Silver(I) 2,2'-(1,2-Phenylenedisulfanediyl)diacetic Acid as a Molecular Building Block for a Silver(I)-Cadmium(II) Coordination Polymer
Abstract
:1. Introduction
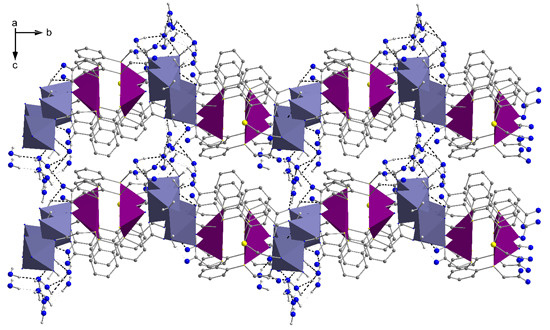
2. Results and Discussion
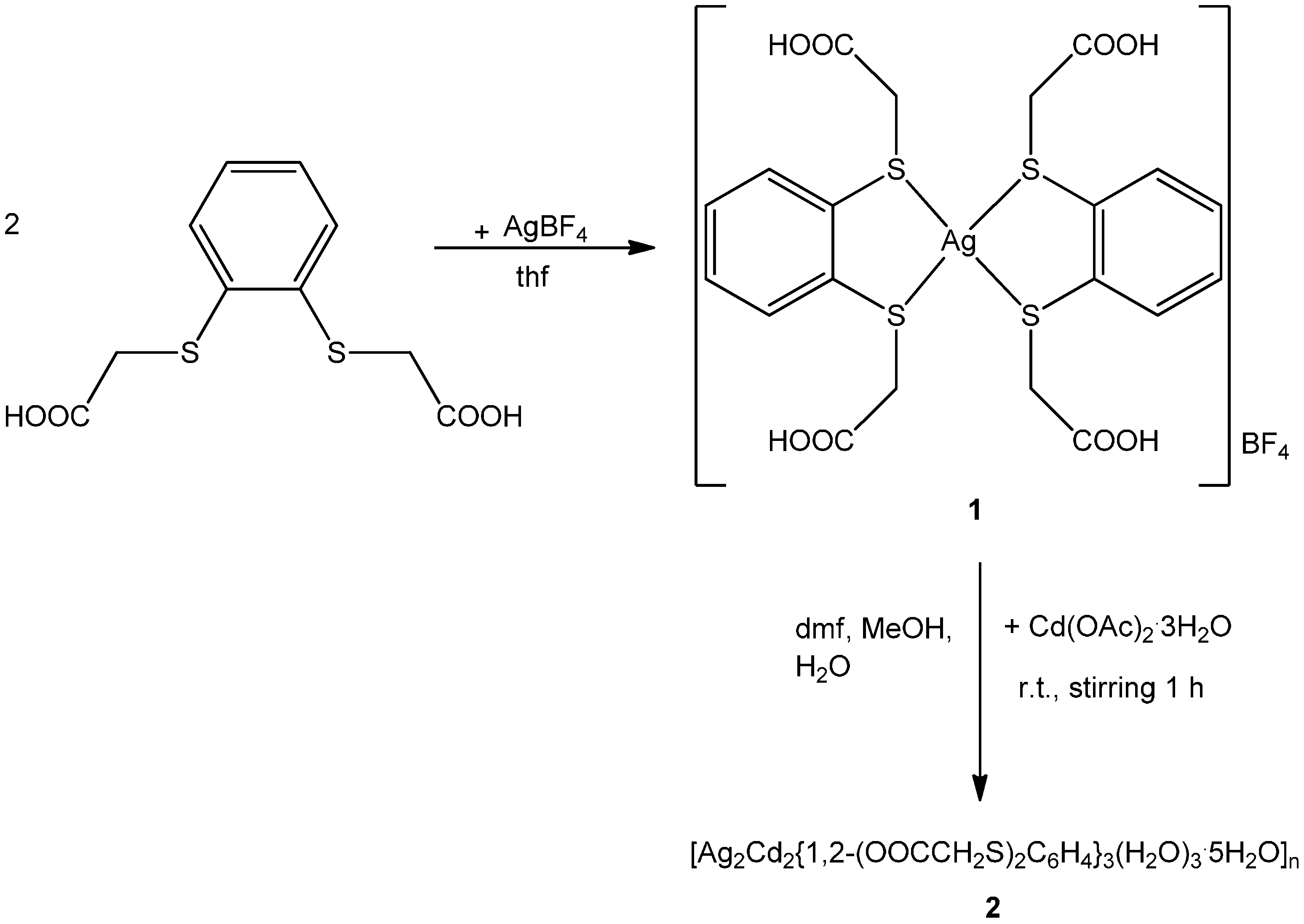
2.1. Synthesis of 1 and 2
2.2. Molecular Structures of 1 and 2
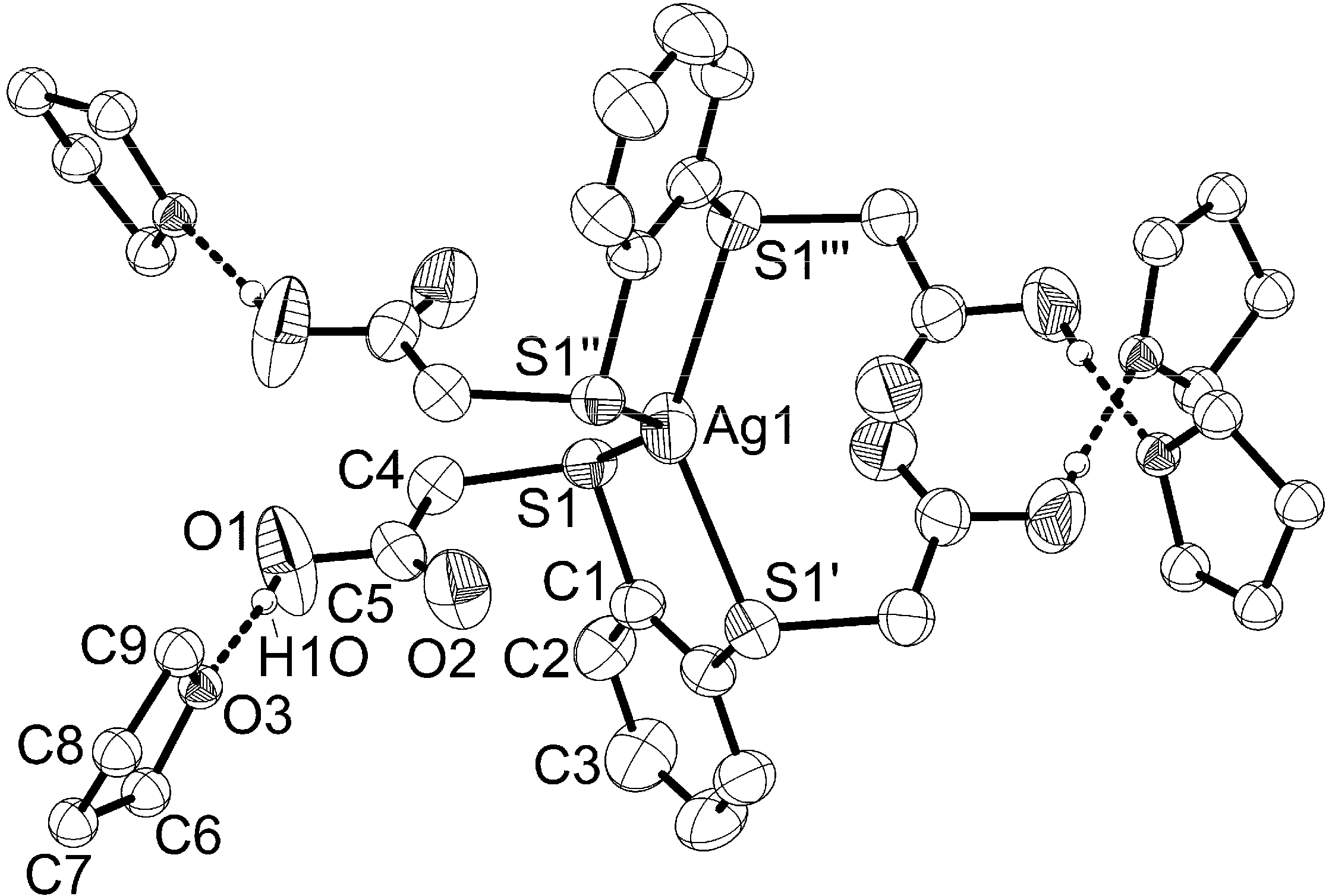
| Ag(1)–S(1) | 2.5518(8) | S(1)–Ag(1)–S(1') | 83.67(3) |
| S(1)–C(1) | 1.778(3) | S(1)–Ag(1)–S(1'') | 140.05(3) |
| S(1)–C(4) | 1.805(3) | S(1)–Ag(1)–S(1''') | 110.10(3) |
| F(1)–B(1) | 1.343(3) | F(1)–B(1)–F(1') | 109.8(1) |
| O(1)–C(5) | 1.307(4) | ||
| O(1)–H(1o) | 0.82(5) | ||
| O(2)–C(5) | 1.182(4) | ||
| O(3)–C(9) | 1.394(7) | ||
| O(3)–C(6) | 1.427(8) |
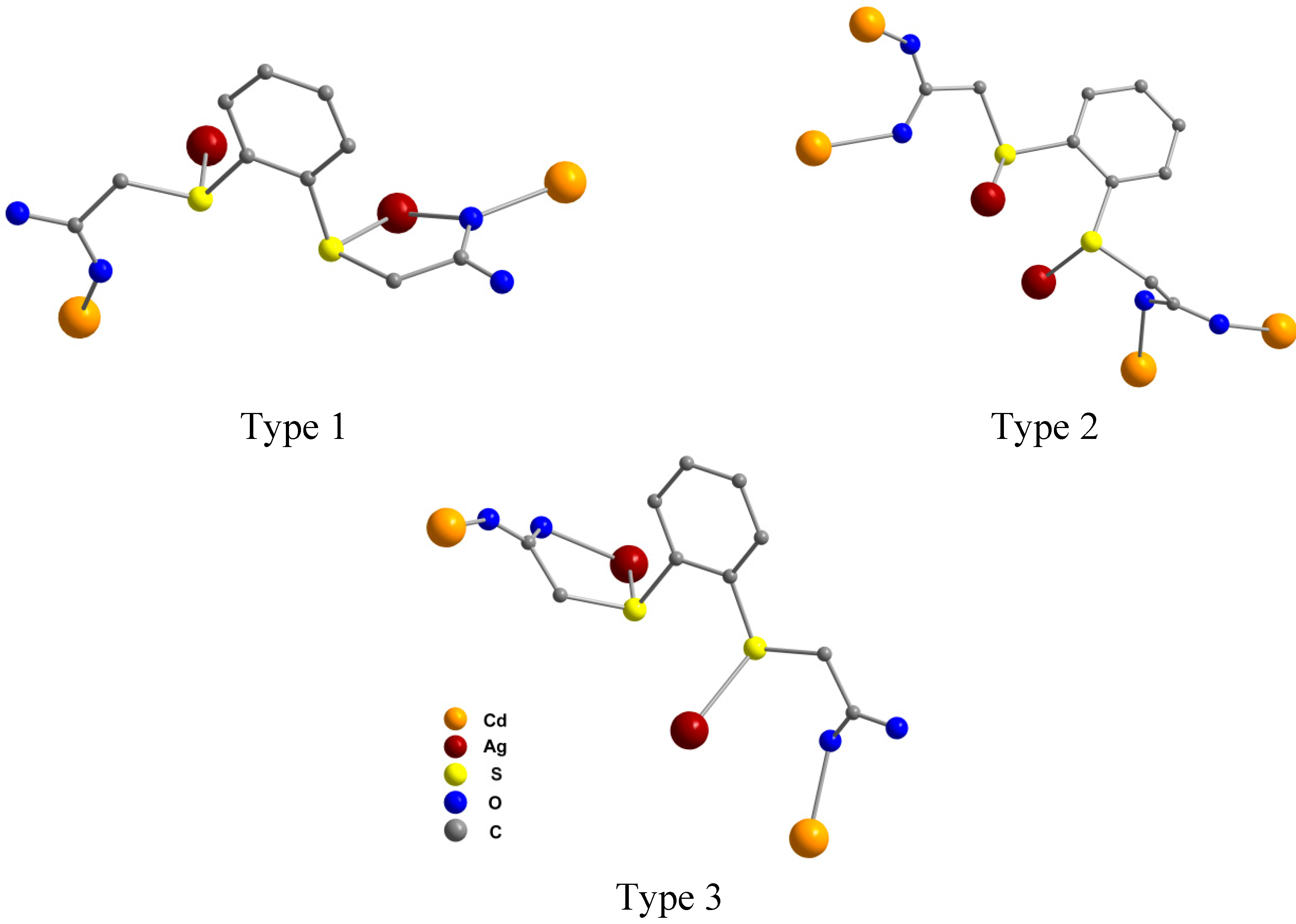
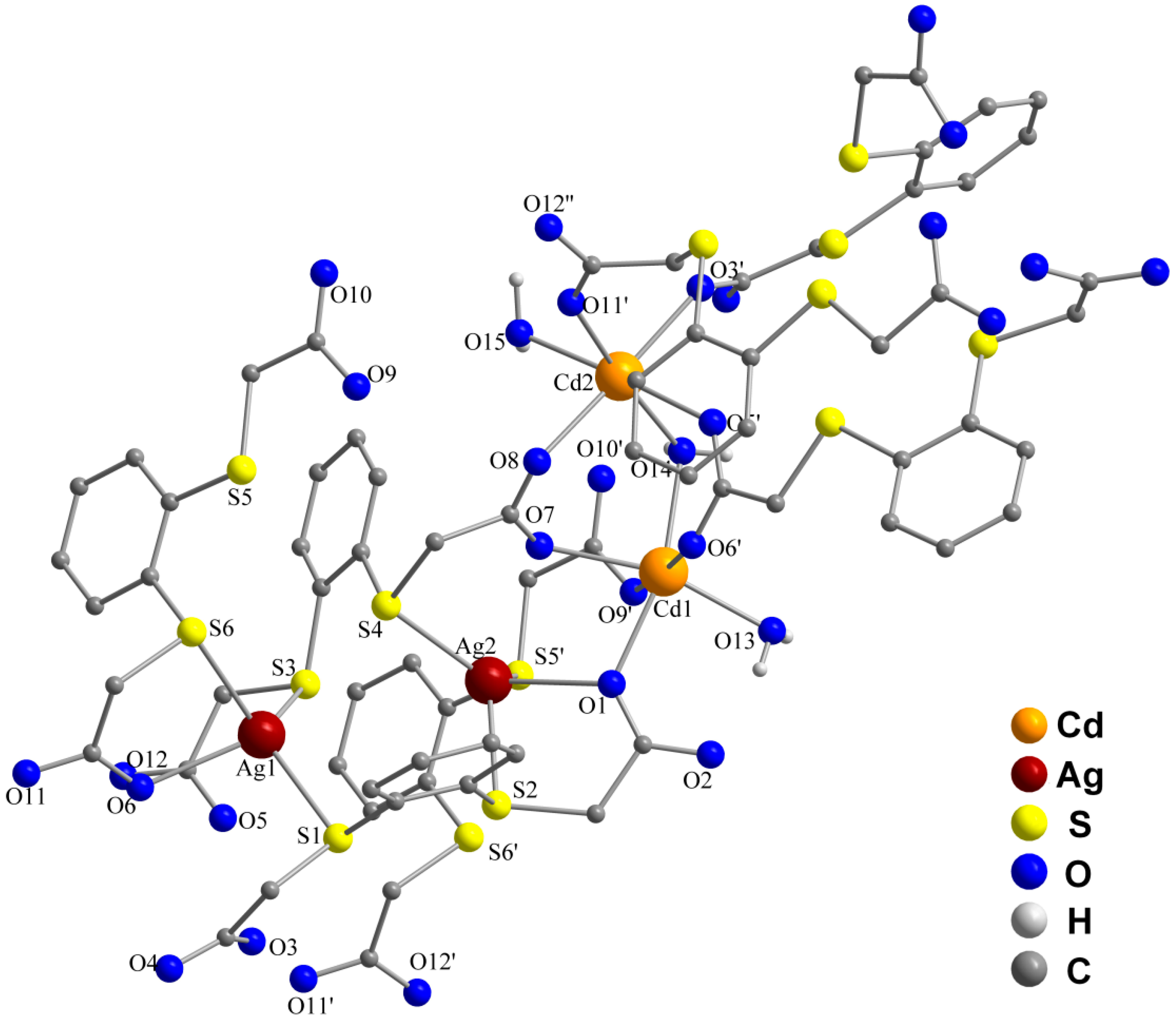
| Cd(1)–O(1) | 2.282(2) | O(1)–Cd(1)–O(7) | 76.97(7) |
| Cd(1)–O(6') | 2.255(2) | O(6')–Cd(1)–O(13) | 90.66(8) |
| Cd(1)–O(7) | 2.295(2) | O(7)–Cd(1)–O(13) | 162.48(8) |
| Cd(1)–O(9') | 2.275(2) | O(7)–Cd(1)–O(9) | 79.70(7) |
| Cd(1)–O(13) | 2.247(2) | O(9)–Cd(1)–O(13) | 91.32(8) |
| Cd(1)–O(14) | 2.301(2) | O(13)–Cd(1)–O(14) | 107.07(8) |
| Cd(2)–O(3') | 2.263(2) | O(3')–Cd(2)–O(8) | 176.54(8) |
| Cd(2)–O(5') | 2.319(2) | O(3')–Cd(2)–O(11') | 92.15(8) |
| Cd(2)–O(8) | 2.289(2) | O(3')–Cd(2)–O(15) | 97.57(1) |
| Cd(2)–O(11') | 2.260(2) | O(5')–Cd(2)–O(8) | 91.68(8) |
| Cd(2)–O(14) | 2.344(2) | O(8)–Cd(2)–O(15) | 82.73(1) |
| Cd(2)–O(15) | 2.268(3) | O(11')–Cd(2)–O(14) | 174.21(8) |
| Ag(1)–O(12) | 2.434(2) | O(12)–Ag(1)–S(1) | 97.80(5) |
| Ag(1)–S(1) | 2.534(8) | O(12)–Ag(1)–S(3) | 124.95(5) |
| Ag(1)–S(3) | 2.571(7) | S(1)–Ag(1)–S(3) | 116.24(2) |
| Ag(1)–S(6) | 2.592(8) | S(1)–Ag(1)–S(6) | 126.86(2) |
| Ag(2)–O(1) | 2.482(2) | O(1)–Ag(2)–S(4) | 118.42(6) |
| Ag(2)–S(2) | 2.604(8) | O(1)–Ag(2)–S(2) | 73.15(5) |
| Ag(2)–S(4) | 2.548(7) | S(2)–Ag(2)–S(5')) | 120.37(2) |
| Ag(2)–S(5') | 2.527(7) | S(4)–Ag(2)–S(2) | 106.58(2) |
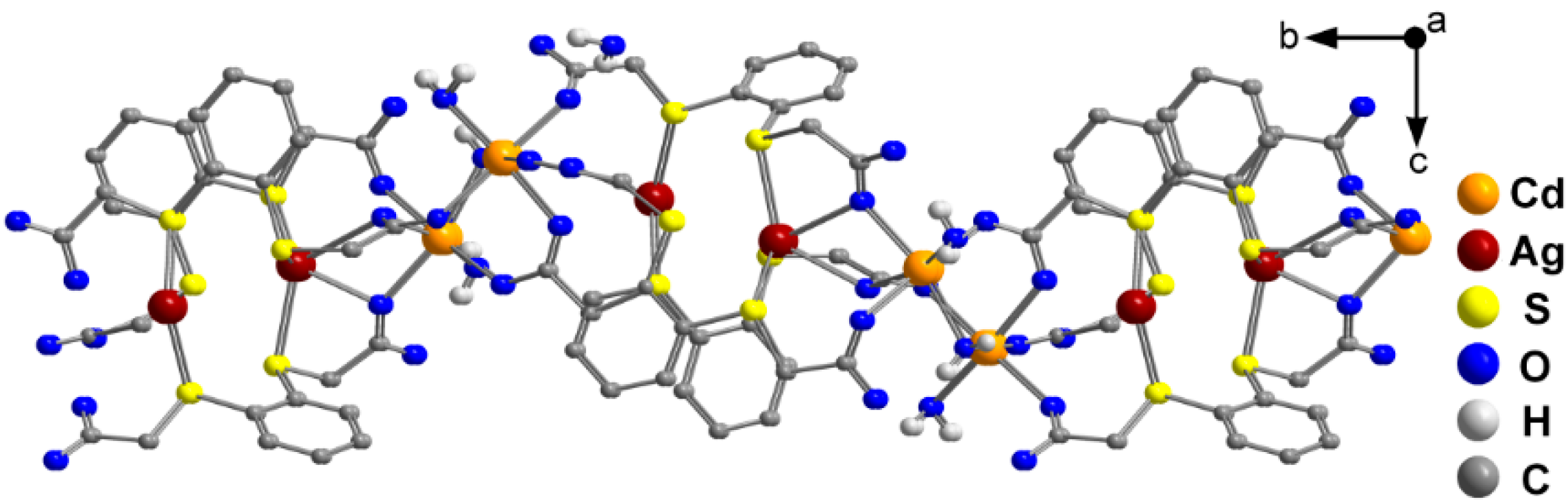
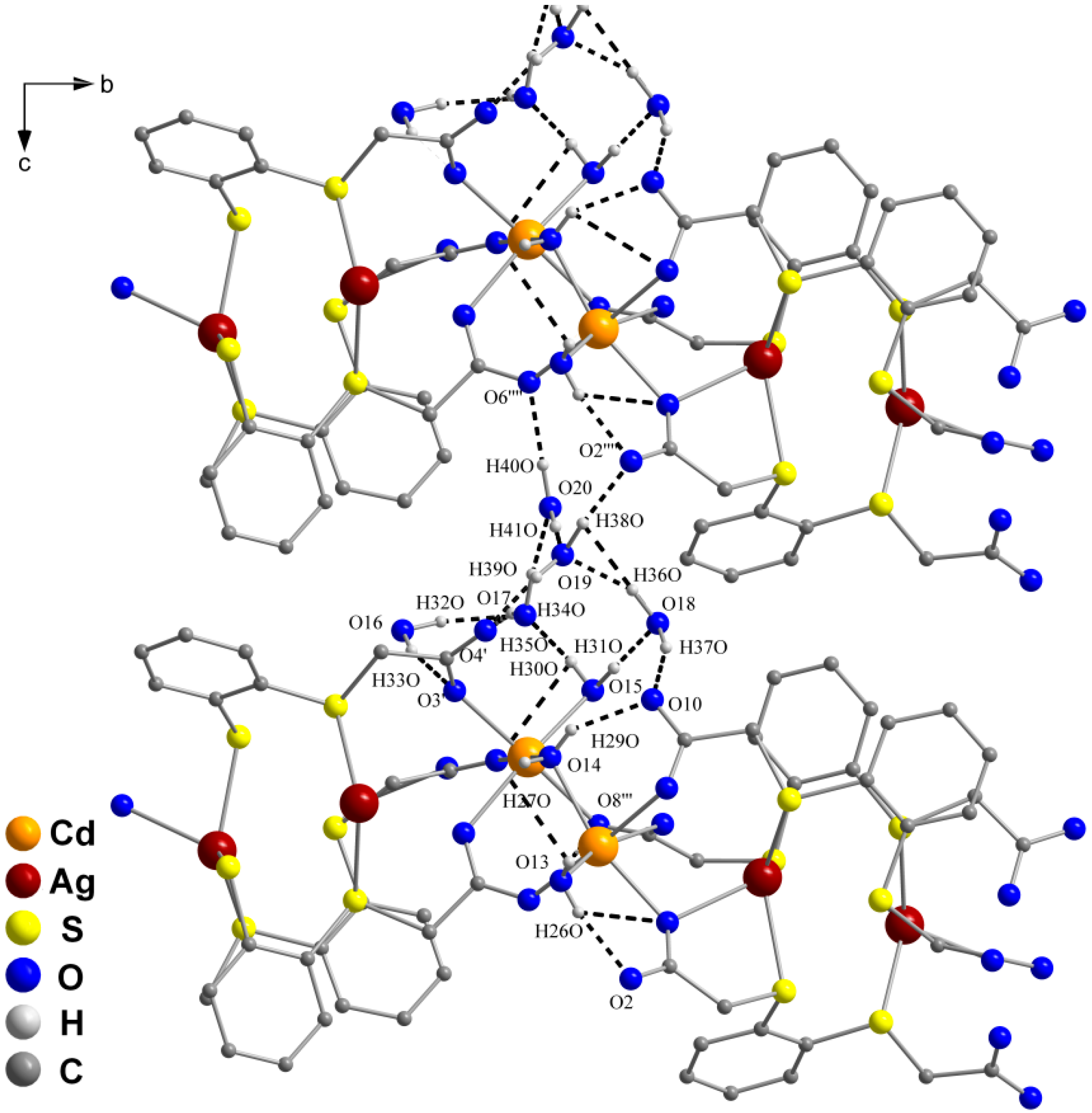
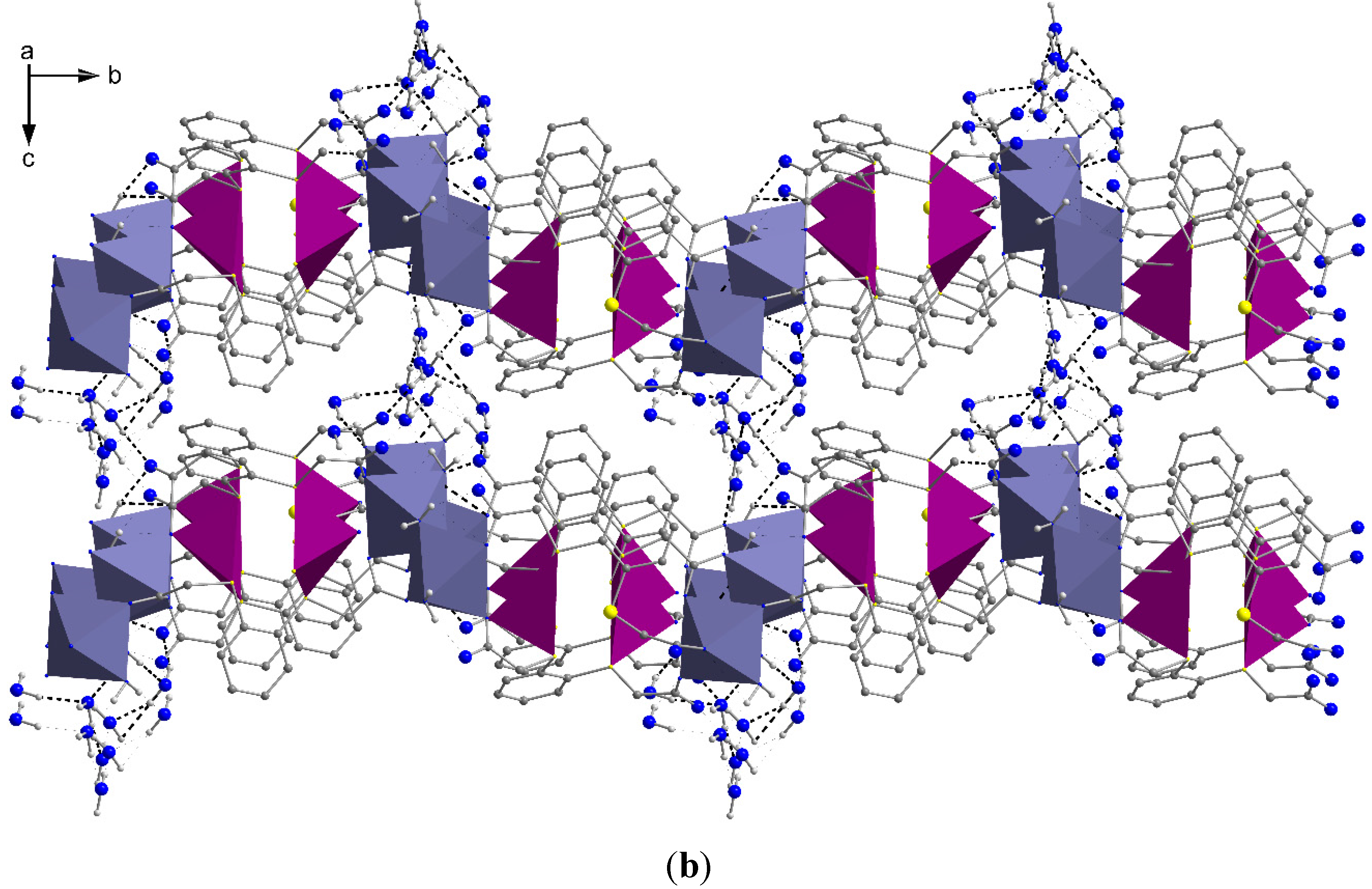
3. Experimental Section
3.1. General Procedures
3.2. Synthesis of (RS,RS,RS,RS/SS,SS,SS,SS)-[Ag{1,2-C6H4(SCH2COOH)2-κ2S,S'}2]BF4 (1)
3.3. Synthesis of [Ag2Cd2{1,2-(OOCCH2S)2C6H4}3(H2O)3.5H2O]n (2)
3.4. X-ray Structure Determination
| 1 | 2 | |
|---|---|---|
| Empirical formula | C20H20AgBF4O8S4 4thf | C30H30Ag2Cd2O15S6 5H2O |
| M/g mol−1 | 999.70 | 1353.52 |
| T/K | 213(2) | 130(2) |
| Crystal system | tetragonal | monoclinic |
| Space group | n2 | P21 |
| a/Å | 12.0071(6) | 7.6790(1) |
| b/Å | 12.0071(6) | 24.2111(3) |
| c/Å | 15.219(1) | 11.7500(2) |
| α/° | 90 | 90 |
| β/° | 90 | 102.175(1) |
| γ/° | 90 | 90 |
| V/Å3 | 2194.1(2) | 2135.39(5) |
| Z | 2 | 2 |
| Dcalcd/Mg m−3 | 1.513 | 2.105 |
| μ/mm−1 | 0.723 | 2.256 |
| F(000) | 1032 | 1332 |
| Reflections collected | 11088 | 48203 |
| Independent reflections | 2238 [R(int) = 0.0209] | 12977 [R(int) = 0.0290] |
| Restraints/parameters | 0/152 | 8/557 |
| Goodness of fit on F2 | 1.061 | 0.985 |
| Final R indices [I > 2 σ(I)] | R1 = 0.0310 | R1 = 0.0227 |
| wR2 = 0.0756 | wR2 = 0.0519 | |
| R indices (all data) | R1 = 0.0379 | R1 = 0.0255 |
| wR2 = 0.086 | wR2 = 0.0524 | |
| Largest diff. peak and hole/e Å−3 | 0.241 and −0.186 | 2.186 and −0.759 |
| Absolute structure parameter | −0.01(4) | –0.02(1) |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Cook, T.R.; Zheng, Y.-R.; Stang, P.J. Metal-organic frameworks and self assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis and functionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Kitagawa, S.; Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 2005, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Noro, S.; Kitagawa, S.; Akutagawa, T.; Nakamura, T. Coordination polymers constructed from transition metal ions and organic N-containing heterocyclic ligands: Crystal structures and microporous properties. Prog. Polym. Sci. 2009, 34, 240–279. [Google Scholar] [CrossRef]
- Ho, C.-H.; Wong, W.-Y. Facile tuning of photophysical traits and emerging applications in organic electronics and photonics. Coord. Chem. Rev. 2011, 255, 2469–2502. [Google Scholar] [CrossRef]
- Bureekaev, S.; Shimomura, S.; Kitagawa, S. Chemistry and application of flexible porous coordination polymers. Sci. Technol. Adv. Mater. 2008, 9, 14108–14120. [Google Scholar] [CrossRef]
- Ma, L.; Abney, C.; Lin, W. Enantioselective catalysis with homochiral metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Murray, K.S. Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- Kurmoo, M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.K.; Rao, C.N.R.; Feller, R.K. Structural diversity and chemical trends in hybrid inorganic-organic framework materials. Chem. Commun. 2006, 4780–4795. [Google Scholar] [CrossRef]
- Shimizu, G.K.H. Assembly of metal ions and ligands with adaptable coordinative tendencies as route to functional metal-organic solids. J. Solid State Chem. 2005, 178, 2519–2526. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Steto, K.C.; Kongshaung, K.O.; Jakobsen, S.; Tilset, M.; Lillerud, K.P. Design, synthesis and characterization of a Pt-Gd metal-organic framework containing potentially catalytically active sites. Dalton Trans. 2008, 2054–2060. [Google Scholar]
- Oh, M.; Carpenter, G.B.; Sweigart, D.A. A coordination network containing metal-organometallic secondary building units based on π-bonded benzoquinone complexes. Chem. Commun. 2002, 2168–2169. [Google Scholar] [CrossRef]
- Oh, M.; Carpenter, G.B.; Sweigart, D.A. A novel 3D brick-wall coordination network based on nodes with square-pyramidal connectivity. Angew. Chem. Int. Ed. 2003, 42, 2026–2028. [Google Scholar] [CrossRef]
- Oh, M.; Carpenter, G.B.; Sweigart, D.A. Metal-mediated self-assembly for π-bonded benzoquinone complexes into polymers with tunable properties. Angew. Chem. Int. Ed. 2001, 40, 3191–3194. [Google Scholar] [CrossRef]
- Bickley, J.; Bonar-Law, R.; McGrath, T.; Singh, N.; Steiner, A. Dirhodium (II) carboxylate complexes as building blocks cis-Chelating dicarboxylic acids designed to bridge the dinuclear core. New J. Chem. 2004, 28, 425–433. [Google Scholar]
- Lin, W.; Rieter, W.J.; Taylor, K.L.M. Modular synthesis of functional nanoscale coordination polymers. Angew. Chem. Int. Ed. 2009, 48, 650–658. [Google Scholar] [CrossRef]
- Chandler, B.T.; Cramb, D.T.; Shimizu, G.K.H. Microporous metal-organic frameworks formed in a step-wise manner from luminescent building blocks. J. Am. Chem. Soc. 2006, 128, 10403–10421. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yuan, L.; Li, H.; Sun, J. A 3D heterometallic metal–organic framework constructed from luminescent building blocks, exhibiting reversible dehydration and rehydration procedure. Inorg. Chem. Commun. 2007, 10, 1281–1284. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Maggini, S.; Proserpio, D.M.; Visconti, M. Heterometallic modular metal-organic 3D frameworks assembled via new tris-β-diketonate metalloligands: Nanoporous materials for anion exchange and scaffolding of selected anionic guests. Chem. Eur. J. 2010, 16, 12328–12341. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-P.; Li, D.-J.; Gao, D.-Z.; Chen, J.; Liu, Z.-Q.; Liao, D.-Z.; Jiang, Z.-H.; Yan, S.-P. One-dimensional diamagnetic-metal nitronyl nitroxide radical complexes with dicyanoargentate(I) bridges: M(NIT4Py)2[Ag(CN)2]2 (M = Zn, Cd). Z. Anorg. Allg. Chem. 2005, 631, 1702–1705. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Wei, Z.-H.; Ng, S.W. Poly[bis(μ3-thiocyanato-κ3N:S:S')(μ2-thiocyanato-κ2N:S)(4'-p-tolyl-2,2':6',2''-terpyridine-κ3N,N',N'')cadmium(II)silver(I)]. Acta Crystallogr. Sect. E: Struct. Rep. Online 2010, 66, m1313–m1314. [Google Scholar] [CrossRef]
- Hahn, T. International Tables for Crystallography, 5th ed.; The International Union of Crystallography and Springer: Dordrecht, The Netherlands, 2005; Volume A. [Google Scholar]
- Li, J.-R.; Bu, X.-H.; Jiao, J.; Du, W.-P.; Xu, X.-H.; Zhang, R.-H. Novel dithioether–silver(I) coordination architectures: Structural diversities by varying the spacers and terminal groups of ligands. Dalton Trans. 2005, 464–474. [Google Scholar] [CrossRef]
- Hittenhausen, H.; van der Meer, H. Silver complex of 4,7-dithiadecane-1,10-dicarboxylic acid, C20H35AgO8S4. Cryst. Struct. Commun. 1978, 7, 385–387, (CCDC code TDECAG). [Google Scholar]
- Brunner, H.; Hollman, A.; Zabel, M.; Nuber, B. The ligand [Cp2MoH2] in complexes with Ag-S bonds. J. Organomet. Chem. 2000, 609, 44–52. [Google Scholar] [CrossRef]
- Gao, S.; Liu, J.-W.; Huo, L.-H.; Zhao, H.; Zhao, J.-G. A one-dimensional chain CdII polymer: Catena-poly[[tris(1H-imidazole-κN3)cadmium(II)]-μ-benzene-1,4-dioxyacetato-κ3O,O':O'']. Acta Crystallogr. Sect. E: Struct. Rep. Online 2004, 60, m1308–m1310. [Google Scholar] [CrossRef]
- Niu, S.Y.; Chi, Y.X.; Jin, J.; Yang, G.D.; Ye, L. Synthesis and structure of the coordinatively unsaturated boron subphthalocyanine cation, [B(SubPc)]. Struct. Chem. 2006, 17, 209–216. [Google Scholar] [CrossRef]
- Wang, L.; Yang, M.; Li, G.; Shi, Z.; Feng, S. Synthesis and characterization of f-element iodate architectures with variable dDimensionality, α- and β-Am(IO3)3. Inorg. Chem. 2006, 45, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- Hunger, J.; Krautscheid, H.; Sieler, J. Hydrothermal synthesis and structure of coordination polymers by combination of bipyrazole and aromatic dicarboxylate ligands. Cryst. Growth Des. 2009, 9, 4613–4625. [Google Scholar] [CrossRef]
- Rietmeijer, F.J.; Birker, P.J.M.W.L.; Gorter, S.; Reedijk, J. Copper(I) and copper(II) chelates containing imidazole and thioether groups; Synthesis of the ligand 1,2 bis(benzimidazol-2'-ylmethylthio)-benzene (bbtp) and the X-ray crystal structure at −52 °C of [Cu(bbtp)(H2O)][ClO4]2·5EtOH. J. Chem. Soc. Dalton Trans. 1982, 1191–1198. [Google Scholar] [CrossRef]
- Cabiddu, M.G.; Cabiddu, S.; Cadoni, E.; de Montis, S.; Fattuoni, C.; Melis, S.; Sotgiu, F. Metallation reactions. XXVI. α,α'-Dimetallation of 1,2-bis(methylthiobenzene). Tetrahedron 1999, 55, 14069–14078. [Google Scholar] [CrossRef]
- SMART, Area-Detector Software Package, Siemens Industrial Automation, Inc.: Madison, WI, USA, 1993.
- SAINT, Area-Detector Integration Software, Version 6.01; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1999.
- Sheldrick, G.M. SADABS, Program for Scaling and Correction of Area-detector Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- CrysAlisPro: Data collection and data reduction software package, Agilent Technologies, including a numerical adsorption correction (Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. Sect. A: Found. Crystallogr. 1995, 51, 887–897. [Google Scholar]).
- SHELX includes SHELXS97, SHELXL97; Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] .
- SIR92: A program for crystal structure solution; Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.J. Completion and refinement of crystal structures with SIR92. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] .
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND 3—Crystal and Molecular Structure Visualization; Crystal Impact GbR: Bonn, Germany.
- Cambridge Crystallographic Data Centre. Available online: https://summary.ccdc.cam.ac.uk/structure-summary-form (accessed on 4 May 2015).
- Samples Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosu, I.G.; Berghof, C.; Lönnecke, P.; Silaghi-Dumitrescu, L.; Hey-Hawkins, E. Silver(I) 2,2'-(1,2-Phenylenedisulfanediyl)diacetic Acid as a Molecular Building Block for a Silver(I)-Cadmium(II) Coordination Polymer. Molecules 2015, 20, 8020-8032. https://doi.org/10.3390/molecules20058020
Grosu IG, Berghof C, Lönnecke P, Silaghi-Dumitrescu L, Hey-Hawkins E. Silver(I) 2,2'-(1,2-Phenylenedisulfanediyl)diacetic Acid as a Molecular Building Block for a Silver(I)-Cadmium(II) Coordination Polymer. Molecules. 2015; 20(5):8020-8032. https://doi.org/10.3390/molecules20058020
Chicago/Turabian StyleGrosu, Ioana Georgeta, Christiane Berghof, Peter Lönnecke, Luminita Silaghi-Dumitrescu, and Evamarie Hey-Hawkins. 2015. "Silver(I) 2,2'-(1,2-Phenylenedisulfanediyl)diacetic Acid as a Molecular Building Block for a Silver(I)-Cadmium(II) Coordination Polymer" Molecules 20, no. 5: 8020-8032. https://doi.org/10.3390/molecules20058020
APA StyleGrosu, I. G., Berghof, C., Lönnecke, P., Silaghi-Dumitrescu, L., & Hey-Hawkins, E. (2015). Silver(I) 2,2'-(1,2-Phenylenedisulfanediyl)diacetic Acid as a Molecular Building Block for a Silver(I)-Cadmium(II) Coordination Polymer. Molecules, 20(5), 8020-8032. https://doi.org/10.3390/molecules20058020