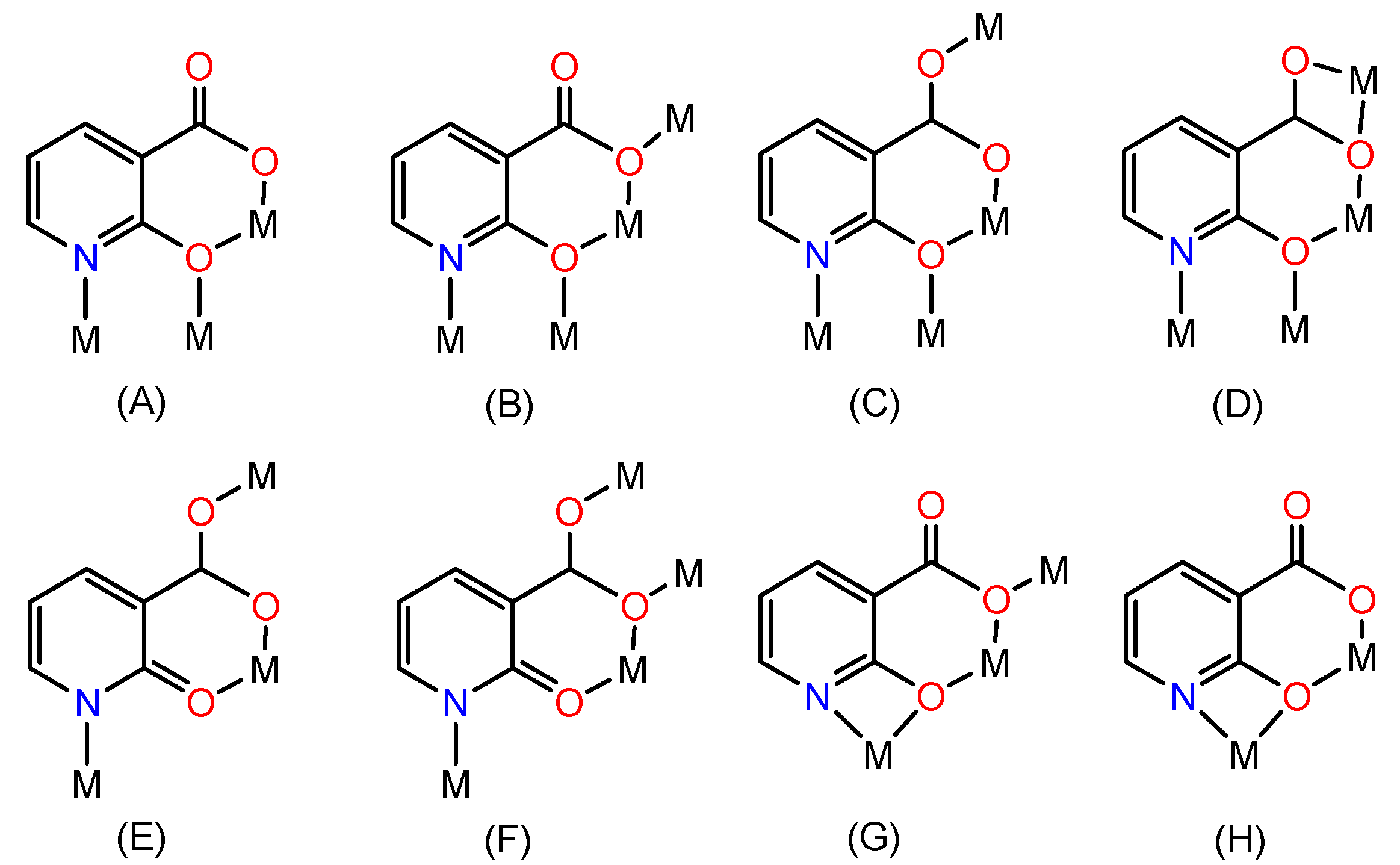
2. Results and Discussion
2.1. Synthesis

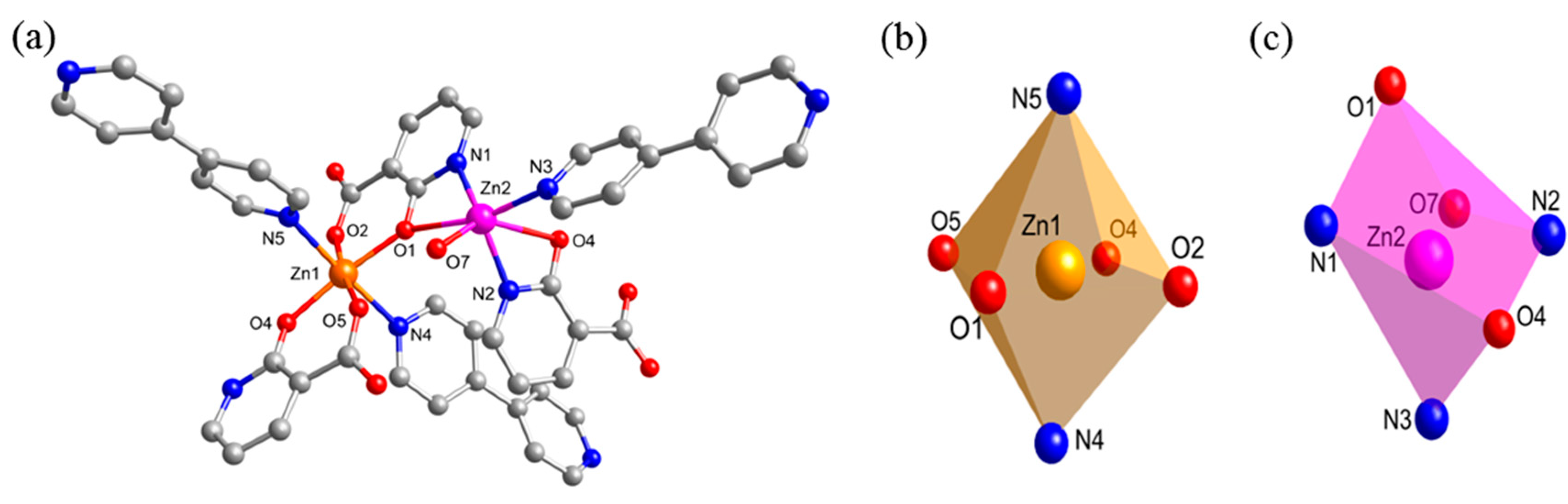
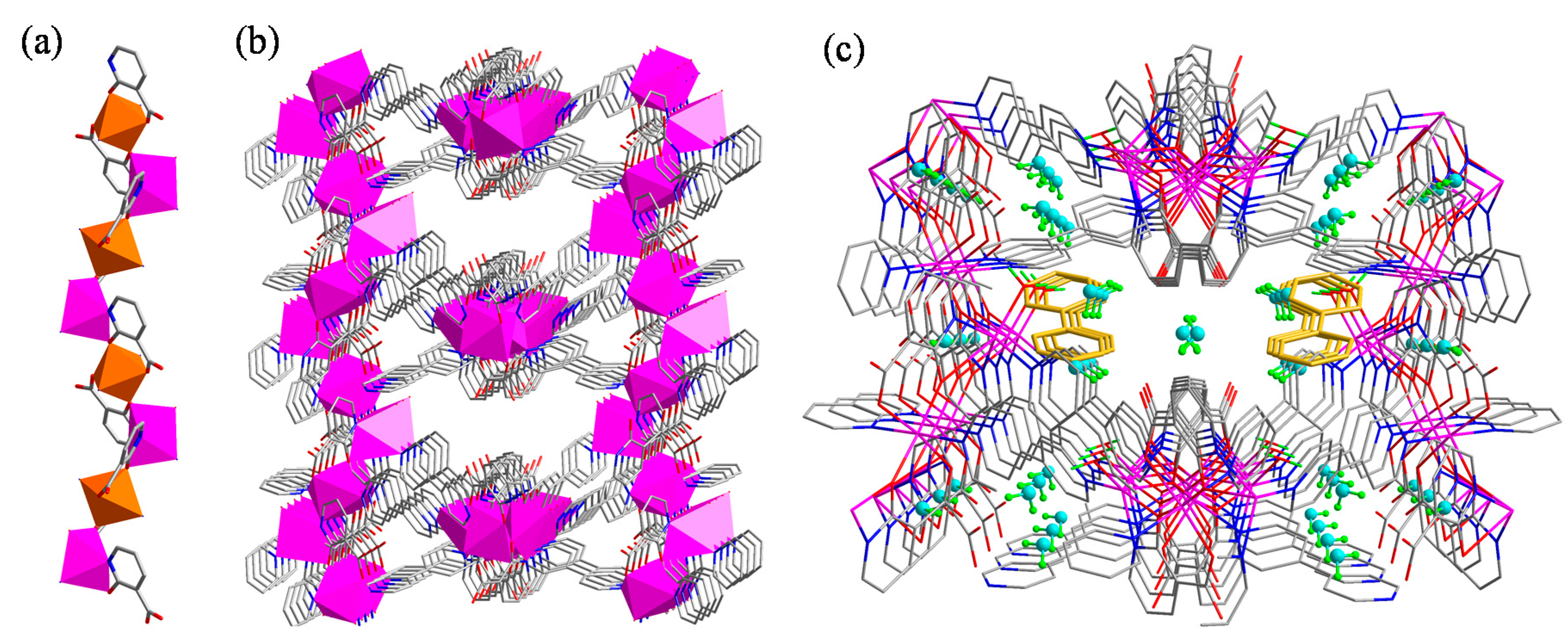
2.2. Crystal Structure
| Compound | 1 |
|---|---|
| chemical formula | C32H30N6O10Zn2 |
| formula weight | 789.36 |
| crystal system | Orthorhombic |
| space group | Fdd2 |
| temperature | 100.0(2) |
| a (Å) | 26.630(5) |
| b (Å) | 35.928(7) |
| c (Å) | 13.001(3) |
| V (Å3) | 12,439(4) |
| Z | 16 |
| Dcalcd (g/cm3) | 1.686 |
| θ range/(°) | 1.83–27.10 |
| µ (mm−1) | 1.614 |
| F (000) | 6464 |
| reflns collected | 6515 |
| unique reflns | 6776 |
| parameters | 451 |
| Rint | 0.0426 |
| R1, wR2a (I > 2σ(I)) | 0.0211, 0.0492 |
| R1, wR2a (all data) | 0.0227, 0.0497 |
| GOF | 1.071 |
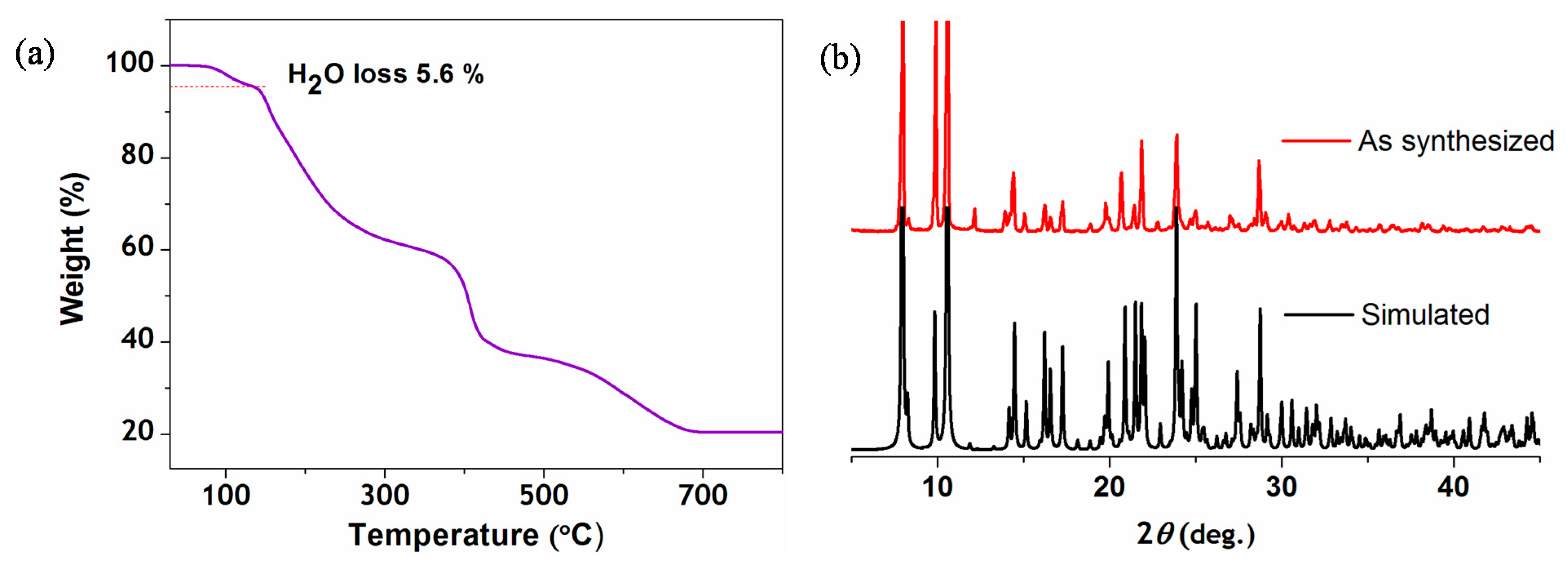
2.3. Thermal Stability
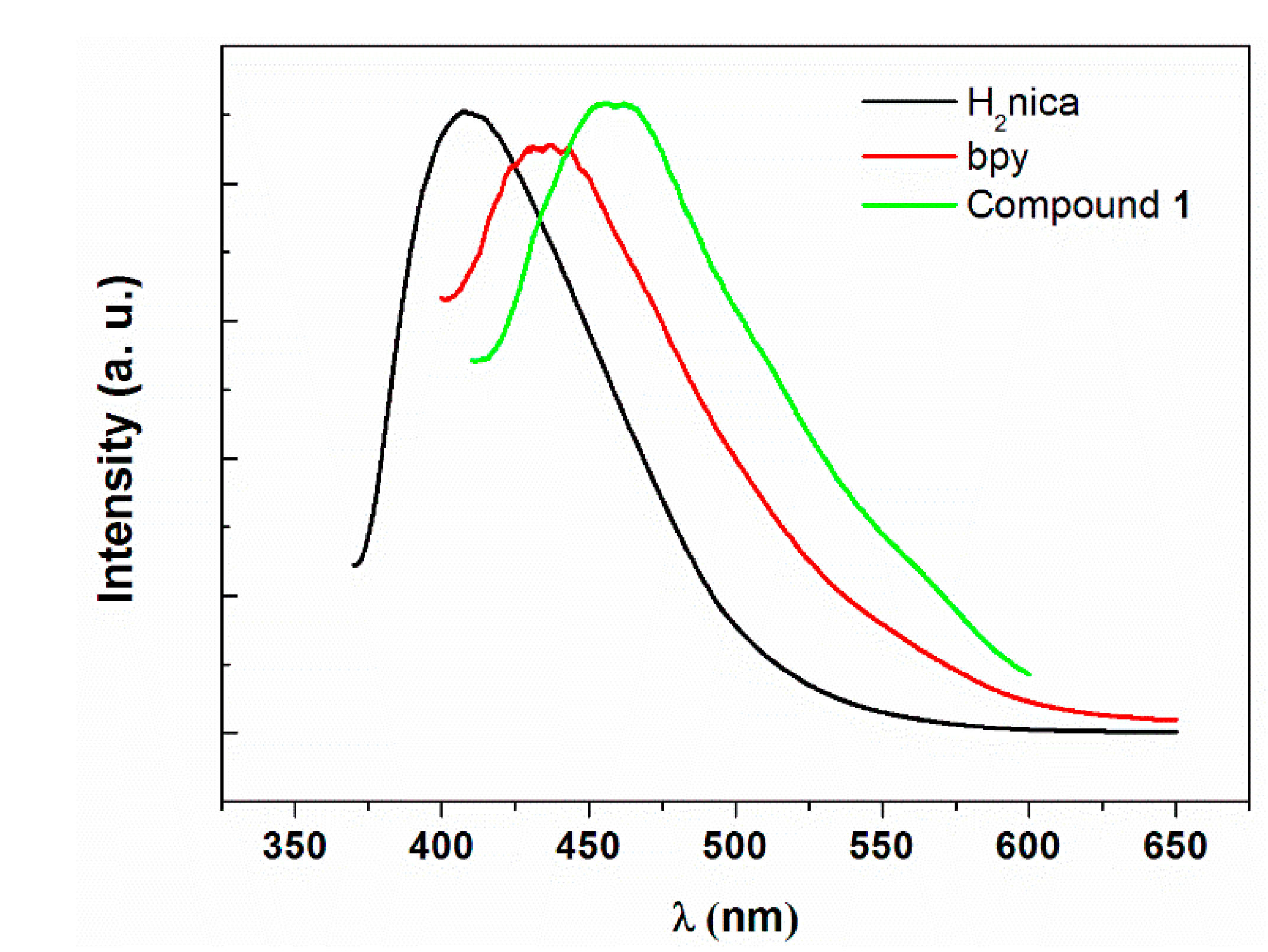
2.4. Photoluminescence Studies
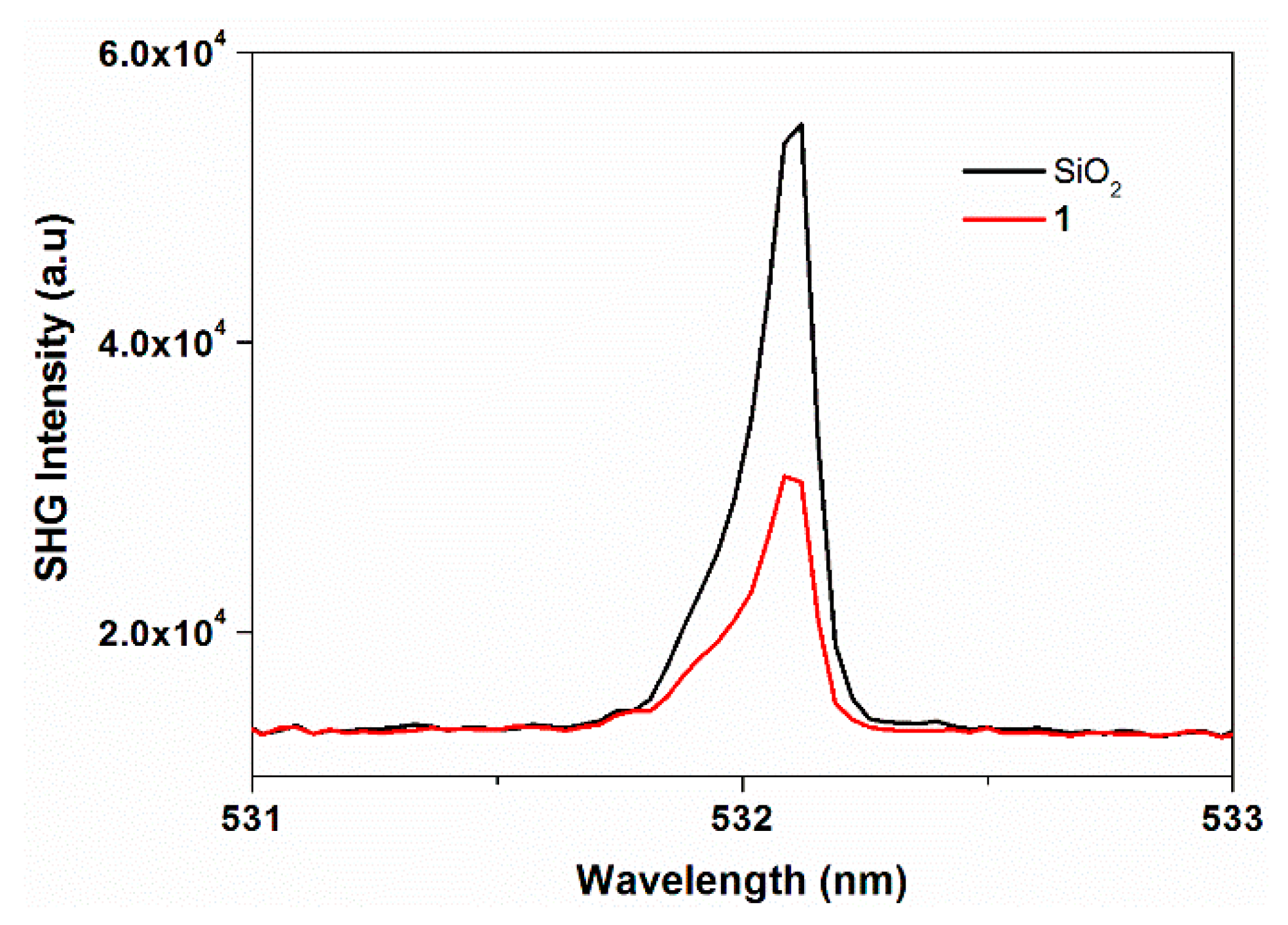
2.5. Non-Linear Optical Studies
3. Experimental Section
3.1. General Information
3.2. Synthesis of {[Zn2(nica)2(bpy)1.5(H2O)]·0.5(bpy)ʷ3H2O}n (1)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, C.Y.; Wang, X.L.; Zhang, X.; Qin, C.; Li, P.; Su, Z.M.; Zhu, D.X.; Shan, G.G.; Shao, K.Z.; Wu, H.; et al. Efficient and tunable white-light emission of metal-organic frameworks by iridium-complex encapsulation. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Ma, W.; Jiang, Q.; Yu, P.; Yang, L.; Mao, L. Zeolitic imidazolate framework-based electrochemical biosensor for in vivo electrochemical measurements. Anal. Chem. 2013, 85, 7550–7557. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Lee, J.H.; Moon, D.; Moon, H.R. Luminescent Li-based metal-organic framework tailored for the selective detection of explosive nitroaromatic compounds: Direct observation of interaction sites. Inorg. Chem. 2013, 52, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lin, X.; Lewis, W.; Suyetin, M.; Bichoutskaia, E.; Parker, J.E.; Tang, C.C.; Allan, D.R.; Rizkallah, P.J.; Hubberstey, P.; et al. A partially interpenetrated metal-organic framework for selective hysteretic sorption of carbon dioxide. Nat. Mater. 2012, 11, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.L.; Rieter, W.J.; Lin, W. Manganese-based nanoscale metal-organic frameworks for magnetic resonance imaging. J. Am. Chem. Soc. 2008, 130, 14358–14359. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Luo, T.T.; Wu, H.C.; Jao, Y.C.; Huang, S.M.; Tseng, T.W.; Wen, Y.S.; Lee, G.H.; Peng, S.M.; Lu, K.L. Self-assembled arrays of single-walled metal-organic nanotubes. Angew. Chem. Int. Ed. 2009, 48, 9461–9464. [Google Scholar] [CrossRef]
- Jones, J.T.A.; Hasell, T.; Wu, X.; Bacsa, J.; Jelfs, K.E.; Schmidtmann, M.; Chong, S.Y.; Adams, D.J.; Trewin, A.; Schiffman, F.; et al. Modular and predictable assembly of porous organic molecular crystals. Nature 2011, 474, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, S.; Han, L.; Sun, Z.; Luo, J.; Hong, M. A non-centrosymmetric dual-emissive metal-organic framework with distinct nonlinear optical and tunable photoluminescence properties. Cryst. Growth Des. 2013, 13, 106–110. [Google Scholar] [CrossRef]
- Yu, J.; Cui, Y.; Wu, C.; Yang, Y.; Wang, Z.; O’Keeffe, M.; Chen, B.; Qian, G. Second-order nonlinear optical activity induced by ordered dipolar chromophores confined in the pores of an anionic metal-organic framework. Angew. Chem. Int. Ed. 2012, 51, 10542–10545. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Schwartzberg, A.; Stavila, V.; Talin, A.A. A roadmap to implementing metal-organic frameworks in electronic devices: Challenges and critical directions. Chem. Eur. J. 2011, 17, 11372–11388. [Google Scholar] [CrossRef] [PubMed]
- Agranovich, V.M.; Gartstein, Y.N.; Litinskaya, M. Hybrid resonant organic-inorganic nanostructures for optoelectronic applications. Chem. Rev. 2011, 111, 5179–5214. [Google Scholar] [CrossRef] [PubMed]
- Delaire, J.A.; Nakatani, K. Linear and nonlinear optical properties of photochromic molecules and materials. Chem. Rev. 2000, 100, 1817–1846. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta, S.; Usman, M.; Luo, T.T.; Chang, B.C.; Lee, S.F.; Lin, Y.C.; Lu, K.L. Anion-controlled dielectric behavior of homochiral tryptophan-based metal–organic frameworks. Cryst. Growth Des. 2014, 14, 1572–1579. [Google Scholar] [CrossRef]
- Mendiratta, S.; Usman, M.; Luo, T.T.; Lee, S.F.; Lin, Y.C.; Lu, K.L. Guest dependent dielectric properties of nickel(II)-based supramolecular networks. Cryst. Eng. Comm 2014, 16, 6309–6315. [Google Scholar] [CrossRef]
- Mendiratta, S.; Usman, M.; Tseng, T.W.; Luo, T.T.; Lee, S.F.; Zhao, L.; Wu, M.K.; Lee, M.M.; Sun, S.S.; Lin, Y.C.; Lu, K.L. Low dielectric behavior of a robust, guest-free magnesium(II)-organic framework: A potential application of an alkaline-earth metal compound. Eur. J. Inorg. Chem. 2015, 1669–1674. [Google Scholar] [CrossRef]
- Usman, M.; Lee, C.H.; Hung, D.S.; Lee, S.F.; Wang, C.C.; Luo, T.T.; Zhao, L.; Wu, M.K.; Lu, K.L. Intrinsic low dielectric behaviour of a highly thermally stable Sr-based metal-organic framework for interlayer dielectric materials. J. Mater. Chem. C 2014, 2, 3762–3768. [Google Scholar] [CrossRef]
- Usman, M.; Mendiratta, S.; Lu, K.L. Metal-organic frameworks: New interlayer dielectric materials. ChemElectroChem 2015. [Google Scholar] [CrossRef]
- Long, S.; Zhou, P.; Theiss, K.L.; Siegler, M.A.; Li, T. Solid-state identity of 2-hydroxynicotinic acid and its polymorphism. CrystEngComm 2015. [Google Scholar] [CrossRef]
- Quintal, S.M.O.; Nogueira, H.I.S.; Félix, V.; Drew, M.G.B. Coordination modes of 2-hydroxynicotinic acid in second- and third-row transition metal complexes. Polyhedron 2002, 21, 2783–2791. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Liu, G.F.; Zheng, S.L.; Ye, B.H.; Zhang, X.M.; Chen, X.M. Helical ribbons of cadmium(II) and zinc(II) dicarboxylates with bipyridyl-like chelates—Syntheses, crystal structures and photoluminescence. Eur. J. Inorg. Chem. 2003, 2003, 2965–2971. [Google Scholar] [CrossRef]
- Bertoncello, R.; Bettinelli, M.; Casarin, M.; Gulino, A.; Tondello, E.; Vittadini, A. Hexakis(acetato)oxotetrazinc, a well-tailored molecular model of zinc oxide. An experimental and theoretical investigation of the electronic structure of Zn4O(acetate)6 and ZnO by means of UV and X-ray photoelectron spectroscopies and first principle local density molecular cluster calculations. Inorg. Chem. 1992, 31, 1558–1565. [Google Scholar] [CrossRef]
- Tessore, F.; Roberto, D.; Ugo, R.; Mussini, P.; Quici, S.; Ledoux-Rak, I.; Zyss, J. Large, concentration-dependent enhancement of the quadratic hyperpolarizability of [Zn(CH3CO2)2(L)2] in CHCl3 on substitution of acetate by triflate. Angew. Chem. Int. Ed. 2003, 42, 456–459. [Google Scholar] [CrossRef]
- Dragonetti, C.; Balordi, M.; Colombo, A.; Roberto, D.; Ugo, R.; Fortunati, I.; Garbin, E.; Ferrante, C.; Bozio, R.; Abbotto, A.; et al. Two-photon absorption properties of Zn(II) complexes: Unexpected large TPA cross section of dipolar [ZnY2(4,4ʹ-bis(para-di-n-butylaminostyryl)-2,2ʹ-bipyridine)] (Y = Cl, CF3CO2). Chem. Phys. Lett. 2009, 475, 245–249. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Failla, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Bella, S.D. Synthesis, characterization, optical absorption/fluorescence spectroscopy, and second-order nonlinear optical properties of aggregate molecular architectures of unsymmetrical Schiff-base zinc(II) complexes. Dalton. Trans. 2014, 43, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhou, Y.L.; Zeng, M.H.; Kurmooc, M. The concept of mixed organic ligands in metal-organic frameworks: Design, tuning and functions. Dalton. Trans. 2015, 44, 5258–5275. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, Q.X.; Zeng, M.H. Iodine release and recovery, influence of polyiodide anions on electrical conductivity and nonlinear optical activity in an interdigitated and interpenetrated bipillared-bilayer metal-organic framework. J. Am. Chem. Soc. 2012, 134, 4857–4863. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.K.; Perry, T.T. A powder technique for the evaluation of nonlinear optical materials. J. Appl. Phys. 1968, 39, 3798–3813. [Google Scholar] [CrossRef]
- Corn, R.M.; Higgins, D.A. Optical second harmonic generation as a probe of surface chemistry. Chem. Rev. 1994, 94, 107–125. [Google Scholar] [CrossRef]
- Cariati, E.; Dragonetti, C.; Lucenti, E.; Nisic, F.; Righetto, S.; Roberto, D.; Tordin, E. An acido-triggered reversible luminescent and nonlinear optical switch based on a substituted styrylpyridine: Efish measurements as an unusual method to reveal a protonation-deprotonation NLO contrast. Chem. Commun. 2014, 50, 1608–1610. [Google Scholar] [CrossRef]
- Zhou, W.W.; Chen, J.T.; Xu, G.; Wang, M.S.; Zou, J.P.; Long, X.F.; Wang, G.J.; Guo, G.C.; Huang, J.S. Nonlinear optical and ferroelectric properties of a 3-D Cd(II) triazolate complex with a novel (63)2(610.85) topology. Chem. Commun. 2008, 24, 2762–2764. [Google Scholar] [CrossRef]
- Lin, W.; Evans, O.R.; Xiong, R.G.; Wang, Z. Supramolecular engineering of chiral and acentric 2D networks. Synthesis, structures, and second-order nonlinear optical properties of bis(nicotinato)zinc and bis{3-[2-(4-pyridyl)ethenyl]benzoato}cadmium. J. Am. Chem. Soc. 1998, 120, 13272–13273. [Google Scholar] [CrossRef]
- Cariati, E.; Roberto, D.; Ugo, R.; Ford, P.C.; Galli, S.; Sironi, A. X-ray structures and emissive and second-order nonlinear optical properties of two inorganic-organic polymeric adducts of CuI with 4-acetylpyridine. The role of both “Intrastrand” charge transfers and structural motifs on the nonlinear optical response of Cu(I) polymeric adducts with pseudoaromatic η1-nitrogen donor ligands. Chem. Mater. 2002, 14, 5116–5123. [Google Scholar] [CrossRef]
- Hu, S.; Zou, H.H.; Zeng, M.H.; Wang, Q.X.; Liang, H. Molecular packing variation of crimpled 2D Layers and 3D uncommon 65.8 topology: effect of ligand on the construction of metal-quinoline-6-carboxylate polymers. Cryst. Growth Des. 2008, 8, 2346–2351. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Crystallographic Data Centre. Available online: http://www.ccdc.cam.ac.uk/conts/-retrieving.html (accessed on 30 March 2015).
- Sample Availability: Samples of compound {[Zn2(nica)2(bpy)1.5(H2O)]·0.5(bpy)·3H2O}n (1) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendiratta, S.; Lee, C.-H.; Lee, S.-Y.; Kao, Y.-C.; Chang, B.-C.; Lo, Y.-H.; Lu, K.-L. Structural Characteristics and Non-Linear Optical Behaviour of a 2-Hydroxynicotinate-Containing Zinc-Based Metal-Organic Framework. Molecules 2015, 20, 8941-8951. https://doi.org/10.3390/molecules20058941
Mendiratta S, Lee C-H, Lee S-Y, Kao Y-C, Chang B-C, Lo Y-H, Lu K-L. Structural Characteristics and Non-Linear Optical Behaviour of a 2-Hydroxynicotinate-Containing Zinc-Based Metal-Organic Framework. Molecules. 2015; 20(5):8941-8951. https://doi.org/10.3390/molecules20058941
Chicago/Turabian StyleMendiratta, Shruti, Cheng-Hua Lee, Sih-Ying Lee, Ya-Chuan Kao, Bor-Chen Chang, Yih-Hsing Lo, and Kuang-Lieh Lu. 2015. "Structural Characteristics and Non-Linear Optical Behaviour of a 2-Hydroxynicotinate-Containing Zinc-Based Metal-Organic Framework" Molecules 20, no. 5: 8941-8951. https://doi.org/10.3390/molecules20058941
APA StyleMendiratta, S., Lee, C.-H., Lee, S.-Y., Kao, Y.-C., Chang, B.-C., Lo, Y.-H., & Lu, K.-L. (2015). Structural Characteristics and Non-Linear Optical Behaviour of a 2-Hydroxynicotinate-Containing Zinc-Based Metal-Organic Framework. Molecules, 20(5), 8941-8951. https://doi.org/10.3390/molecules20058941





