Abstract
Readily available chiral ammonium salts derived from cinchona alkaloids have proven to be effective phase transfer catalysts in the asymmetric Michael reaction of 3-substituted isoindolinones. This protocol provides a convenient method for the construction of valuable asymmetric 3,3-disubstituted isoindolinones in high yields and moderate to good enantioselectivity. Diastereoselectivity was also investigated in the construction of contiguous tertiary and quaternary stereocenters. The use of acrolein as Michael acceptor led to an interesting tricyclic derivative, a pyrroloisoindolinone analogue, via a tandem conjugated addition/cyclization reaction.
1. Introduction
The construction of chiral tetrasubstituted carbons represents one of the most challenging and demanding topics in the synthesis of natural products and chiral drugs [1,2,3,4,5]. The development of such a new catalytic enantioselective synthesis of isoindolinones with this feature appeared to be of great value. Besides unsubstituted [6,7] and monosubstituted isoindolinones [7], many asymmetric 3,3-disubstituted isoindolinones show a wide spectra of biological activities as represented by the general structure 1, a family of inhibitors of phosphatidylinositolo 3-kinase [8]; by 2, a drug for the treatment of cardiac arrhythmias [9]; by 3, which is a HIV-reverse transcriptase inhibitor [10]; and by 4, which is a renin inhibitor [11] (Figure 1).
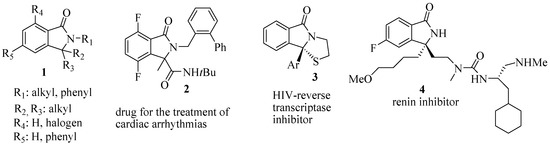
Figure 1.
Examples of synthetic pharmacalogically active chiral 3,3-disubstituted isoindolinones.
The preparation of these compounds in enantioenriched form has traditionally been pursued by kinetic resolution of racemates or with chiral acids or bases [12,13,14] or by the use chiral auxiliaries [15,16,17], while few catalytic asymmetric methodologies have been reported [18,19,20,21,22,23,24,25]. In particular, the construction of tetrasubstituted stereocenters in the heterocyclic ring in the presence of a chiral Pd(II) complex in an aerobic aza-Wacker-type cyclization performed on alkylidene ortho-substituted benzamides has been reported in 2012 by Zhang et al. [19]. In 2013 Nishimura et al. found that a chiral hydroxorhodium complex was effective in the synthesis of 3,3-diaryl substituted isoindolinones [20]. Only one organocatalytic method for the asymmetric Friedel–Crafts alkylation of indoles with 3-alkyl-3-hydroxyisoindolin-1-ones, showing good enantioselectivity has been described by Zhou et al. in 2011 [21]. The limited number of catalytic methodologies for the construction of quaternary stereocenters on the isoindolinone ring prompted us to tackle this challenge, considering the possible use of compounds of general structure 5 as nucleophiles in asymmetric reactions (Figure 2).
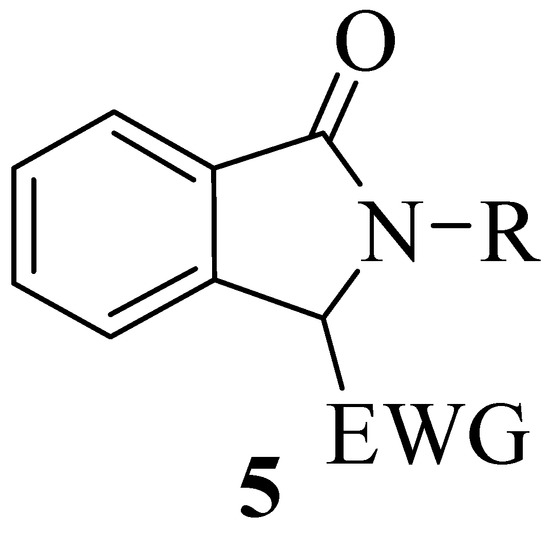
Figure 2.
General structure of potentially nucleophilic 3-substituted isoindolinones
The electron-withdrawing group should activate the benzylic carbon in the 3 position of 5 for asymmetric transformations in the presence of chiral organocatalysts or chiral phase transfer catalysts. As part of our ongoing studies on the asymmetric synthesis of isoindolinones and related compounds [24,25,26,27,28], we report herein the first example of an enantioselective Michael reaction of rac-3-substituted isoindolinones in the presence of chiral phase transfer catalysts for the construction of 3,3-disubstituted chiral derivatives, highlighting the scope and the limitations of the procedure.
2. Results and Discussion
In order to prove the synthetic utility of compounds of general structure 5 in asymmetric transformations, we started our investigation by testing the reactivity of the readily available isoindolinone 5a [29] taken as model compound. The choice to study the asymmetric Michael reaction has been inspired by the number of asymmetric methodologies using cyclic β-keto esters and activated phthalides, which can be performed under both organocatalytic [30,31,32] or chiral phase transfer conditions [33,34,35]. Accordingly, we firstly tested quinine and bifunctional organocatalyst 7 (Figure 3), under the conditions of Table 1. Pleasingly, the reaction of 5a with methyl vinyl ketone in DCM led to the adduct 6a in moderate ee. However, rather low yields, very long reaction times and incomplete conversions were observed (Table 1).

Table 1.
Chinchona based organocatalysts in the and identified Michael reaction of 3-substituted isoindolinones. 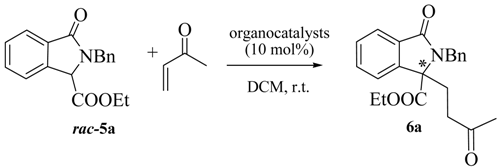

| Entry | Cat. (10 mol %) | t (h) | Yield (%) a | ee (%) b |
|---|---|---|---|---|
| 1 | quinine | 96 | 51 | 48 |
| 2 | 7 | 96 | 55 | 45 |
a Isolated yield. b Determined by HPLC on chiral column.
Then, for comparison, we turned our attention to the use of the chiral phase transfer catalyst 8a (Figure 3) in combination with the inorganic base K2CO3, a catalytic system also employed in Michael reaction of cyclic β-keto esters with good results [33,34,35]. Nicely enough, the expected Michael adduct 6a was obtained in high yield and with higher enantioselectivity (56% ee) than when quinine and 7 were used and in a shorter reaction time (compare Entry 1 of Table 2 with the data of Table 1). Considering the promising results obtained under asymmetric phase transfer conditions, we tested other readily available chiral ammonium salts, widely used in asymmetric reactions [33,34,35,36,37,38,39]. The O-allyl ether derivative 8b was less effective in terms of yield and enantioselectivity, emphasizing the importance of maintaining free the -OH group at the C-9 position of the catalyst (Entry 2). The quasi-enantiomer cinchoninium salt 9a showed a comparable efficiency with respect to 8a, giving ent-6a with a −55% ee (Entry 3), while 8c had a negative effect on the enantioselectivity (entry 4).
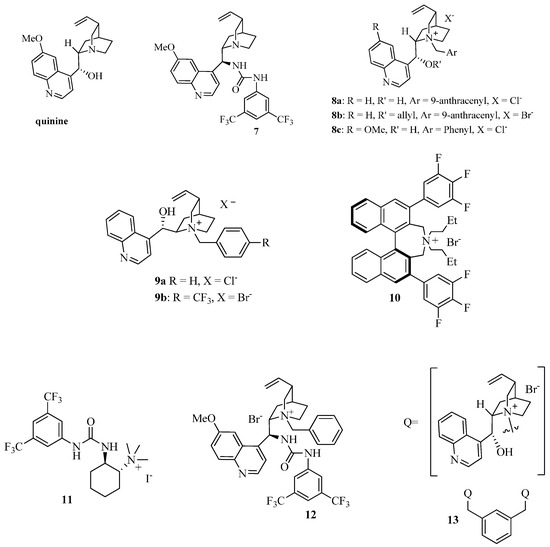
Figure 3.
A survey of chiral phase transfer catalysts and organocatalysts.

Table 2.
Phase transfer catalyzed asymmetric Michael reactions. 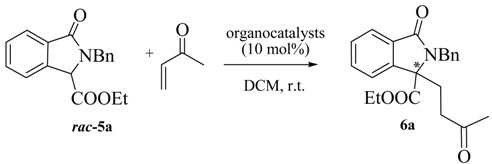

| Entry | PTC (10 mol %) | Solvent | T (°C) | t (h) | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|---|
| 1 | 8a | CH2Cl2 | r.t. | 2 | 90 | 56 |
| 2 | 8b | CH2Cl2 | r.t. | 24 | 95 | 11 |
| 3 | 9a | CH2Cl2 | r.t. | 2 | 89 | −55 |
| 4 | 8c | CH2Cl2 | r.t. | 48 | 92 | 38 |
| 5 | 10 | CH2Cl2 | r.t. | 30 | 91 | 10 |
| 6 | 11 | CH2Cl2 | r.t. | 8 | 83 | rac. |
| 7 | 12 | CH2Cl2 | r.t. | 8 | 96 | −11 |
| 8 c | 8a | CH2Cl2 | r.t. | 3 | 96 | 56 |
| 9 d | 8a | CH2Cl2 | r.t. | 24 | 93 | 54 |
| 10 | 8a | CH2Cl2/H2O | r.t. | 1 | 92 | 40 |
| 11 | 8a | CH2Cl2 | −20 | 3 | 91 | 60 |
| 12 | 8a | CH2Cl2 | −40 | 24 | 97 | 73 |
| 13 | 8a | CH2Cl2 | −50 | 48 | 97 | 68 |
| 14 | 9b | CH2Cl2 | −50 | 48 | 97 | −25 |
| 15 e | 8a | CH2Cl2 | −40 | 48 | 97 | 65 |
| 16 f | 8a | CH2Cl2 | −40 | 48 | 62 | 33 |
| 17 | 8a | CHCl3 | −40 | 72 | 96 | 68 |
| 18 | 8a | 1,2-DCE | r.t. | 24 | 97 | 51 |
| 19 | 8a | Toluene | −40 | 72 | 87 | 61 |
| 20 g | 8a | CH2Cl2 | −40 | 36 | 95 | 68 |
| 21 h | 8a | CH2Cl2 | −40 | 7 | 94 | 63 |
a Isolated yield. b Determined by HPLC on chiral column. c 8a was used at 5 mol %. d 8a was used at 2 mol %. e Cs2CO3 was used. f iPr2NEt was used. g [5a] = 7 mM instead of 14 mM of entry 12. h [5a] = 28 mM.
The structurally different Maruoka’s catalyst 10 [33] also employed in a number of asymmetric transformations, gave almost a racemic compound (entry 5). We also investigated the bifunctional chiral ammonium salts 11 [36,37] and 12 [38] derived from (R,R)-diamino cyclohexane and from quinine, respectively. Despite the possibility of giving a more ordered TS with the additional hydrogen bonds of the urea group [39], unsatisfactory results were obtained (Entries 6 and 7). Thus, focusing on 8a, we were able to perform the reaction even at 2 mol % with only a slight decrease in the ee (Entries 8 and 9). The DCM/H2O system was less effective in terms of enantioselectivity (entry 10). Only with the decreasing of the temperature we observed an increase of the enantioselectivity with a maximum of 73% ee at −40 °C (Entries 11–13). Under these conditions, the PTC 9b was less effective (entry 14). Other combinations of bases like Cs2CO3 or iPr2NEt with DCM and solvents like CHCl3, 1,2-DCE or toluene with K2CO3, gave less satisfactory results, even if in some cases they have positive effects on asymmetric Michael reactions of methyl vinyl ketone (Entries 15–19) [34]. Also the effect of the molar concentration was analyzed: the best result is represented by Entry 12 in comparison with those of Entries 20 and 21. Then, the scope of the reaction was analyzed by screening several Michael acceptors and isoindolinones, in the presence of 8a, under different conditions (Table 3).

Table 3.
Scope of phase transfer catalyzed asymmetric Michael reaction. 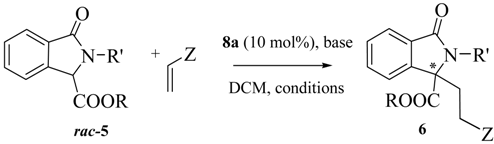

| Entry | 5 | R | R' | Z | T (°C) | t (h) | 6 | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5a | Et | Bn | COMe | −40 | 24 | 6a | 97 | 73 |
| 2 | 5b | Me | Bn | COMe | −40 | 24 | 6b | 98 | 70 |
| 3 | 5c | t-Bu | Bn | COMe | −40 | 48 | 6c | 60 | 45 |
| 4 | 5a | Et | Bn | COEt | −40 | 24 | 6d | 95 | 58 |
| 5 | 5a | Et | Bn | CHO | −40 | 18 | 6e | 90 | 33 |
| 6 | 5a | Et | Bn | CO2Me | r.t. | 24 | - | No reac. | - |
| 7 | 5d | Et | n-Bu | CO2Me | r.t. | 24 | 6f | 75 | 50 |
| 8 | 5d | Et | n-Bu | CO2Me | −20 | 48 | 6f | 75 | 21 |
| 9 | 5d | Et | n-Bu | COMe | r.t. | 1 | 6g | 90 | 61 |
| 10 c | 5d | Et | n-Bu | COMe | r.t. | 24 | 6g | 95 | 59 |
| 11 | 5d | Et | n-Bu | COMe | −20 | 8 | 6g | 95 | 76 |
| 12 | 5d | Et | n-Bu | COMe | −40 | 24 | 6g | 96 | 71 |
| 13 d | 5d | Et | n-Bu | COMe | r.t. | 4 | 6g | 96 | 38 |
| 14 | 5d | Et | n-Bu | CN | −20 | 18 | 6h | 97 | 13 |
| 15 | 5d | Et | n-Bu | CN | r.t. | 5 | 6h | 94 | 36 |
| 16 | 5e | Et | H | COMe | −40 | 8 | 6i | 97 | 20 |
a Isolated yield. b Determined by HPLC on chiral column. c Reaction performed in the presence of Na2CO3. d Reaction performed with 10 mol % of PTC 13 instead of 8a.
Isoindolinone 5c with the hindered t-butyl ester group was less effective in terms of reactivity and enantioselectivity than the analogues with ethyl and methyl groups 5a and 5b, respectively (Table 3, Entries 1–3). Other Michael acceptors were tested. Ethyl vinyl ketone and acrolein gave the expected adducts in very good yields, but with progressively lower ees than methyl vinyl ketone (Entries 4 and 5). Methyl acrylate did not react with 5a (Entry 6) and a structural change of the isoindolinone scaffold was necessary to guarantee a higher reactivity.In this case, in the presence of the n-butyl substituent on the amide in 5d instead of a benzyl group, the final adduct was obtained in good yield and moderate ee in a reasonable reaction time (Entries 7 and 8). The isoindolinone 5d, in the presence of methyl vinyl ketone, slightly affected the enantioselectivity in a positive manner, giving the good value of 76% at −20 °C in very high yield (Entries 9–12). Under these new conditions, Na2CO3 was slightly less effective than K2CO3 (entry 10), while the dimeric cinchonidinium salt 13, synthesized according to reported procedures [40] was less satisfactory than 8a (entry 13). Acrylonitrile also showed a very good reactivity, with higher enantioselectivity being observed at r.t. (entries 14 and 15). We also tested the isoindolinone 5e synthesized according to reported procedures [41] in order to investigate the effect of a further structural change on the reactivity and enantioselectivity. Despite the high reactivity toward methyl vinyl ketone, the free NH group was not beneficial for the enantioselectivity of the process, (entry 16). On the other hand the presence of the NH in 5e was particularly useful because a further cyclization reaction with the acrolein led to the tricyclic derivative 6j in high yield (Scheme 1). As observed by 1H-NMR analysis only one diastereomer was detected, but with very low enantioselectivity. A similar reactivity was observed with cynnamaldehyde, leading to the tricyclic derivative in high yield, but with low enantioselectivity, confirming the negative trend of 5e with this type of catalysis [42].
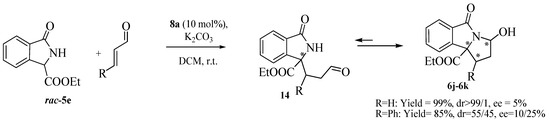
Scheme 1.
Synthesis of pyrroloisoindolinone analogue.
Nevertheless, this outcome is particular promising. The obtained compound is an analog of the pyrroloisoindolinone scaffold found, for example, in the cyclin dependent kinase 1,2,4,6 inhibitor [43]. Other studies are in course to improve the enantioselectivity and to enlarge the scope of this class of heterocyclic compounds, also considering eventual transformations of the existing functional groups [42].
In the last part of the discussion we focused on reactions of the Michael acceptor 3-penten-2-one in order to study the diastereoselectivity and possibly to obtain adducts with contiguous tertiary and quaternary stereocenters (Table 4). Also in this case the behavior was rather unexpected. According to the data reported in Table 3, the reactivity was strongly dependent on the isoindolinone structure. Probably due to the congested steric situation at the nucleophilic carbon, N-substituted isoindolinones 5a and 5d did not react at all, while the substrate 5e gave smoothly the expected product 6l with the contiguous quaternary and tertiary stereocenters in high yield and with good diastereoselectivity. Unfortunately, also in this case, a very low enantioselectivity was observed, leaving this challenge to future investigations.

Table 4.
Reactivity of 3-penten-2-one. 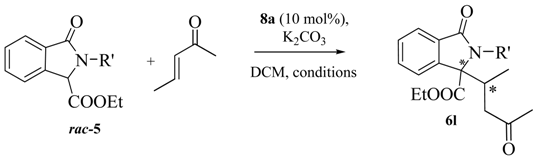

| Entry | 5 | Rʹ | t (h) | T (°C) | Yield (%) a | d.r. b | ee (%) c |
|---|---|---|---|---|---|---|---|
| 1 | 5a | Bn | 24 | r.t. | - | - | - |
| 2 | 5d | n-Bu | 24 | r.t. | - | - | - |
| 3 | 5e | H | 4 | r.t. | 95 | 9/1 | 4 |
| 4 | 5e | H | 24 | −40 | 92 | 92/8 | 8 |
a Isolated yield. b Determined by 1H-NMR on the crude. c Determined by HPLC on chiral column.
3. Experimental Section
3.1. General Information
All reactions were performed using commercially available compounds without further purification. Column chromatographic purification of products was carried out using silica gel 60 (70–230 mesh, Merck, Darmastdt, Germany). The NMR spectra were recorded on Bruker (Rheinstetten, Germany) DRX 400, 300, 250 spectrometers (400 MHz, 300 MHz, 250 MHz, 1H; 100 MHz, 75 MHz, 62.5 MHz 13C). Spectra were referenced to residual CHCl3 (7.26 ppm, 1H, 77.23 ppm, 13C). Coupling constants J are reported in Hz. Yields are given for isolated products showing one spot on a TLC plate and no impurities detectable in the NMR spectrum. Mass spectral analyses were carried out using a Waters 4 micro quadrupole electrospray spectrometer (Waters, Milford, MA, USA). Elemental analyses were performed with a FLASHEA 1112 series for CHNS-O apparatus (Thermo Scientific, Waltham, MA, USA). Polarimeter Jasco P-2000 (Tokio, Japan), HPLC Waters dual 1485 (Waters).
3.2. Synthesis
The isoindolinones 5a–e were synthesized according to reported procedures and spectroscopic were in agreement with literature [29,41,44]. Only 5c and 5d have never been described and the corresponding spectroscopic data are given below.
tert-Butyl-2-benzyl-3-oxoisoindoline-1-carboxylate (5c). To a solution of compound 5b (56 mg, 0.2 mmol) in absolute EtOH (1 mL), NaOH 0.5 M (1 mL) was added and the mixture was stirred for 2 h at room temperature. The solvent was removed and the residue was taken up with water, acidified to pH 1 and extracted twice with dichloromethane. The organic layers were combined and evaporated to give a white solid (52 mg, 0.18 mmol) which was resolubilized in dichloromethane (1 mL). Then EDC (40 mg, 0.204 mmol), DMAP (2 mg, 0.02 mmol) and tert-butanol (30 μL, 0.670 mmol, in excess) were added to the solution and the mixture was allowed to stir at room temperature for 3 h. Purification by chromatography (ethyl acetate–hexane 1:4) gave a waxy solid. Yield: 52%. 1H-NMR (400 MHz, CDCl3) δ: 7.89 (d, 1H, J = 6.5 Hz), 7.35–7.52 (m, 4H), 7.34–7.27 (m, 4H), 5.49 (d, 1H, J = 16 Hz), 4.76 (s, 1H), 4.27 (d, 1H, J = 16 Hz), 1.47 (s, 9H). MS (ESI): m/z = 324 (M+H)+. Anal. Calcd. for C20H21NO3 C, 74.28; H, 6.55; N, 4.33; found C, 74.33; H, 6.46; N, 4.30.
Ethyl 2-butyl-3-oxoisoindoline-1-carboxylate (5d). Yield 85%; oil. 1H-NMR (400 MHz, CDCl3) δ: 7.99–7.95 (m, 1H), 7.74–7.45 (m, 3H), 5.31 (s, 1H), 4.47–4.24 (m, 2H), 4.23–4.15 (m, 1H), 3.43–3.32 (m, 1H), 1.79–1.69 (m, 2H), 1.54–1.35 (m, 5H), 1.07 (t, 3H, J = 7.2 Hz). 13C-NMR (100 MHz, CDCl3) δ: 168.7, 168.5, 139.5, 132.2, 131.9, 129.3, 124.0, 122.7, 62.3, 62.2, 41.3, 30.2, 20.3, 14.3, 13.9. MS (ESI): m/z = 262 (M+H)+. Anal. calcd for C15H19NO3 C, 68.94; H, 7.33; N, 5.36; found C, 68.83; H, 7.46; N, 5.30.
3.3. Procedure for Preparation of rac-6
The Michael acceptor (1.5 eq) was added at r.t. to a solution of isoindolinones 5 (0.05 mmol) and K2CO3 (0.5 eq) in CH3CN (1 mL). The reaction was stirred overnight, the solvent was evaporated and the mixture purified directly on a chromatographic column eluting with 4:1 hexane–ethyl acetate, to afford rac-6 in yields ranging from 70%–90% [45].
3.4. Procedure for Enantioselective Michael Reactions of 5 to Afford 6
The Michael acceptor (1.5 eq) was added at −40 °C to a stirred solution of 5 (0.1 mmol, 1 eq.), K2CO3 (0.1 mmol, 1 eq.) and 8a (10% mol) in CH2Cl2 (1.5 mL). The reaction was monitored by TLC until the disappearance of 5. Then, the mixture was purified directly by flash chromatography eluting with 4:1 hexane–ethyl acetate to affording compounds 6 as waxy solids in yields ranging from 60%–99%.
Ethyl 2-benzyl-3-oxo-1-(3-oxobutyl)isoindoline-1-carboxylate (6a). Yield: 35 mg (97%);
= −2.0 (c = 0.1 M CHCl3); ee: 73%; 1H-NMR (400 MHz, CDCl3) δ: 7.91 (d, 1H, J = 8 Hz,), 7.55–7.46 (m, 2H), 7.4–7.36 (m, 3H), 7.27 (q, 3H, J = 7.6 Hz), 5.1 (d, 1H, J = 15.2 Hz,), 4.3 (d, 1H, J = 15.2 Hz,), 4.0 (q, 2H, J = 7.2 Hz), 2.74–2.67 (m, 1H), 2.39–2.35 (m, 1H), 1.61 (s, 3H), 1.48–1.41 (m, 2H), 1.09 (t, 3H, J = 7.0 Hz); 13C-NMR (100 MHz, CDCl3) δ: 206.5, 169.8, 169.1, 142.9, 137.2, 132.2, 131.5, 129.2, 129.0, 128.4, 127.6, 123.9, 121.4, 71.6, 62.1, 44.7, 35.9, 29.4, 26.2, 13.7; MS (ESI): m/z = 366 (M+H)+. Anal. calcd for C22H23NO4 C, 72.31; H, 6.34; N, 3.83; found C, 72.20; H, 6.45; N, 3.70. Chiral HPLC: ODH column, hexane–iPrOH (4:1), flow: 0.6 mL/min, t: 15.6 min and 17.5 min.
Methyl 2-benzyl-3-oxo-1-(3-oxobutyl)isoindoline-1-carboxylate (6b). Yield: 34 mg (98%);
= −2.7 (c = 0.7 M CHCl3); ee: 70%; 1H-NMR (400 MHz, CDCl3) δ: 7.91 (d, 1H, J = 7.4 Hz), 7.54–7.36 (m, 3H), 7.28–7.22 (m, 5H), 4.98 (d, 1H, J = 14.9 Hz), 4.42 (d, 1H, J = 15.1 Hz), 3.46 (s, 3H), 2.77–2.65 (m, 1H), 2.48–2.42 (m, 1H), 1.65 (s, 3H), 1.53–1.43 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ: 206.7, 170.7, 169.3, 143.2, 137.4, 132.6, 131.9, 129.5, 129.4, 128.8, 127.9, 124.3, 121.8, 71.6, 53.1, 44.9, 36.2, 29.7, 26.4; MS (ESI): m/z = 352 (M+H)+. Anal. calcd for C21H21NO4 C, 71.78; H, 6.02; N, 3.99; found C, 71.60; H, 6.11; N, 3.87; Chiral HPLC: ODH column, hexane–iPrOH (4:1), flow: 0.6 mL/min, t: 19.2 min and 22.6 min.
tert-Butyl 2-benzyl-3-oxo-1-(3-oxobutyl)isoindoline-1-carboxylate (6c). Yield: 24 mg (60%);
= −56.0 (c = 0.8 M CHCl3); ee: 45%; 1H-NMR (400 MHz, CDCl3) δ: 7.87 (d, 1H, J = 6.7 Hz), 7.85–7.35 (m, 3H), 7.28–7.18 (m, 5H), 5.18 (d, 1H, J = 15.5 Hz), 4.23 (d, 1H, J = 15.0 Hz), 2.66–2.30 (m, 1H), 2.29–2.14 (m, 1H), 1.5 (m, 5H), 1.28 (s, 9H); 13C-NMR (100 MHz, CDCl3) δ: 206.7, 169.3, 168.7, 143.3, 137.5, 132.1, 131.6, 129.1, 128.5, 127.5, 123.9, 121.4, 83.1, 72.5, 44.9, 36.2, 29.6, 27.5, 26.3; MS (ESI): m/z = 394 (M+H)+. Anal. calcd for C24H27NO4 C, 73.26; H, 6.92; N, 3.56; found C, 73.40; H, 6.80; N, 3.65; Chiral HPLC: ODH column, hexane–iPrOH (4:1), flow: 0.6 mL/min. t: 13.9 min and 14.8 min.
Ethyl 2-benzyl-3-oxo-1-(3-oxopentyl)isoindoline-1-carboxylate (6d). Yield: 36 mg (95%); = −1.8 (0.1 M, CHCl3); ee: 58%; 1H-NMR (400 MHz, CDCl3) δ: 7.89 (d, 1H, J = 1.5 Hz), 7.87–7.43 (m, 3H), 7.38–7.35 (m, 1H), 7.27–7.20 (m, 4H), 5.06 (d, 1H, J = 15.3 Hz), 4.31 (d, 1H, J = 15.3 Hz), 3.97–3.92 (m, 2H), 2.72–2.67 (m, 1H), 2.44–2.33 (m, 1H), 1.80–1.76 (m, 2H), 1.45–1.36 (m, 2H), 1.08–1.02 (m, 3H), 0.72 (t, 3H, J = 7.3 Hz); 13C-NMR (100 MHz, CDCl3) δ: 209.6, 170.2, 169.5, 143.7, 137.9, 132.5, 131.9, 129.5, 129.4, 128.7, 127.8, 124.2, 121.9, 72.0, 62.4, 45.1, 35.7, 35.0, 26.5, 14.0, 8.2; MS (ESI): m/z = 380 (M+H)+. Anal. calcd for C23H25NO4 C, 72.80; H, 6.64; N, 3.69; found C, 72.95; H, 6.60; N, 3.59; Chiral HPLC: ODH column, hexane–iPrOH (4:1), flow: 0.6 mL/min, t: 13.1 min and 14.5 min.
Ethyl 2-benzyl-3-oxo-1-(3-oxopropyl)isoindoline-1-carboxylate (6e).Yield: 32 mg (90%);
= +0.52 (0.9 M, CHCl3); ee: 33%; 1H-NMR (300 MHz, CDCl3) δ: 9.10 (s, 1H), 7.89–7.86 (m, 1H), 7.88–7.41 (m, 6H), 7.37–7.20 (m, 2H), 5.07 (d, 1H, J = 15.2 Hz), 4.31 (d, 1H, J = 15.2 Hz), 4.01–3.88 (m, 2H), 2.75–2.65 (m, 1H), 2.44–2.34 (m, 1H), 1.48 (t, 2H, J = 7.7 Hz), 1.05 (t, 3H, J = 7.1 Hz); 13C-NMR (75 MHz, CDCl3) δ: 200.0, 170.1, 169.4, 143.0, 137.4, 132.6, 131.9, 129.7, 129.3, 128.8, 128.0, 124.4, 121.6, 71.7, 62.5, 45.1, 37.2, 24.8, 14.0; MS (ESI): m/z = 352 (M+H)+. Anal. calcd for C21H21NO4 C, 71.78; H, 6.02; N, 3.99, found C, 71.90; H, 6.12; N, 3.87; Chiral HPLC: ODH column, hexane–iPrOH (4:1), f: 0.6 mL/min., t: 21.4 min and 23.6 min.
Ethyl 2-butyl-1-(3-methoxy-3-oxopropyl)-3-oxoisoindoline-1-carboxylate (6f).Yield: 26 mg (75%);
= −25.3 (0.7 M, CHCl3); ee: 50%; 1H-NMR (250 MHz, CDCl3) δ: 7.82 (d, 1H, J = 6.5 Hz), 7.54–7.40 (m, 3H), 4.19–4.08 (m, 2H), 3.55 (s, 3H), 3.47–3.32 (m, 2H), 2.94–2.84 (m, 1H), 2.62–2.51 (m, 1H), 1.95–1.83 (m, 1H), 1.73–1.62 (m, 3H), 1,4 (q, 2H, J = 7.3 Hz), 1.15 (t, 3H, J = 7.1 Hz). 0.92 (t, 3H, J = 7.1 Hz); 13C-NMR (60 MHz, CDCl3) δ: 172.6, 170.2, 169.1, 142.5, 132.1, 131.9, 129.2, 123.7, 121.4, 71.3, 62.2, 51.6, 41.5, 30.3, 27.5, 27.4, 20.5, 13.7, 13.6; MS (ESI): m/z = 348 (M+H)+. Anal. calcd for C19H25NO5 C, 65.69; H, 7.25; N, 4.03; found C, 65.80; H, 7.40; N, 4.09; Chiral HPLC: ODH column, hexane–iPrOH (4:1), flow: 0.6 mL/min, t: 29.2 min and 33.7 min.
Ethyl 2-butyl-3-oxo-1-(3-oxobutyl)isoindoline-1-carboxylate (6g). Yield: 31 mg (95%);
= −1.4 (0.1 M CHCl3); ee: 76%; 1H-NMR (400 MHz, CDCl3) δ: 7.84 (d, 1H, J = 7.4 Hz), 7.56–7.47 (m, 2H), 7.40 (d, 1H, J = 7.1 Hz), 4.18–4.09 (m, 2H), 3.44–3.36 (m, 1H), 3.34–3.31 (m, 1H), 2.86–2.78 (m, 1H), 2.54–2.47 (m, 1H), 2.03–1.94 (m, 1H), 1.75 (s, 3H), 1.73–1.68 (m, 2H), 1.61–1.55 (m, 1H), 1.39 (q, 2H, J = 7.5 Hz), 1.16 (t, 3H, J = 7.1 Hz), 0.94 (t, 3H, J = 7.3 Hz); 13C-NMR (100 MHz, CDCl3) δ: 206.6, 170.2, 169.1, 142.8, 132.1, 132.0, 129.2, 123.6, 121.6, 71.3, 62.1, 41.5, 36.4, 30.3, 29.9, 25.9, 20.5, 13.8, 13.6; MS (ESI): m/z = 332 (M+H)+. Anal. calcd for C19H25NO4 C, 68.86; H, 7.60; N, 4.23; C, 68.94; H, 7.75; N, 4.15; Chiral HPLC: IA-3 column, hexane–iPrOH (4:1), f: 0.6 mL/min, t: 11.3 min and 15.9 min.
Ethyl 2-butyl-1-(2-cyanoethyl)-3-oxoisoindoline-1-carboxylate (6h).Yield: 29 mg (97%);
= −10.1 (1.6 M CHCl3); ee: 36%; 1H-NMR (250 MHz, CDCl3) δ: 7.86–7.83 (m, 1H), 7.84–7.50 (m, 2H), 7.44–7.41 (m, 1H), 4.20–4.05 (m, 2H), 3.51–3.42 (m, 1H), 3.37–3.27 (m, 1H), 2.98–2.86 (m, 1H), 2.65–2.53 (m, 1H), 1.99–1.86 (m, 1H), 1.73–1.65 (m, 2H), 1.41 (q, 2H, J =7.2 Hz), 1.25 (m, 1H), 1.15 (t, 3H, J = 7.1 Hz), 0.92 (t, 3H, J = 7.1 Hz); 13C-NMR (60 MHz, CDCl3) δ: 169.6, 168.9, 141.4, 132.5, 132.2, 129.9, 124.2, 121.2, 118.1, 70.7, 62.6, 60.3, 41.6, 30.5, 28.5, 20.5, 13.7, 11.0; MS (ESI): m/z = 315 (M+H)+. Anal. calcd for C18H22N2O3 C, 68.77; H, 7.05; N, 8.91; C, 68.90; H, 7.14; N, 8.82; Chiral HPLC: IE-3 column, hexane–iPrOH (4:1), f: 0.6 mL/min. t: 10.0 min and 11.4 min.
Ethyl 3-oxo-1-(3-oxobutyl)isoindoline-1-carboxylate (6i).Yield: 25 mg (97%); = −0.3 (0.5 M CHCl3); ee: 20%; 1H-NMR (400 MHz, CDCl3) δ: 7.82 (d, 1H, J = 7.28 Hz), 7.63–7.51 (m, 3H), 6.68 (brs, 1H), 4.22 (q, 2H, J = 6.6 Hz), 2.56–2.25 (m, 2H), 2.19–2.12 (m, 2H), 2.03 (s, 3H), 1.26 (t, 3H, J = 7.1 Hz);. 13C-NMR (100 MHz, CDCl3) δ: 206.8, 170.3, 169.8, 144.1, 132.6, 131.0, 129.3, 123.8, 123.3, 67.0, 62.4, 37.1, 30.9, 29.9, 13.9; MS (ESI): m/z = 276 (M+H)+. Anal. calcd for C15H17NO4 C, 65.44; H, 6.22; N, 5.09; C, 65.54; H, 6.34; N, 5.15; Chiral HPLC: IA3 column, hexane–iPrOH (4:1), flow 0.6 mL/min. t: 19.2 min and 29.4 min.
Ethyl 2,3,5,9b-tetrahydro-3-hydroxy-5-oxo-1H-pyrrolo[2,1-a]isoindole-9b-carboxylate (6j). Yield: 26 mg (99%);
= −0.1 (1 M CHCl3); ee: 5%; 1H-NMR (300 MHz, CDCl3) δ: 7.77 (d, 1H, J = 7.1 Hz), 7.61–7.45 (m, 3H), 5.71 (q, 1H, J = 6.3 Hz), 4.26–4.21 (m, 2H), 3.63 (d, 1H, J = 6.1 Hz), 2.80–2.75 (m, 1H), 2.68–2.63 (m, 1H), 2.24–2.18 (m, 1H), 1.72–1.67 (m, 1H), 1.25 (t, 3H, J = 6.9 Hz); 13C-NMR (100 MHz, CDCl3) δ: 171.4, 171.0, 145.1, 133.2, 131.9, 129.7, 124.9, 123.3, 80.2, 75.5, 62.7, 37.9, 34.2, 14.2; MS (ESI): m/z = 262 (M+H)+. Anal. calcd for C14H15NO4: C, 64.36; H, 5.79; N, 5.36; found: C, 64.51; H, 5.65; N, 5.43; Chiral HPLC: colonna IE-3 column, hexane–iPrOH (4:1) flow: 0.6 mL/min. t: 32.2 min and 37.6 min.
Ethyl 2,3,5,9b-tetrahydro-3-hydroxy-5-oxo-1-phenyl-1H-pyrrolo[2,1-a]isoindole-9b-carboxylate (6k). The title compound was obtained as a mixture of two diastereomers. Yield: 29 mg (85%). ee: 10/25%. 1H-NMR (300 MHz, CDCl3) δ: 7.85–7.82 (m, 1H, minor diast.), 7.59–7.55 (m, 1H), 7.43–7.40 (m, minor diast), 7.29–7.22 (m, 3H), 7.10–6.98 (m, 3H), 6.79 (dd, 2H, J2 = 1.5 Hz, J1 = 6.2 Hz), 6.12 (q, 1H, J = 6.4 Hz), 5.82 (t, 1H, J = 5.3 Hz, minor diast.), 4.39–4.20 (m, 3H), 3.99 (m, minor diast.), 3.64 (d, 1H, J = 6.3 Hz), 3.13 (q, J = 7.0 Hz, minor diast.), 2.90 (ddd, 1H, J3 = 2.6 Hz, J2 = 6.7 Hz, J1 = 12 Hz), 2.70 (ddd, 1H, J3 = 4.7 Hz, J2 = 7.7 Hz, J1 = 13 Hz), 1.35 (t,3H, J = 7.1 Hz) 0.93 (t, 3H, J = 7.1 Hz, minor diast.); 13C-NMR (75 MHz, CDCl3) δ: 171.7, 170.0, 143.3, 141.9, 138.2, 134.7, 132.2, 132.0, 129.5, 129.0, 128.6, 128.2, 127.9, 127.7, 127.0, 125.4, 124.3, 124.0, 79.4, 79.0, 62.7, 62.3, 54.5, 50.0, 44.5, 43.2, 14.0, 13.2; MS (ESI): m/z = 338 (M+H)+. Anal. calcd for C20H19NO4: C, 71.20; H, 5.68; N, 4.15; found: C, 71.32; H, 5.77; N, 4.02; Chiral HPLC: IA-3 column, hexane–iPrOH (9:1), flow: 0.6 mL/min. Major diast. t: 12.5 min and 14.2 min. Minor diast. t: 16 min and 19.4 min.
Ethyl 3-oxo-1-(4-oxopentan-2-yl)isoindoline-1-carboxylate (6l). Yield: 27 mg (95%); 1H-NMR (400 MHz, CDCl3) δ: 7.81 (d, 1H, J = 7.5 Hz), 7.69 (d, 1H, J = 7.7 Hz), 7.60 (t, 1H, J = 7.5 Hz), 7.53 (t, 1H, J = 7.5 Hz), 7.18 (brs, 1H), 7.07 (brs, 1H, minor diastereomer), 4.22–4.16 (m, 2H), 3.19–3.14 (m, 1H), 2.50 (dd, J = 16.8 Hz, 3.6.Hz, 1H), 2.41 (dd, J = 16.8 Hz, 9.2 Hz, 1H), 2.17 (s, 3H), 1.87 (s, 1H, minor diastereomer), 1.26–1.19 (m, 3H), 1.08 (d, 2H, J = 9.2 Hz), 0.59 (d, 3H, J = 6.7 Hz); 13C-NMR (100 MHz, CDCl3) δ: 206.6, 170.9, 170.8, 144.4, 132.7, 131.6, 129.5, 123.8, 123.7, 71.5, 62.6, 46.3, 35.7, 30.9, 14.2, 13.6; MS (ESI): m/z = 290 (M+H)+. Anal. calcd for C16H19NO4: C, 64.42; H, 6.62; N, 4.84; found: 64.55; H, 6.52; N, 4.71; Chiral HPLC: IE-3 column, hexane–iPrOH (4:1), f: 0.8 mL/min. Major diast. t: 8.9.5 min and 13.0 min. Minor diast. t: 9.7 min and 16.4 min.
4. Conclusions
In conclusion, 3-substituted isoindolinones have been used for the first time as nucleophiles in asymmetric Michael reactions under phase transfer catalyzed conditions in the synthesis of adducts with tetrasubstituted stereocenters. Several electron-deficient olefins were tested. Excellent chemical yields were obtained in the presence of chiral phase transfer catalysts, while organocatalysts were less effective. However, variable enantioselectivities were observed, with good values only occurring in the presence of methyl vinyl ketone. Other studies are in course with the aim to enlarge the substrate scope and field of application of these isoindolinone-based nucleophiles in different asymmetric reactions.
Supplementary Materials
Supplementary materials are available at: http://www.mdpi.com/1420-3049/20/05/8484/s1.
Acknowledgments
MIUR and University of Salerno for their financial support.
Author Contributions
FS performed the synthetic work, spectral data analysis and collected data. ADM contributed with literature research and with the synthetic work. LP and AM analysed and discussed results. AM assisted with overall planning, planned the synthetic route, designed the new derivatives, discussed results, wrote and reviewed the manuscript. All authors contributed to the paper and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest
References
- Corey, E.J.; Guzman-Perez, A. The Catalytic Enantioselective Construction of Molecules with Quaternary Carbon Stereocenters. Angew. Chem. Int. Ed. 1998, 37, 388–401. [Google Scholar] [CrossRef]
- Christoffers, J.; Mann, A. Enantioselective Construction of Quaternary Stereocenters. Angew. Chem. Int. Ed. 2001, 40, 4591–4597. [Google Scholar] [CrossRef]
- Cozzi, P.G.; Hilgraf, R.; Zimmermann, N. Enantioselective Catalytic Formation of Quaternary Stereogenic Centers. Eur. J. Org. Chem. 2007, 5969–5994. [Google Scholar] [CrossRef]
- Christoffers, J.; Baro, A. Quaternary Stereocenters: Challenges and Solution for Organic Synthesis; Christoffers, J., Baro, A., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 83–115. [Google Scholar]
- Bella, M.; Gasperi, T. Organocatalytic Formation of Quaternary Stereocenters. Synthesis 2009, 10, 1583–1614. [Google Scholar] [CrossRef]
- Shi, L.; Hu, L.; Wang, J.; Cao, X.; Gu, H. Highly Efficient Synthesis of N-Substituted Isoindolinones and Phthalazinones Using Pt Nanowires as Catalysts. Org. Lett. 2012, 14, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, A.; Palombi, L.; Massa, A. Active Methylene Compounds in the Synthesis of 3-substituted Isobenzofuranones, Isoindolinones and Related Compounds. Curr. Org. Chem. 2012, 16, 2302–2320. [Google Scholar] [CrossRef]
- Aronov, A.; Come, J.H.; Davies, R.J.; Pierce, A.C; Collier, P.N.; Grey, R.L.; O’Dowd, H.; Henderson, J.A.; Krueger, E.B.; Le Tiran, A.; et al. Isoindolinone Inhibitors of Phosphatidylinosytol 3-kinase. U.S. Patent 2012/0202784 A1, 9 August 2012. [Google Scholar]
- Bjoere, A.; Bostroem, J.; Davidsson, O.; Emtenaes, H.; Gran, H.; Iliefski, T.; Kajanus, J.; Olsson, R.; Sandberg, L.; Strandlund, G.; et al. Isoindoline Derivatives for the Treatment of Arrhythmias. WO2008008022, 17 January 2008. [Google Scholar]
- Mertens, A.; Zilch, J.H.; Konig, B.; Schafer, W.; Poll, T.; Kampe, W.; Seidel, S.; Leser, U.; Leinert, H. Selective Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors. New 2,3-Dihydrothiazolo[2,3-a]isoindol-5(9bH)-ones and Related Compounds with Anti-HIV-1 Activity. J. Med. Chem. 1993, 36, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.J.; Claremon, D.A.; Tice, C.M.; Cacatian, S.; Dillard, L.H.; Ishchenko, A.V.; Yuan, J.; Xu, Z.; Mcgeehan, G.; Zhao, W.; et al. Renin Inhibitors. WO2008156816, 27 March 2008. [Google Scholar]
- Yagishita, F.; Ishikawa, H.; Onuki, T.; Hachiya, S.; Mino, T.; Sakamoto, M. Total spontaneous resolution by deracemization of isoindolinones. Angew. Chem. Int. Ed. 2012, 51, 13023–13025. [Google Scholar] [CrossRef]
- Belliotti, T.R.; Brink, W.A.; Kestern, S.R.; Rubin, J.R.; Wistrow, D.J.; Zoski, K.T.; Whetzel, S.Z.; Corbin, A.E.; Pugsley, A.T.; Heffner, T.G.; et al. Isoindolinone enantiomers having affinity for the dopamine D4 receptor. Bioorg. Med. Chem. Lett. 1998, 8, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Kanamitsu, N.; Osaki, T.; Itsuji, Y.; Yoshimura, M.; Tsujimoto, H.; Soga, M. Novel water-soluble sedative-hypnotic agents: Isoindolin-1-one derivatives. Chem. Pharm. Bull. 2007, 55, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, M.; Couture, A.; Deniau, E.; Grandclaudon, P. Alternative and complementary approaches to the asymmetric synthesis of C3 substituted NH free or N-substituted isoindolin-1-one. Tetrahedron: Asymmetry 2008, 19, 111–123. [Google Scholar] [CrossRef]
- Nieto, S.; Sayago, F.J.; Laborda, P.; Soler, T.; Cativiela, C.; Urriolabeitia, E.P. Efficient access to (1H)-isoindolin-1-one-3-carboxylic acid derivatives by orthopalladation and carbonylation of methyl arylglycinate substrates. Tetrahedron 2011, 67, 4185–4191. [Google Scholar] [CrossRef]
- Lorion, M.; Couture, A.; Deniau, E.; Grandclaudon, P. Complementary synthetic approaches to constitutionally diverse N-amino-alkylated isoindolinones: Application to the synthesis of falipamil and 5-HT1A receptor ligand analogues. Synthesis 2009, 2009, 1897–1903. [Google Scholar] [CrossRef]
- Guo, S.; Xie, Y.; Hu, X.; Xia, C.; Huang, H. Diastereo- and Enantioselective Catalytic Tandem Michael Addition/Mannich Reaction: Access to Chiral Isoindolinones and Azetidines with Multiple Stereocenters. Angew. Chem. Int. Ed. 2010, 49, 2728–2731. [Google Scholar] [CrossRef]
- Yang, G.; Shen, C.; Zhang, W. An Asymmetric Aerobic Aza-Wacker-Type Cyclization: Synthesis of Isoindolinones Bearing Tetrasubstituted Carbon Stereocenters. Angew. Chem. Int. Ed. 2012, 51, 9141–9145. [Google Scholar] [CrossRef]
- Nishimura, T.; Noishiki, A.; Ebe, Y.; Hayashi, T. Hydroxorhodium/Chiral Diene Complexes as Effective Catalysts for the Asymmetric Arylation of 3-Aryl-3-hydroxyisoindolin-1-ones. Angew. Chem. Int. Ed. 2013, 52, 1777–1780. [Google Scholar] [CrossRef]
- Yu, X.; Lu, A.; Wang, Y.; Wu, G.; Song, H.; Zhou, Z.; Tang, C. Organocatalyzed Enantioselective Synthesis of Quaternary Carbon-Containing Isoindolin-1-ones. Eur. J. Org. Chem. 2011, 16, 3060–3066. [Google Scholar] [CrossRef]
- Mirabal-Gallardo, Y.; Piérola, J.; Shankaraiah, N.; Santos, L.S. Enantioselective total synthesis of (S)-(+)-lennoxamine through asymmetric hydrogenation mediated by l-proline-tetrazole ruthenium catalyst. Tetrahedron Lett. 2012, 53, 3672–3675. [Google Scholar] [CrossRef]
- Chen, M.W.; Chen, Q.A.; Duan, Y.; Ye, Z.S.; Zhou, Y.G. Asymmetric hydrogenolysis of racemic tertiary alcohols, 3-substituted 3-hydroxyisoindolin-1-ones. Chem. Commun. 2012, 48, 1698–1700. [Google Scholar] [CrossRef]
- More, V.; Rohlmann, R.; García Mancheño, O.; Petronzi, C.; Palombi, L.; de Rosa, A.; di Mola, A.; Massa, A. The first organocatalytic asymmetric synthesis of 3-substituted isoindolinones. RSC Adv. 2012, 2, 3592–3595. [Google Scholar] [CrossRef]
- Tiso, S.; Palombi, L.; Vignes, C.; di Mola, A.; Massa, A. Organocatalysts and sequential asymmetric cascade reactions in the synthesis of functionalized isoindolinones and benzoindolizidinones. RSC Adv. 2013, 3, 19380–19387. [Google Scholar] [CrossRef]
- Petronzi, C.; Collarile, S.; Croce, G.; Filosa, R.; de Caprariis, P.; Peduto, A.; Palombi, L.; Intintoli, V.; Di Mola, A.; Massa, A. Synthesis and Reactivity of the 3-Substituted Isoindolinone Framework to Assemble Highly Functionalized Related Structures. Eur. J. Org. Chem. 2012, 27, 5357–5365. [Google Scholar] [CrossRef]
- Antico, P.; Capaccio, V.; Di Mola, A.; Massa, A.; Palombi, L. Electrochemically Initiated Tandem and Sequential Conjugate Addition Processes: One-Pot Synthesis of Diverse Functionalized Isoindolinones. Adv. Synth. Catal. 2012, 354, 1717–1724. [Google Scholar] [CrossRef]
- Massa, A.; Rizzo, P.; Monaco, G.; Zanasi, R. Absolute configuration assignment made easier by the VCD of coupled oscillating carbonyls: the case of (−)-propanedioic acids, 2-(2,3)-dihydro-3-oxo-1H-isoindol-1-yl)-1,3-dimethyl ester. Tetrahedron Lett. 2013, 54, 6242–6246. [Google Scholar] [CrossRef]
- Rammah, M.M.; Othman, M.; Ciamala, K.; Strohmann, C.; Rammah, M.B. Silver-catalyzed spirolactonization: First synthesis of spiroisoindole-γ-methylene-γ-butyrolactones. Tetrahedron 2008, 64, 3505–3516. [Google Scholar] [CrossRef]
- Almasi, D.; Alonso, D.A.; Najera, C. Organocatalytic asymmetric conjugate additions. Tetrahedron: Asymmetry 2007, 18, 299–365. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, C.; Wang, H.; Xu, L.W.; Lu, Y. Direct asymmetric Michael addition of phthalide derivatives to chalcones. Tetrahedron Lett. 2013, 54, 5261–5265. [Google Scholar] [CrossRef]
- Tan, B.; Hernandez-Torres, G.; Barbas, C.F. Rationally Designed Amide Donors for Organocatalytic Asymmetric Michael Reactions. Angew. Chem. Int. Ed. 2012, 51, 5381–5385. [Google Scholar] [CrossRef]
- Shirakawa, S.; Maruoka, K. Recent Developments in Asymmetric Phase-Transfer Reactions. Angew. Chem. Int. Ed. 2013, 52, 4312–4348. [Google Scholar] [CrossRef]
- Tarí, S.; Chinchilla, R.; Nájera, C. Enantioselective Michael reaction of β-keto esters organocatalyzed by recoverable Cinchona-derived dimeric ammonium salts. Tetrahedron: Asymmetry 2009, 20, 2651–2654. [Google Scholar] [CrossRef]
- Elsner, P.; Bernardi, L.; Salla, G.D.; Overgaard, J.; Jørgensen, K.A. Organocatalytic Asymmetric Conjugate Addition to Allenic Esters and Ketones. J. Am. Chem. Soc. 2008, 130, 4897–4905. [Google Scholar] [CrossRef] [PubMed]
- Perillo, M.; Di Mola, A.; Filosa, R.; Palombi, L.; Massa, A. Cascade reactions of glycine Schiff bases and chiral phase transfer catalysts in the synthesis of alpha-amino acids 3-substituted phthalides or isoindolinones. RSC Adv. 2014, 4, 4239–4246. [Google Scholar] [CrossRef]
- Novacek, J.; Waser, M. Syntheses and Applications of (Thio)Urea-Containing Chiral Quaternary Ammonium Salt Catalysts. Eur. J. Org. Chem. 2014, 4, 802–809. [Google Scholar] [CrossRef]
- Johnson, K.M.; Rattley, M.S.; Sladojevich, F.; Barber, D.M.; Nunez, M.G.; Goldys, A.M.; Dixon, D.J. A New Family of Cinchona-Derived Bifunctional Asymmetric Phase-Transfer Catalysts: Application to the Enantio- and Diastereoselective Nitro-Mannich Reaction of Amidosulfones. Org. Lett. 2012, 10, 2492–2495. [Google Scholar] [CrossRef]
- Novacek, J.; Waser, M. Bifunctional Chiral Quaternary Ammonium Salt Catalysts: A Rapidly Emerging Class of Powerful Asymmetric Catalysts. Eur. J. Org. Chem. 2013, 637–648. [Google Scholar] [CrossRef]
- Jew, S.S.; Jeong, B.S.; Yoo, M.S.; Huh, H.; Park, H.G. Synthesis and application of dimeric Cinchona alkaloid phase-transfer catalysts: -bis[O(9)-allylcinchonidinium]-o,m or p-xylene dibromide. Chem. Commun. 2001, 1244–1245. [Google Scholar] [CrossRef]
- Conn, E.L.; Hepworth, D.; Qi, Y.; Rocke, B.N.; Ruggeri, R.B.; Zhang, Y. 2-Phenyl Benzoylamides. PCT WO 2011/145022 A1, 24 November 2011. [Google Scholar]
- Scorzelli, F.; Di Mola, A.; Croce, G.; Palombi, L.; Massa, A. Organocatalytic asymmetric synthesis of highly functionalized pyrrolizidines via cascade Michael/hemi-aminalization reactions of isoindolinones. Tetrahedron Lett. 2015, 56, 2787–2790. [Google Scholar] [CrossRef]
- Kawanishi, N.; Sugimoto, T.; Shibata, J.; Nakamura, K.; Masutani, K.; Ikuta, M.; Hirai, H. Structure-based drug design of a highly potent CDK1,2,4,6 inhibitor with novel macrocyclic quinoxalin-2-one structure. Bioorg. Chem. Lett. 2006, 16, 5122–5126. [Google Scholar] [CrossRef]
- Cho, C.S.; Jiang, L.H.; Lee, D.Y.; Shim, S.C. Facile synthesis of isoindolin-1-ones via Palladium Catalyzed Carbonylative Cyclization of 2-bromobenzaldehyde with Primary Amines. Bull. Korean Chem. Soc. 1996, 17, 1095–1096. [Google Scholar]
- Scorzelli, F.; Di Mola, A.; Palombi, L.; Filosa, R.; Massa, A. 3-Carboxylate-Substituted Isoindolinones in K2CO3-Catalyzed Michael Reactions. Synth. Commun. 2015. [Google Scholar] [CrossRef]
- Sample Availability: All samples available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).