A Mild Catalytic Oxidation System: FePcOTf/H2O2 Applied for Cyclohexene Dihydroxylation
Abstract
:1. Introduction
2. Results and Discussion
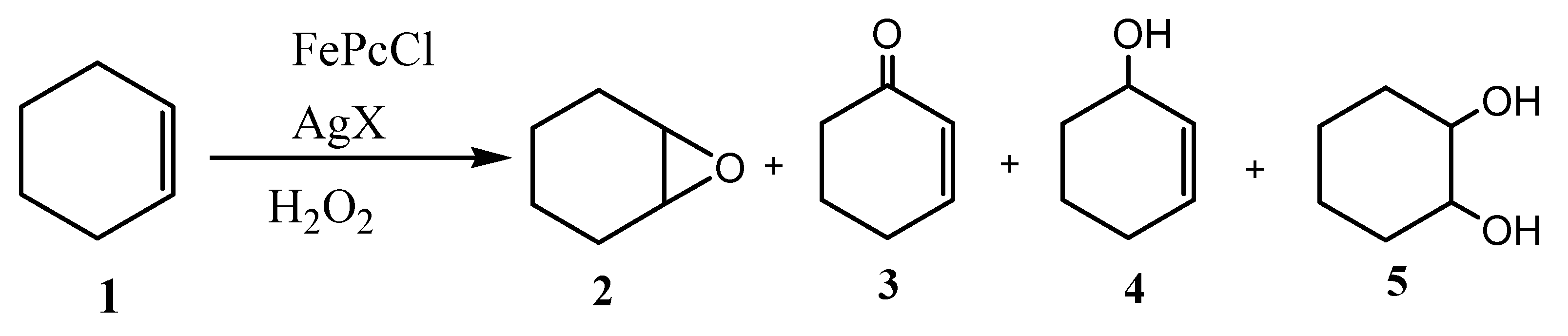
| Entry a | Catalyst | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| Cyclohexene oxide (2) | 2-Cyclohexen-1-ol (3) | 2-Cyclohexen-1-one (4) | Cyclohexane-1,2-diol (5) | |||
| 1 | FePc | 94.0 | 13.7 | 32.4 | 53.9 | / |
| 2 | FePcCl | 85.0 | 59.1 | 16.5 | 21.4 | 3.0 |
| 3 | FePcNO3 | 92.3 | 69.6 | 14.7 | 13.8 | 1.9 |
| 4 | FePcOTf | 97.1 | 20.2 | 18.6 | 15.3 | 45.9 |
| 5 | FePcBF4 | 96.4 | 32.8 | 23.1 | 14.9 | 29.2 |
| 6 | FePcSbF6 | 94.9 | 33.1 | 45.1 | 21.8 | / |
| 7 b | AgCl | trace | ND c | ND | ND | ND |
| Entry a | Oxidant | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| Cyclohexene oxide (2) | 2-Cyclohexen-1-ol (3) | 2-Cyclohexene-1-one (4) | Cyclohexane-1,2-diol (5) | |||
| 1 | TBHP | 86.0 | / | 70.7 | 29.3 | / |
| 2 | m-CPBA | 84.0 | 30.0 | 42.1 | 27.9 | / |
| 3 | 30% H2O2 | 97.1 | 20.2 | 18.6 | 15.3 | 45.9 |
| 4 b | O2 | 46.0 | 13.7 | 32.4 | 53.9 | / |
| Entry a | Reaction temperature (°C) | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| Cyclohexene oxide (2) | 2-Cyclohexen-1-ol (3) | 2-Cyclohexene-1-one (4) | Cyclohexane-1,2-diol (5) | |||
| 1 | 25 | 91.0 | 58.2 | 14.4 | 13.4 | 14.0 |
| 2 | 50 | 97.0 | 45.3 | 20.3 | 18.0 | 17.4 |
| 3 | 80 | 97.1 | 20.2 | 18.6 | 15.3 | 45.9 |
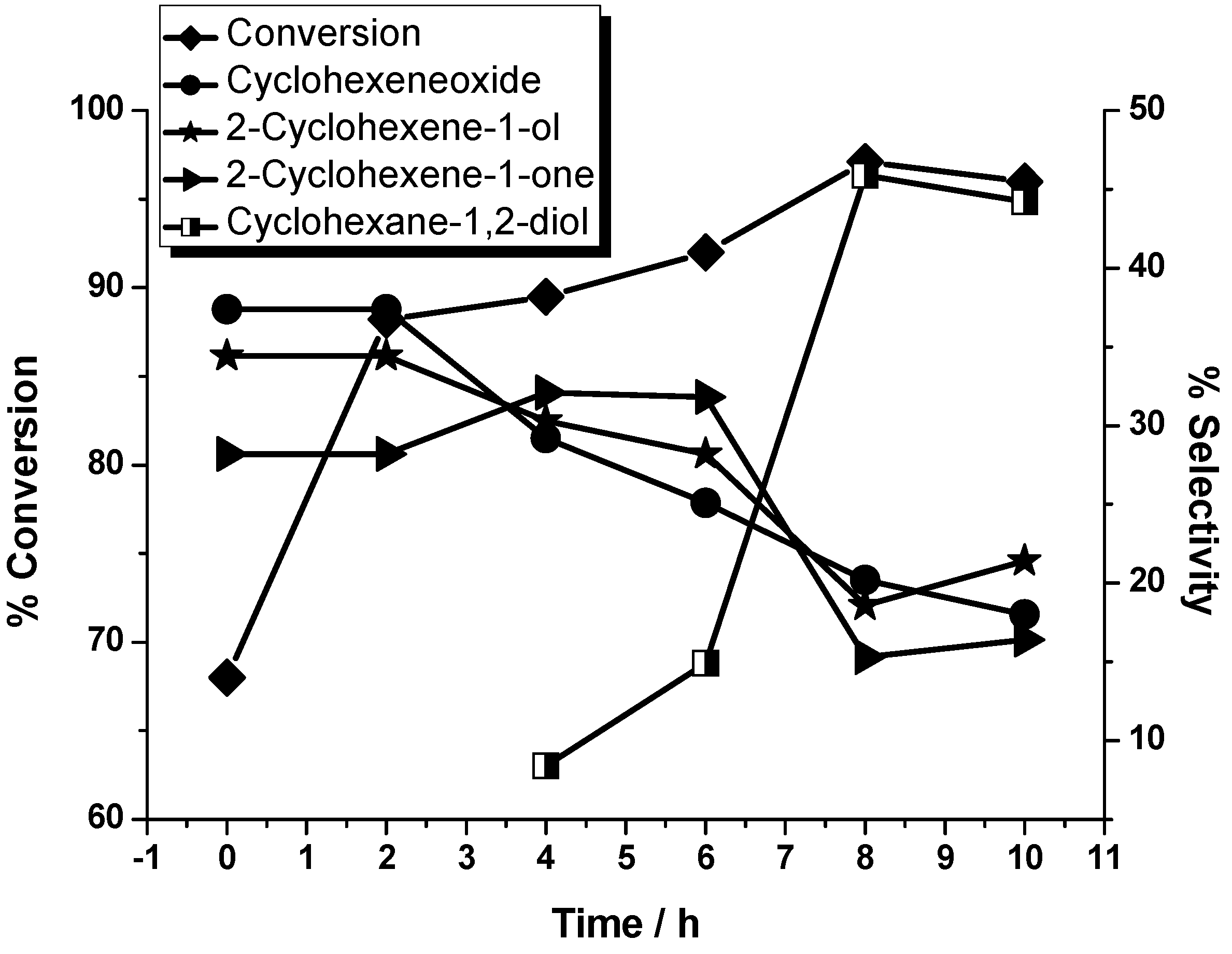
| Entry a | Substrate: oxidant | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| Cyclohexene oxide (2) | 2-Cyclohexen-1-ol (3) | 2-Cyclohexene-1-one (4) | Cyclohexane-1,2-diol (5) | |||
| 1 | 1:1 | 97.1 | 20.2 | 18.6 | 15.3 | 45.9 |
| 2 | 1:2 | 98.0 | 18 | 30.3 | 12.5 | 39.2 |
| 3 | 1:5 | 97.2 | / | 30.5 | 37.5 | 32.0 |
| Entry a | Amount of catalyst (mol %) | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| Cyclohexene oxide (2) | 2-Cyclohexen-1-ol (3) | 2-Cyclohexene-1-one (4) | Cyclohexane-1,2-diol (5) | |||
| 1 | 0.1 | 94.2 | 20.0 | 27.4 | 52.6 | / |
| 2 | 0.5 | 95.0 | 15.2 | 23.1 | 23.2 | 37.5 |
| 3 | 1 | 97.1 | 20.2 | 18.6 | 15.3 | 45.9 |
| 4 | 2 | 95.5 | 20.0 | 23.2 | 19.4 | 37.4 |
| Entry a | Solvent | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| Cyclohexene oxide (2) | 2-Cyclohexen-1-ol (3) | 2-Cyclohexene-1-one (4) | Cyclohexane-1,2-diol (5) | |||
| 1 | CH3OH | 97.5 | 5.0 | 24.5 | 18.5 | 52.0 |
| 2 | CH3CN | 97.2 | 18.2 | 26.4 | 14.0 | 41.2 |
| 3 | DMF | 97.1 | 20.2 | 18.6 | 15.3 | 45.9 |
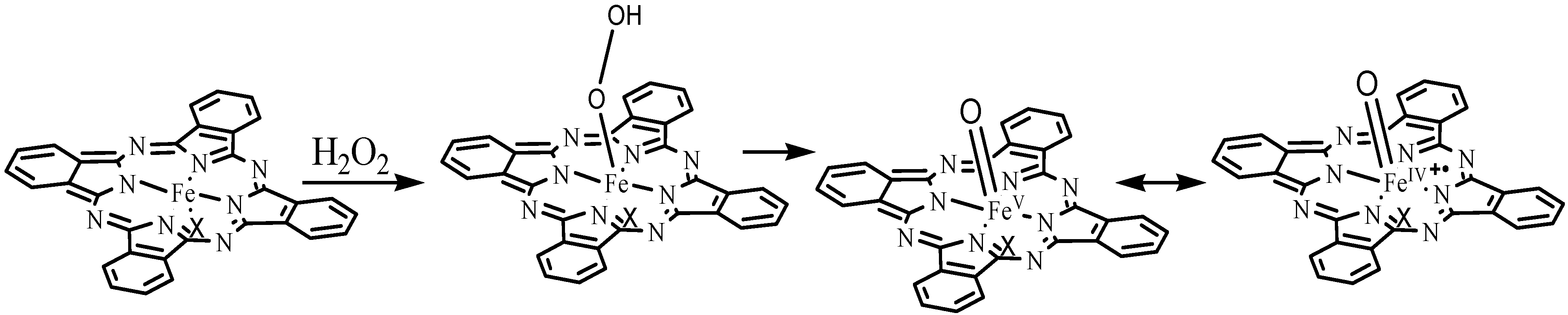
3. Experimental Section
3.1. Materials
3.2. Physical Measurements
3.3. General Procedure for the Preparation of 1,2-Diols
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacobsen, E.N.; Marko, I.; Mungall, W.S.; Schroeder, G.; Sharpless, K.B. Asymmetric dihydroxylation via ligand-accelerated catalysis. J. Am. Chem. Soc. 1988, 110, 1968–1970. [Google Scholar] [CrossRef]
- Jung, J.-K.; Johnson, B.R.; Duong, T.; Decaire, M.; Uy, J.; Gharbaoui, T.P.; Boatman, D.; Sage, C.R.; Chen, R.P.; Richman, J.G.; et al. Analogues of acifran: Agonists of the high and low affinity niacin receptors, GPR109a and GPR109b. J. Med. Chem. 2007, 50, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Zhang, H. Synthesis of some members of the hydroxylated phenanthridone subclass of the amaryllidaceae alkaloid family. J. Org. Chem. 2007, 72, 2570–2582. [Google Scholar] [CrossRef] [PubMed]
- Gancitano, P.; Cirimina, R.; Testa, M.L.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Enhancing selectivity in oxidation catalysis with sol-gel nanocomposites. Org. Biomol. Chem. 2005, 3, 2389–2392. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, P.D.; Shteinman, A.A.; Lawrence, Q.J. Iron-catalyzed olefin cis-dihydroxylation using a bio-inspired N,N,O-ligand. J. Am. Chem. Soc. 2005, 127, 15672–15673. [Google Scholar] [CrossRef] [PubMed]
- Aciro, C.; Davies, S.G.; Kurosawa, W.; Roberts, P.M.; Russel, A.J.; Thomson, J.E. Highly diastereoselective anti-dihydroxylation of 3-N,N-dibenzylaminocyclohex-1-ene N-oxide. Org. Lett. 2009, 11, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Emmanuvel, L.; Shaikh, T.M.A.; Sudalai, A. NaIO4/LiBr-mediated diastereoselective dihydroxylation of olefins: A catalytic approach to the Prevost—Woodward reaction. Org. Lett. 2005, 7, 5071–5074. [Google Scholar] [CrossRef] [PubMed]
- Carl, D.; Robert, R.E. Oxidations with ruthenium tetroxide. J. Am. Chem. Soc. 1953, 75, 3838–3840. [Google Scholar] [CrossRef]
- Tony, K.M.S.; Eric, K.W.T.; Vincent, W.F.T.; Ivan, H.F.C.; Qin, J. Ruthenium-catalyzed cis-dihydroxylation of alkenes: scope and limitations. Chem. Eur. J. 1996, 2, 50–57. [Google Scholar] [CrossRef]
- Plietker, B.; Niggemann, M. An improved protocol for the RuO4-catalyzed dihydroxylation of olefins. Org. Lett. 2003, 5, 3353–3356. [Google Scholar] [CrossRef] [PubMed]
- Venturello, C.; Alneri, E.; Ricci, M. A new effective catalytic system for epoxidation of olefins by hydrogen peroxide under phase transfer conditions. J. Org. Chem. 1983, 8, 3831–3833. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Patil, N.S.; Chaudhari, N.K.; Bhargava, S.K. Biphasic selective epoxidation of styrene by t-butyl hydroperoxide to styrene oxide using potassium chromate or dichromate catalyst in aqueous medium. Catal. Commun. 2004, 5, 205–208. [Google Scholar] [CrossRef]
- Sato, K.; Aoki, M.; Takagi, J.; Noyori, R. Organic solvent- and halide-free oxidation of alcohols with aqueous hydrogen peroxide. J. Am. Chem. Soc. 1997, 119, 12386–12387. [Google Scholar] [CrossRef]
- Ma, N.; Yue, Y.H.; Hua, W.M.; Gao, Z. Selective oxidation of styrene over nanosized spinel-type MgxFe3-xO4 complex oxide catalysts. Appl. Catal. A 2003, 251, 39–47. [Google Scholar] [CrossRef]
- Choudary, B.M.; Reddy, P.N. A new peroxo vanadium catalyst for selective oxidation of aralkenes to benzaldehydes. J. Mol. Catal. A: Chem. 1995, 103, L1–L3. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Weskam, P.T.; Zoller, J.P.; Fishcher, R.W. Methyltrioxorhenium: Oxidative cleavage of CC-double bonds and its application in a highly efficient synthesis of vanillin from biological waste. J. Mol. Catal. A: Chem. 2000, 153, 49–52. [Google Scholar] [CrossRef]
- Safari, N.; Bahadoran, F. Cytochrome P-450 model reactions: a kinetic study of epoxidation of alkenes by iron phthalocyanine. J. Mol. Catal. A: Chem. 2001, 171, 115–151. [Google Scholar] [CrossRef]
- Mirela, F.L.; Litvić, M.; Vinković, V. A highly efficient biomimetic aromatization of Hantzsch-1, 4-dihydropyridines with t-butylhydroperoxide, catalysed by iron (III) phthalocyanine chloride. Bioorg. Med. Chem. 2008, 16, 9276–9282. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.B. Phthalocyanine metal complexes in catalysis. Chem. Rev. 2013, 113, 8152–8191. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, H.J.; Budd, P.M.; McKeown, N.B. Catalysis by microporous phthalocyanine and porphyrin network polymers. J. Mater. Chem. 2008, 18, 573–578. [Google Scholar] [CrossRef]
- Taniguchi, T.; Idota, A.; Ishibashi, H. Iron-catalyzed sulfonyl radical formations from sulfonylhydrazides and oxidative addition to alkenes. Org. Biomol. Chem. 2011, 9, 3151–3153. [Google Scholar] [CrossRef] [PubMed]
- Murahashi, S.; Zhou, X.G.; Komiya, N. Chlorinated phthalocyanine iron (II) complex catalyzed oxidation of alkanes and alkenes with molecular oxygen in the presence of acetaldehyde. Synlett 2003, 3, 321–324. [Google Scholar] [CrossRef]
- Paradine, S.M.; White, M.C. Iron-catalyzed intramolecular allylic C-H amination. J. Am. Chem. Soc. 2012, 134, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Seelan, S.; Agashe, M.S.; Srinivas, D.; Sivasanker, S. Effect of peripheral substitution on spectral and catalytic properties of copper phthalocyanine complexes. J. Mol. Catal. A: Chem. 2001, 168, 61–68. [Google Scholar] [CrossRef]
- Chen, W.; Lv, W.; Yao, Y.; Xu, M. Highly efficient decomposition of organic dyes by aqueous-fiber phase transfer and in situ catalytic oxidation using fiber-supported cobalt phthalocyanine. Environ. Sci. Technol. 2007, 41, 6240–6245. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Chen, W.; Li, N.; Xu, M.; Yao, Y. Oxidative removal of 4-nitrophenol using activated carbon fiber and hydrogen peroxide to enhance reactivity of metallophthalocyanine. Appl. Catal. B Environ. 2009, 87, 146–151. [Google Scholar] [CrossRef]
- Lv, W.; Li, N.; Chen, W.; Yao, Y. The role of multiwalled carbon nanotubes in enhancing the catalytic activity of cobalt tetraaminophthalocyanine for oxidation of conjugated dyes. Carbon 2009, 47, 3337–3345. [Google Scholar] [CrossRef]
- Zhou, B.C.; Chen, W. Preparation and catalytic activity of carbon nanofibers anchored metallophthalocyanine in decomposing acid orange 7. Materials 2014, 7, 1370–1383. [Google Scholar] [CrossRef]
- Kasuga, K.; Tsuboi, K.; Handa, M.; Sugimori, T.; Sogabe, K. Oxygen-oxygenation of cyclohexene catalyzed by manganese (III), iron (III) and cobalt (III) complexes of tetra-tert-butylphthalocyanine in the presence of iso-butyraldehyde. Inorg. Chem. Commun. 1999, 2, 507–509. [Google Scholar] [CrossRef]
- Sehlotho, N.; Nyokong, T. Catalytic activity of iron and cobalt phthalocyanine complexes towards the oxidation of cyclohexene using tert-butylhydroperoxide and chlorperoxybenzoic acid. J. Mol. Catal. A: Chem. 2004, 209, 51–57. [Google Scholar] [CrossRef]
- Nam, W.; Lim, M.H.; Oh, S.Y.; Lee, J.H.; Lee, H.J.; Woo, S.K.; Kim, C.; Shin, W. Remarkable anionic axial ligand effects of iron (III) porphyrin complexes on the catalytic oxygenations of hydrocarbons by H2O2 and the formation of oxoiron (IV) porphyrin intermediates by m-chloroperoxybenzoic acid. Angew. Chem. Int. Ed. 2000, 39, 3646–3649. [Google Scholar] [CrossRef]
- Stephenson, N.A.; Bell, A.T. A study of the mechanism and kinetics of cyclooctene epoxidation catalyzed by iron (III) tetrakispentafluorophenyl porphyrin. J. Am. Chem. Soc. 2005, 127, 8635–8643. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.A.; Bell, A.T. The influence of substrate composition on the kinetics of olefin epoxidation by hydrogen peroxide catalyzed by iron (III) [tetrakis(pentafluorophenyl)] porphyrin. J. Mol. Catal. A: Chem. 2006, 258, 213–235. [Google Scholar]
- Stephenson, N.A.; Bell, A.T. Mechanistic study of iron (III) porphyrin triflate (F20TPP)Fe(OTf) catalyzed cyclooctene epoxidation by hydrogen peroxide. Inorg. Chem. 2007, 46, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.A.; Bell, A.T. Effects of porphyrin composition on the activity and selectivity of the iron (III) porphyrin catalysts for the epoxidation of cyclooctene by hydrogen peroxide. J. Mol. Catal. A: Chem. 2007, 272, 108–117. [Google Scholar] [CrossRef]
- Chen, G.Q.; Xu, Z.J.; Zhou, C.Y.; Che, C.M. Selective oxidation of terminal aryl and aliphatic alkenes to aldehydes catalyzed by iron (III) porphyrines with triflate as a counter anion. Chem. Commun. 2011, 47, 10963–10965. [Google Scholar] [CrossRef]
- Afanasiev, P.; Kudrik, E.V.; Millet, J.M.; Bouchu, D.; Sorokin, A.B. High-valent diiron species generated from N-bridged diiron phthalocyanine and H2O2. Dalton Trans. 2011, 40, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Afanasiev, P.; Kudrik, E.V.; Albrieux, F.; Briois, V.; Koifman, O.I.; Sorokin, A.B. Generation and characterization of high-valent iron oxo phthalocyanines. Chem. Commun. 2012, 48, 6088–6090. [Google Scholar] [CrossRef]
- Skobelev, I.Y.; Kudrik, E.V.; Zalomaeva, O.V.; Albrieux, F.; Afanasiev, P.; Kholdeeva, O.A.; Sorokin, A.B. Efficient epoxidation of olefins by H2O2 catalyzed by iron “helmet” phthalocyanines. Chem. Commun. 2013, 49, 5577–5579. [Google Scholar] [CrossRef]
- Kieler, H.M.; Bierman, M.J.; Guzei, I.A.; Liska, P.J.; MaGaff, R.W. Racemic iron (III) and cobalt (III) complexes containing a new pentadentate “helmet” phthalocyaniate ligand. Chem. Commun. 2006, 31, 3326–3328. [Google Scholar] [CrossRef]
- Brown, E.S.; Robinson, J.R.; McCoy, A.M.; McGaff, R.W. Efficient catalytic cycloalkane oxidation employing a “helmet” phthalocyaninato iron (III) complex. Dalton Trans. 2011, 40, 5921–5925. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Baba, K.; Nakajima, S.; Takanami, T. High-valent metalloporphyrin, Fe(tpp)OTf, catalyzed rearrangement of α,β-epoxy ketones into 1, 2-diketones. Chem. Commun. 2002, 2570–2571. [Google Scholar] [CrossRef]
- Suda, K.; Nakajima, S.; Satoh, Y.; Takanami, T. Metallophthalocyanine complex, Cr(TBPC)OTf: an efficient, recyclable Lewis acid catalyst in the regio- and stereoselective rearrangement of epoxides to aldehydes. Chem. Commun. 2009, 1255–1257. [Google Scholar] [CrossRef]
- Samples Availability: Samples of the compounds (FePcCl, AgNO3, AgOTf, AgBF4, AgSbF6 cyclohexene, cyclohexene oxide, 2-cyclohexen-1-ol, 2-cyclohexen-1-one, and cyclohexane-1,2-diol) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Chen, W. A Mild Catalytic Oxidation System: FePcOTf/H2O2 Applied for Cyclohexene Dihydroxylation. Molecules 2015, 20, 8429-8439. https://doi.org/10.3390/molecules20058429
Zhou B, Chen W. A Mild Catalytic Oxidation System: FePcOTf/H2O2 Applied for Cyclohexene Dihydroxylation. Molecules. 2015; 20(5):8429-8439. https://doi.org/10.3390/molecules20058429
Chicago/Turabian StyleZhou, Baocheng, and Wenxing Chen. 2015. "A Mild Catalytic Oxidation System: FePcOTf/H2O2 Applied for Cyclohexene Dihydroxylation" Molecules 20, no. 5: 8429-8439. https://doi.org/10.3390/molecules20058429
APA StyleZhou, B., & Chen, W. (2015). A Mild Catalytic Oxidation System: FePcOTf/H2O2 Applied for Cyclohexene Dihydroxylation. Molecules, 20(5), 8429-8439. https://doi.org/10.3390/molecules20058429





