Effect of Chum Salmon Egg Lectin on Tight Junctions in Caco-2 Cell Monolayers
Abstract
:1. Introduction
2. Results and Discussion
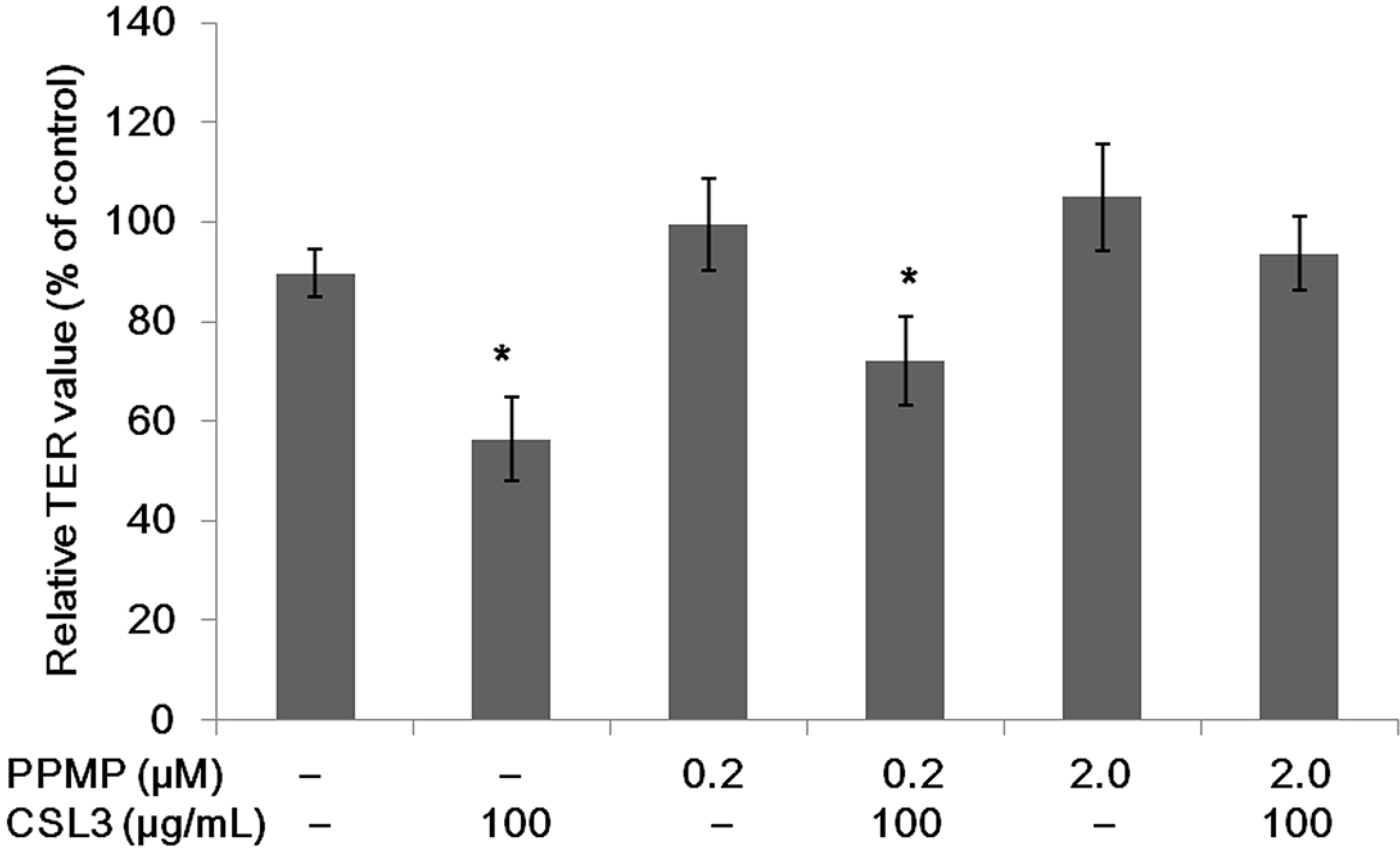
2.1. Effect of Lectins on the TER Values
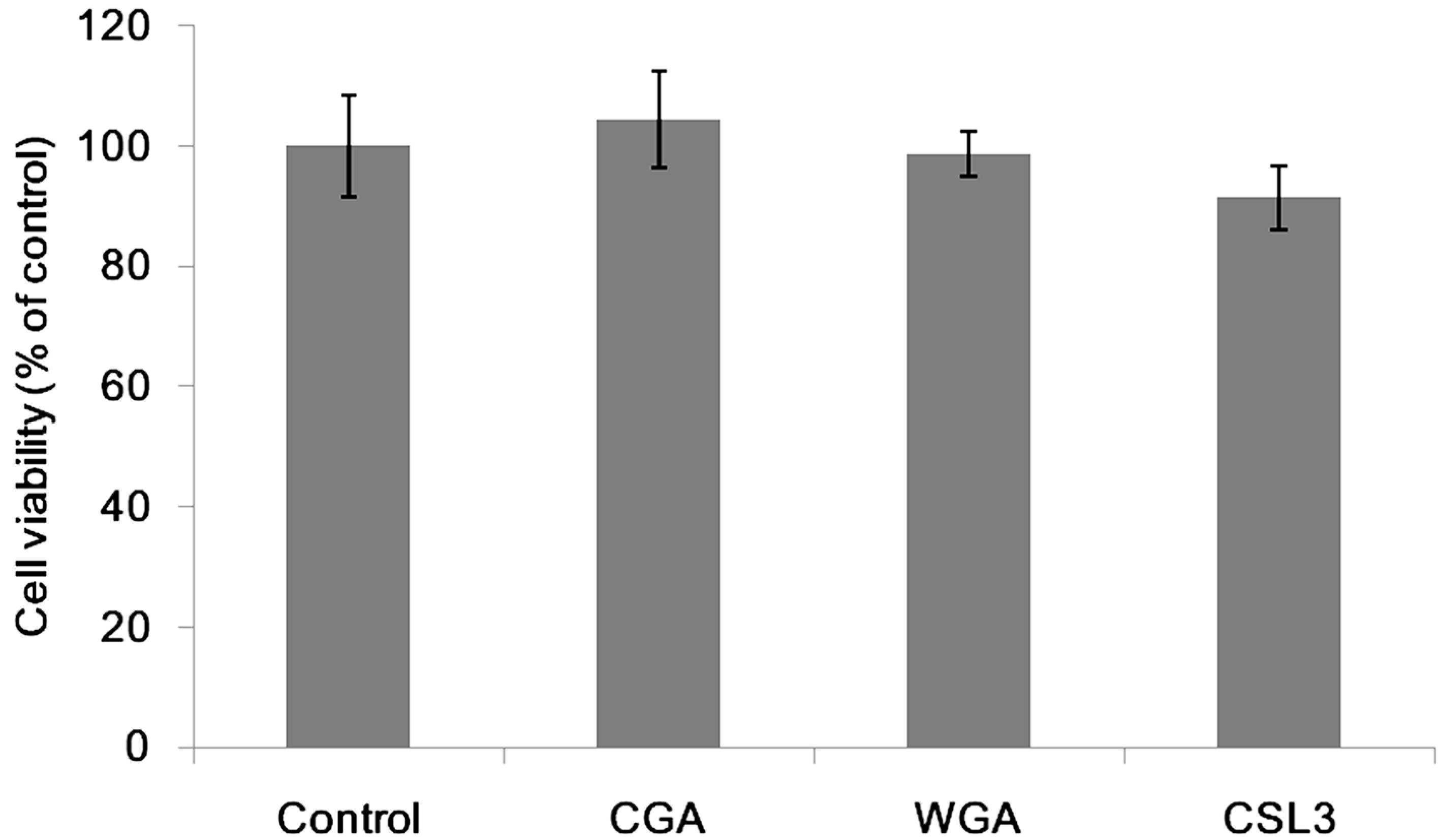
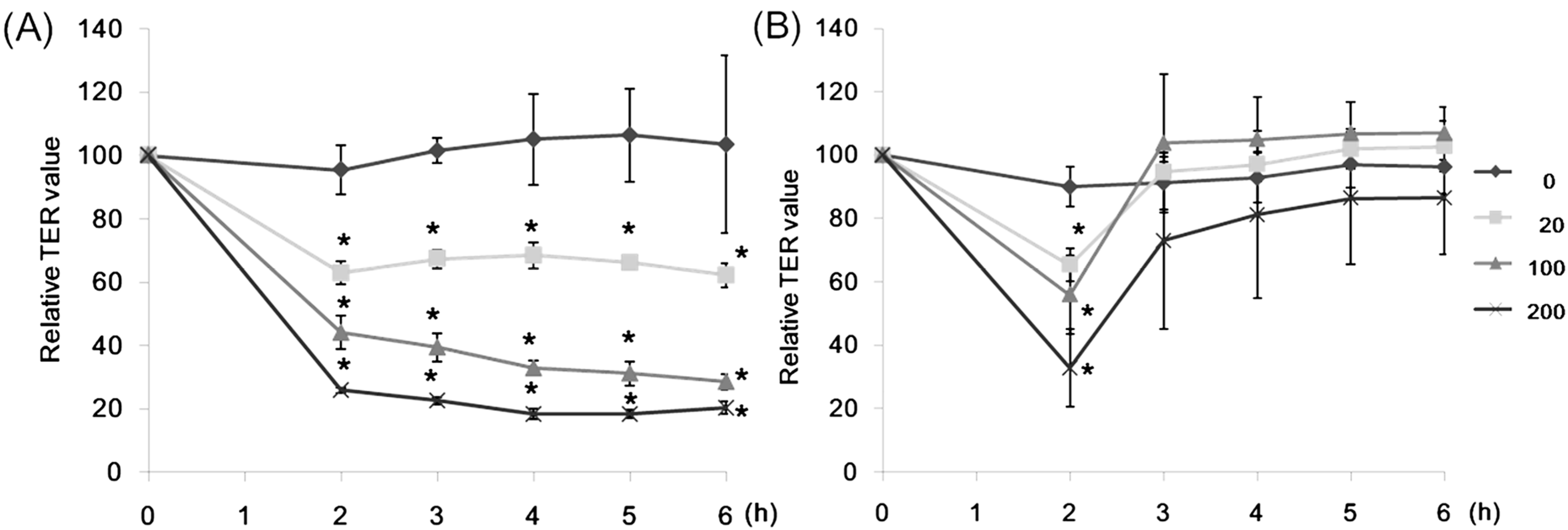
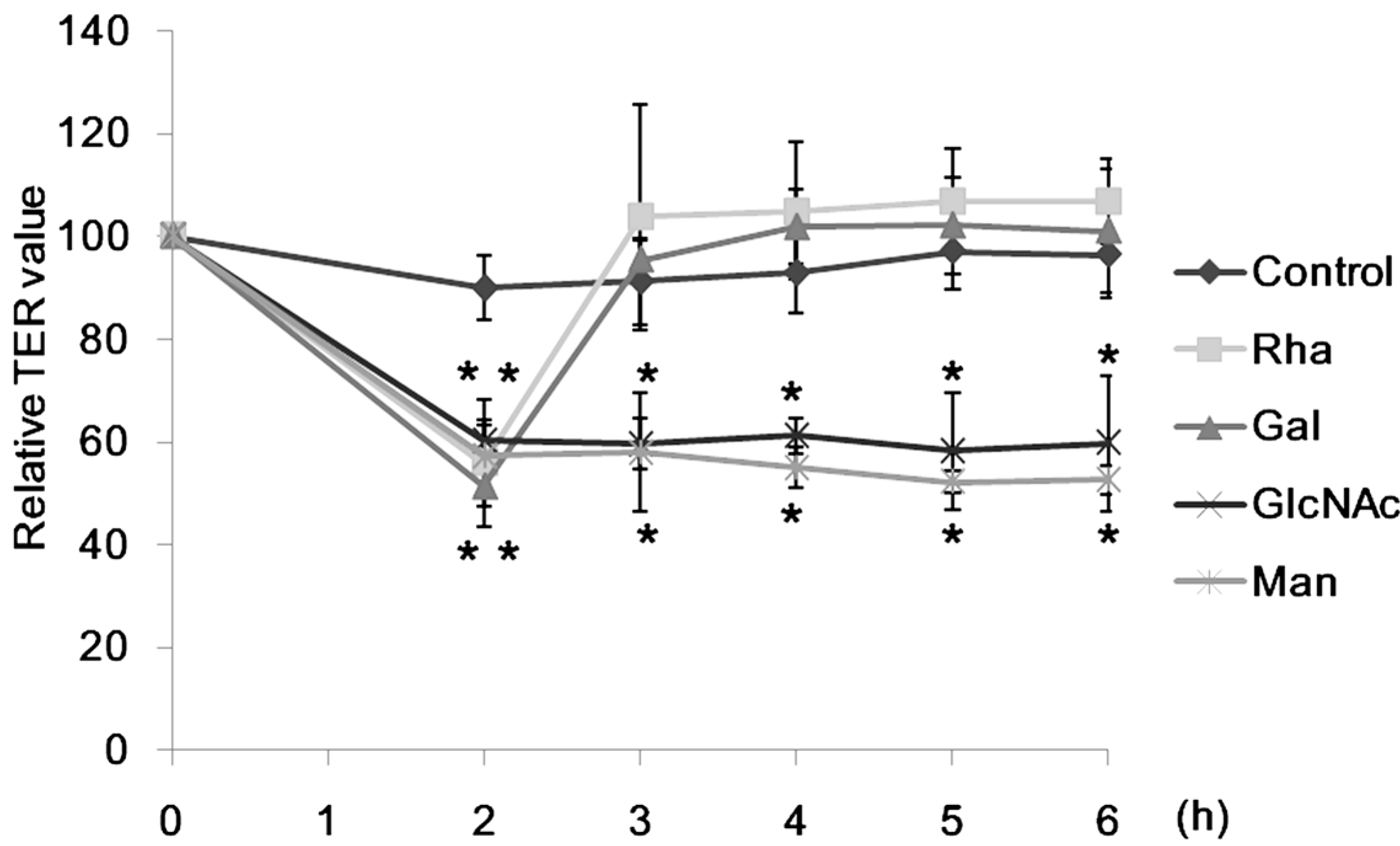
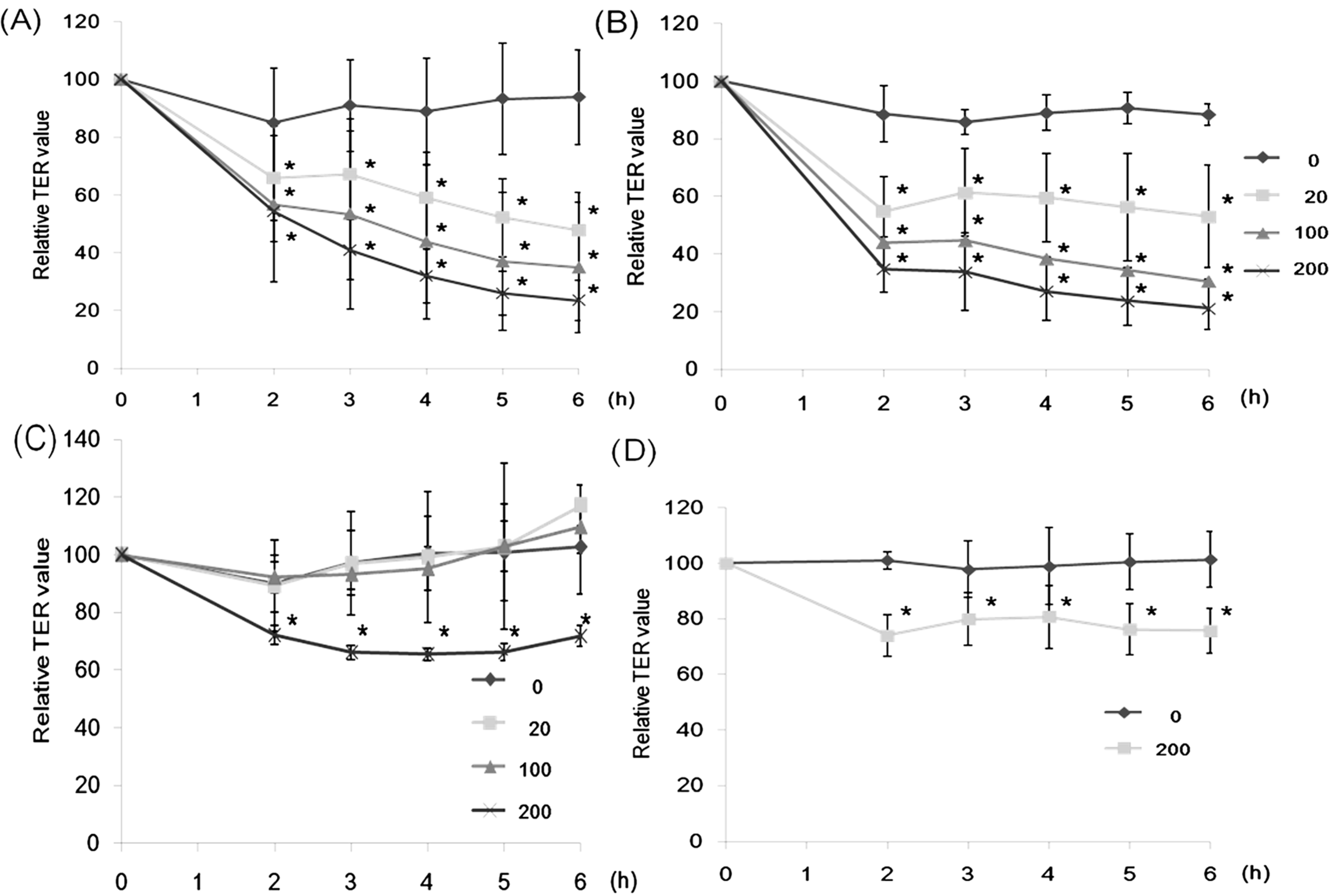
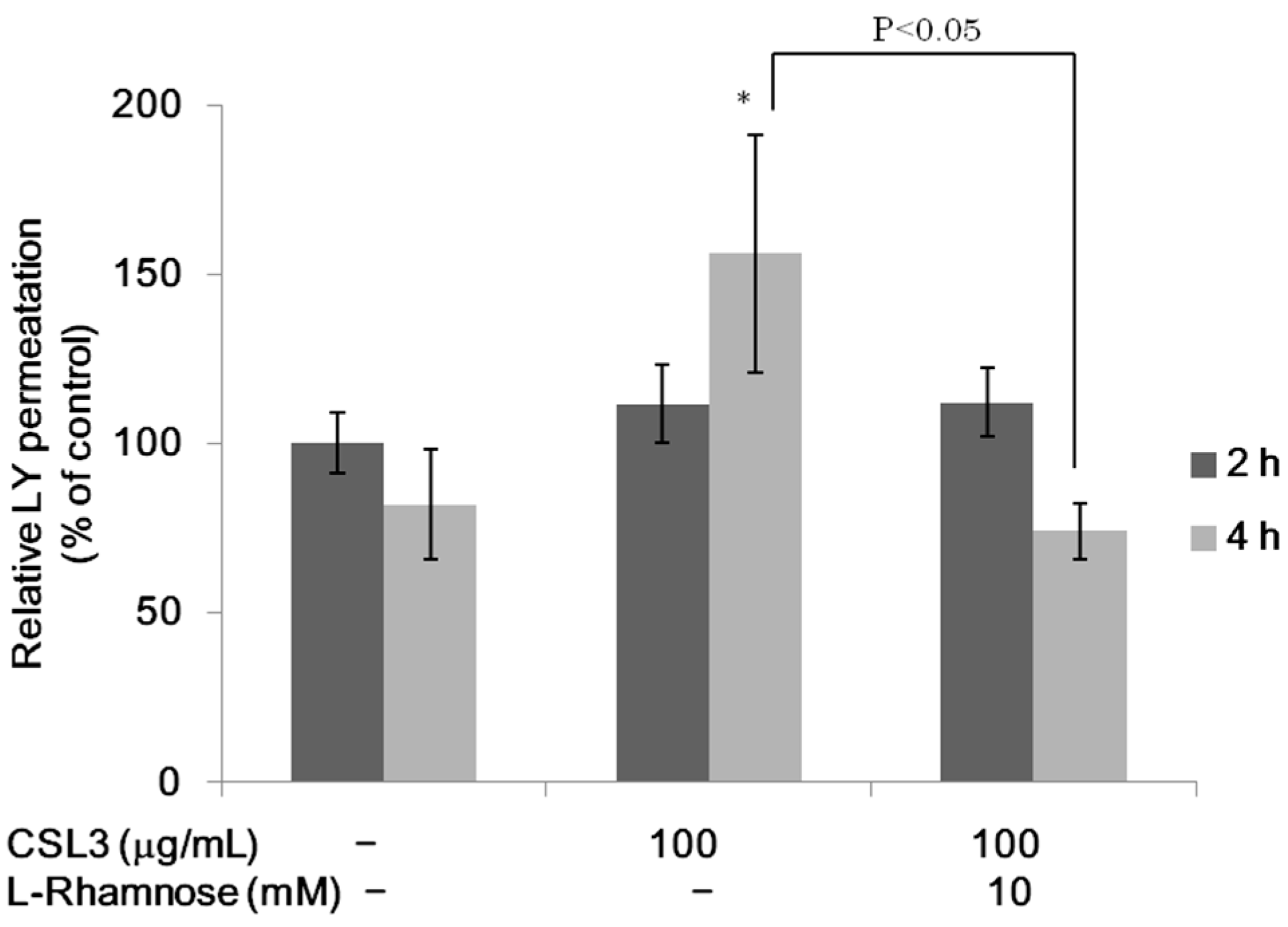
2.2. Effect of CSL3 on LY Transport
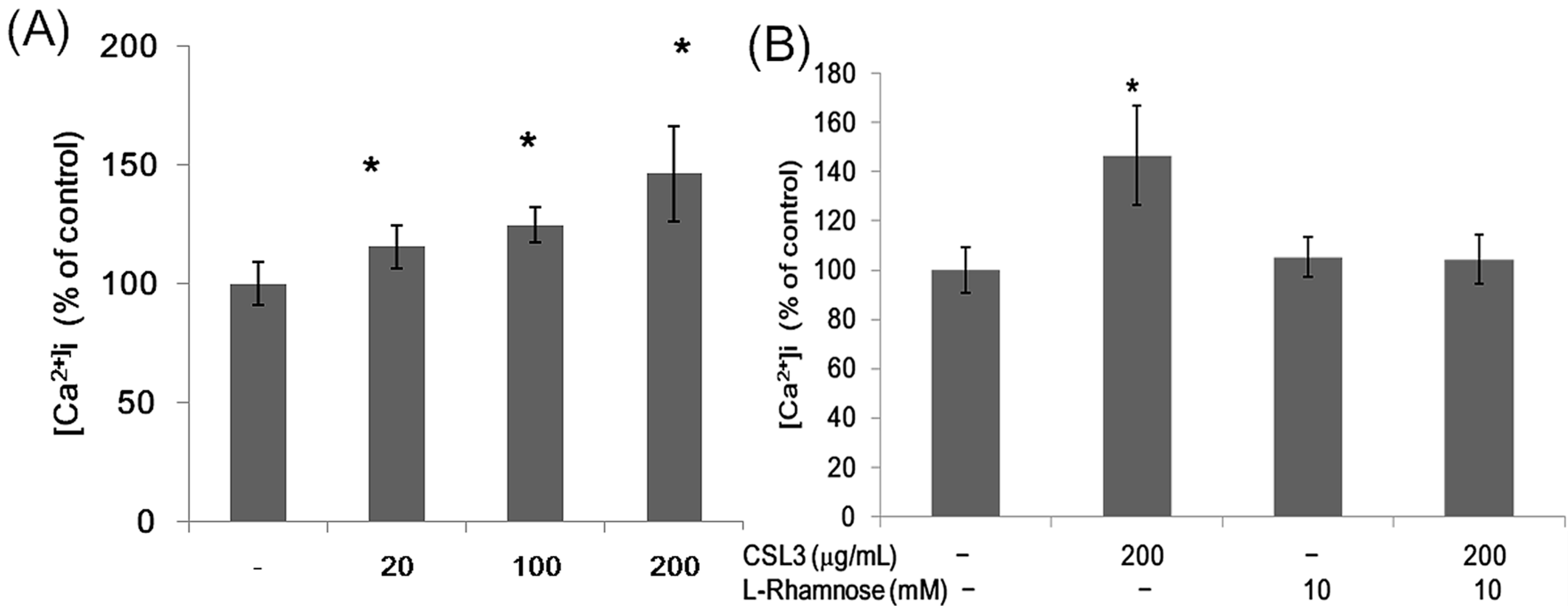
2.3. Effect of CSL3 on the Intracellular Ca2+
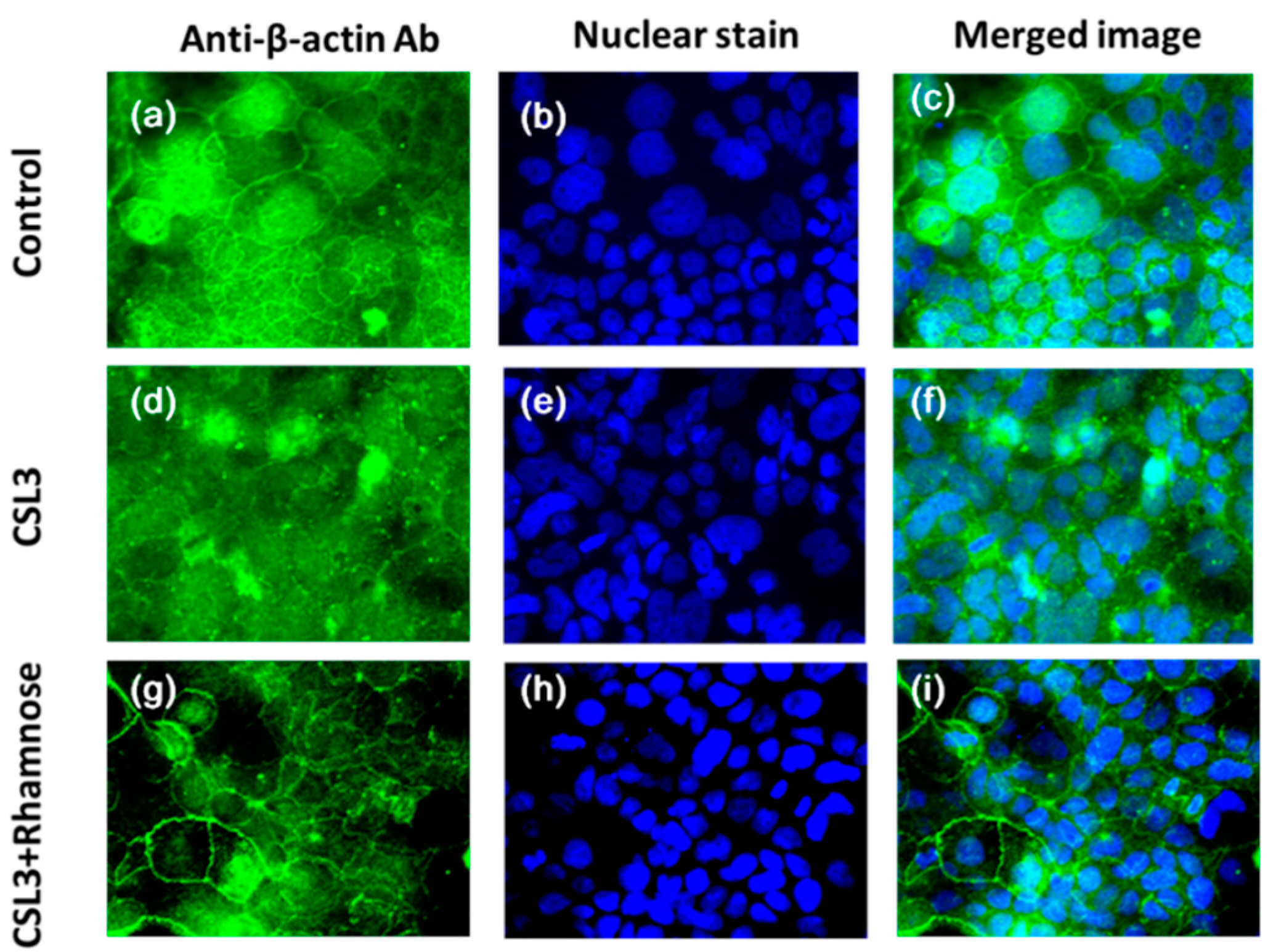
2.4. Effect of CSL3 on β-Actin
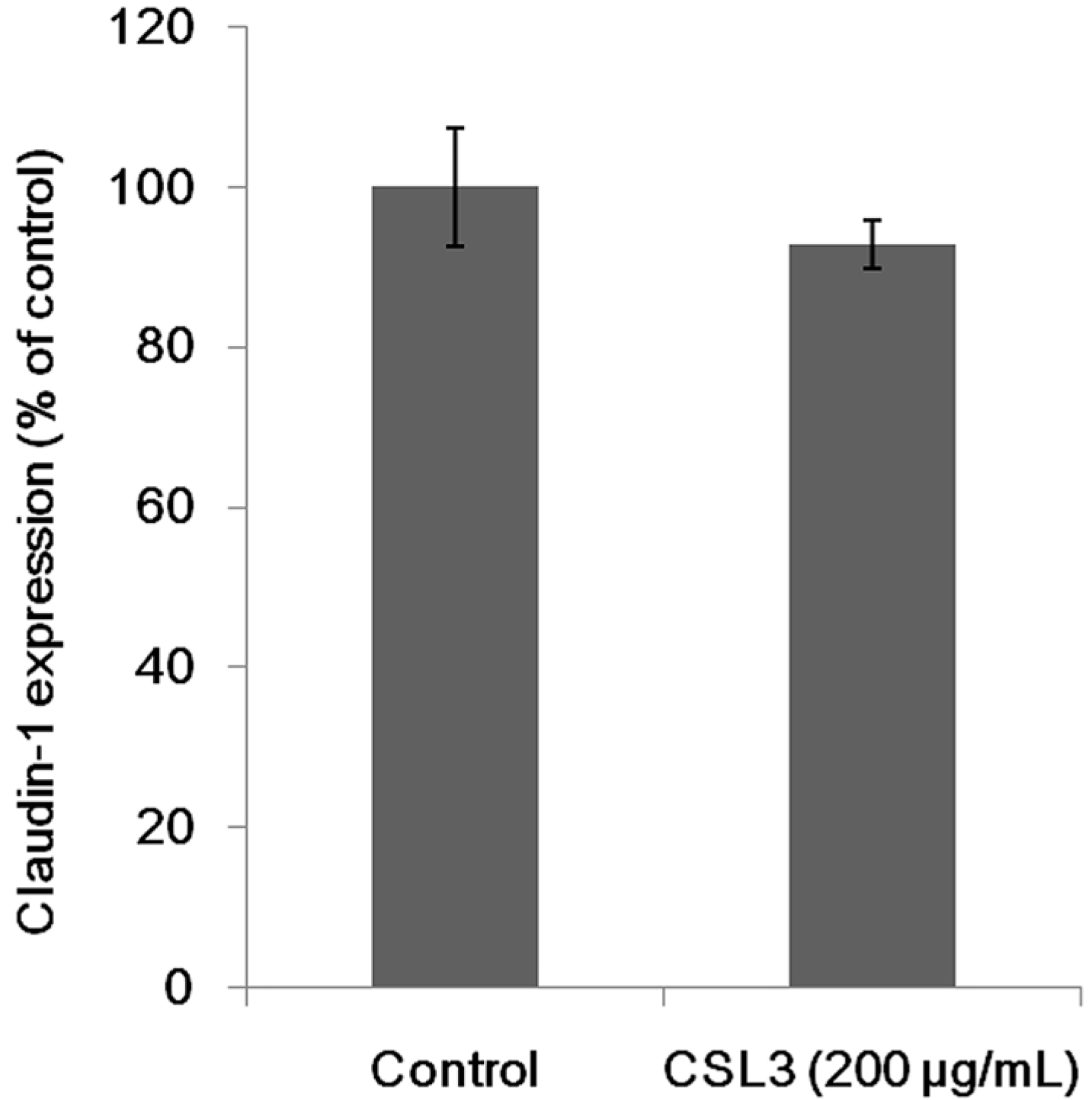
2.5. Effect of CSL3 on Claudin-1 Expression
2.6. Effect of CSL3 on TER Values in Gb3-Defective Caco-2 Cell Monolayers
3. Experimental Section
3.1. Materials
3.2. Cell Culture and TER Measurement
3.3. Effect of Lectins on Tight Junction (TJ)
3.4. LY Transport Experiment
3.5. Intracellular Ca2+ Measurement
3.6. Fluorescence Immunostaining
3.7. Western Blotting
3.8. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharon, N.; Lis, H. Detection, Occurrence and Isolation. In Lectins, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 33–61. [Google Scholar]
- Lectin Classification. In Handbook of Plant Lectins: Properties and Biochemical Applications; van Damme, E.J.M.; Peumans, W.J.; Pusztai, A.; Bardoez, S. (Eds.) John Wiley & Sons: West Sussex, UK, 1998; pp. 31–55.
- Pusztai, A.; Boedocz, S. Biologic effects of plant lectins on the gastrointestinal tract: Metabolic consequences and applications. Trends Glycosci. Glycotechnol. 1997, 8, 149–165. [Google Scholar] [CrossRef]
- Pusztai, A.; Greer, F.; Grant, G. Specific uptake of dietary lectins into the systemic circulation of rats. Biochem. Soc. Trans. 1989, 17, 481–482. [Google Scholar]
- Henney, L.; Ahmed, E.M.; George, D.E.; Kao, K.J.; Sitren, H.S. Tolerance to long-term feeding of isolated peanut lectin in the rat: Evidence for a trophic effect on the small intestines. J. Nutr. Sci. Vitaminol. (Tokyo) 1990, 6, 599–607. [Google Scholar] [CrossRef]
- Vasconcelos, I.M.; Oliveira, J.T.A. Antinutritional properties of plant lectins. Toxicon 2004, 44, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M. Interaction between Food Subbstances and the Intestinal Epithelium. Biosci. Biotechnol. Biochem. 2010, 74, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Robine-Leon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simmon-Assmann, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell. 1983, 47, 323–330. [Google Scholar]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon cancer cell line (Caco-2) as model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [PubMed]
- Konishi, Y.; Hagiwara, K.; Shimizu, M. Transepithelial transport of fluorescein in Caco-2 cell monolayers and use of such transport in in vitro evaluation of phenolic acid availability. Biosci. Biotechnol. Biochem. 2002, 66, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y. Modulation of food-derived substances on intestinal permeability in Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 2297–2299. [Google Scholar] [CrossRef] [PubMed]
- Ulluwishewa, D.; Anderson, R.; McNabb, W.; Moughan, P.; Wells, J.; Roy, N. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Naganuma, T.; Ogawa, T.; Muramoto, K. Effect of lectins on the transport of food factors in Caco-2 cell monolayers. J. Agric. Food Chem. 2006, 54, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tomiyama, M.; Nemoto, R.; Naganuma, T.; Ogawa, T.; Muramoto, K. Effects of food lectins on the transport system of human intestinal Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2013, 77, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Kenmochi, E.; Kabir, S.R.; Ogawa, T.; Naude, R.; Tateno, H.; Hirabayashi, J.; Muramoto, K. Isolation and biochemical characterization of Apios tuber lectin. Molecules 2015, 20, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Shiina, N.; Tateno, H.; Ogawa, T.; Muramoto, K.; Saneyoshi, M.; Kamiya, H. Isolation and characterization of l-rhamonose-binding lectins from chum salmon (Oncorhynchus keta) eggs. Fish. Sci. 2002, 68, 1352–1366. [Google Scholar] [CrossRef]
- Shirai, T.; Watanabe, Y.; Lee, M.; Ogawa, T.; Muramoto, K. Structure of Rhamnose-binding lectin CSL3: Unique pseudo-tetramic architecture of pattern recognition protein. J. Mol. Biol. 2009, 391, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, T.; Tomita, M.; Sadahiro, S.; Hayashi, M.; Awazu, S. Absorption-enhancing effect of sodium caprte and palmitoyl carnitin in rat and human colons. Dig. Dis. Sci. 1998, 43, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, Y.; Han, J.; Bellila, A.; Isoda, H.; Tanaka, T. Cofilin mediates tight-junction opening by redistributing actin and tight-junction protein. Biochem. Biophys. Res. Commun. 2008, 377, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Koninska, A.; Andlauer, W. Modulation of tight junction integrity by food components. Food Res. Int. 2013, 54, 951–960. [Google Scholar] [CrossRef]
- Yamamoto, S.; Naganuma, T.; Ogawa, T.; Muramoto, K. Proteomic analysis of Caco-2 cells treated with food lectins. J. Clin. Biochem. Nutr. 2008, 43 (Suppl. 1), 70–73. [Google Scholar]
- Suzuki, T.; Tanabe, S.; Hara, H. Kaempferol enhances intestinal barrier function through the cytoskeltal association and expression of the tight junction proteins in Caco-2 cells. J. Nutr. 2011, 141, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kunitake, Y.; Hirasaki, N.; Tanaka, M.; Tatsui, T. Theaflavins enhance intestinal barrier of Caco-2 cell monolayers through the expression of AMP-activated protein kinase-mediated occluding, claudin-1, and ZO-1. Biosci. Biotechnol. Biochem. 2015, 79, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [PubMed]
- Samples Availability: CSL3 sample is available from authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemoto, R.; Yamamoto, S.; Ogawa, T.; Naude, R.; Muramoto, K. Effect of Chum Salmon Egg Lectin on Tight Junctions in Caco-2 Cell Monolayers. Molecules 2015, 20, 8094-8106. https://doi.org/10.3390/molecules20058094
Nemoto R, Yamamoto S, Ogawa T, Naude R, Muramoto K. Effect of Chum Salmon Egg Lectin on Tight Junctions in Caco-2 Cell Monolayers. Molecules. 2015; 20(5):8094-8106. https://doi.org/10.3390/molecules20058094
Chicago/Turabian StyleNemoto, Ryo, Shintaro Yamamoto, Tomohisa Ogawa, Ryno Naude, and Koji Muramoto. 2015. "Effect of Chum Salmon Egg Lectin on Tight Junctions in Caco-2 Cell Monolayers" Molecules 20, no. 5: 8094-8106. https://doi.org/10.3390/molecules20058094
APA StyleNemoto, R., Yamamoto, S., Ogawa, T., Naude, R., & Muramoto, K. (2015). Effect of Chum Salmon Egg Lectin on Tight Junctions in Caco-2 Cell Monolayers. Molecules, 20(5), 8094-8106. https://doi.org/10.3390/molecules20058094





