Cytotoxic Lignan from the Non-Transformed Root Culture of Phyllanthus amarus
Abstract
:1. Introduction
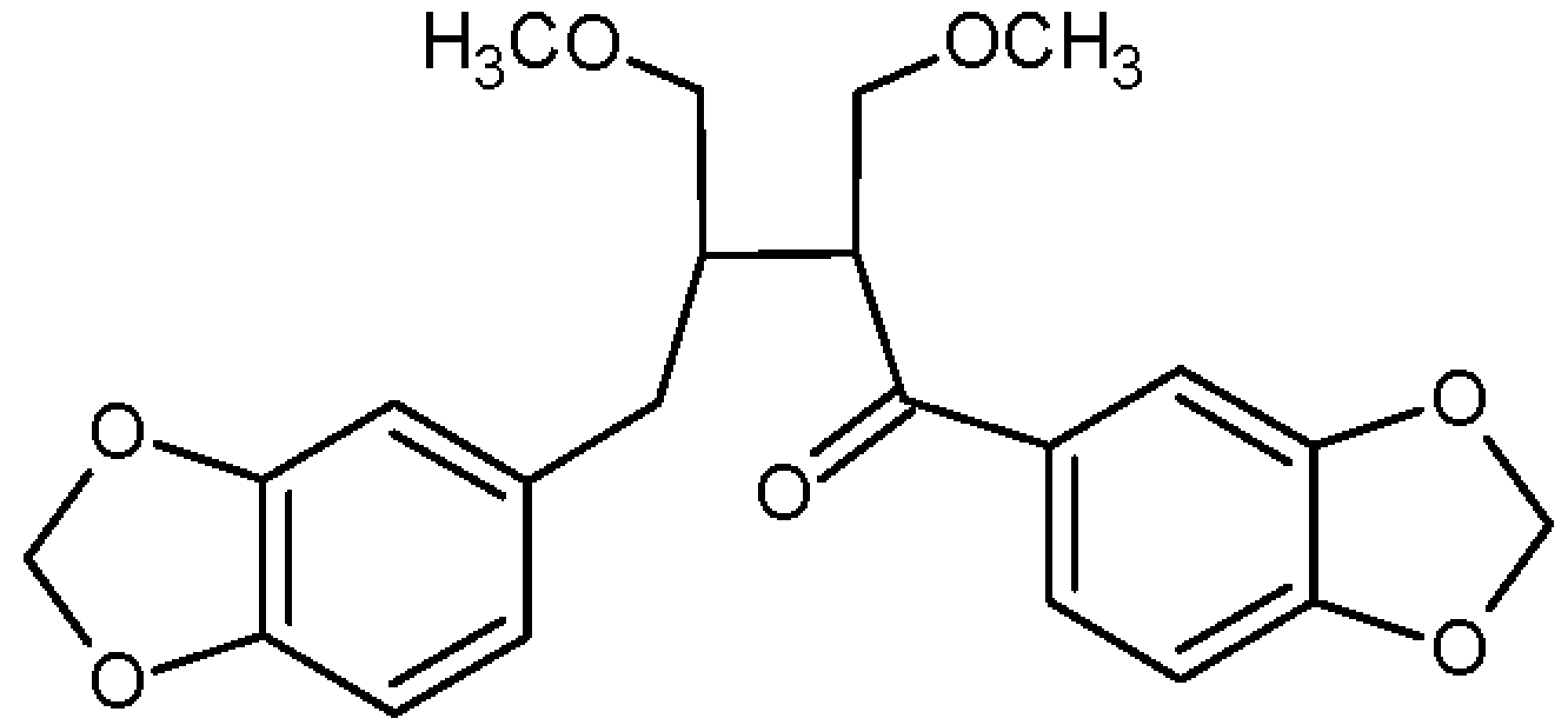
2. Result and Discussion
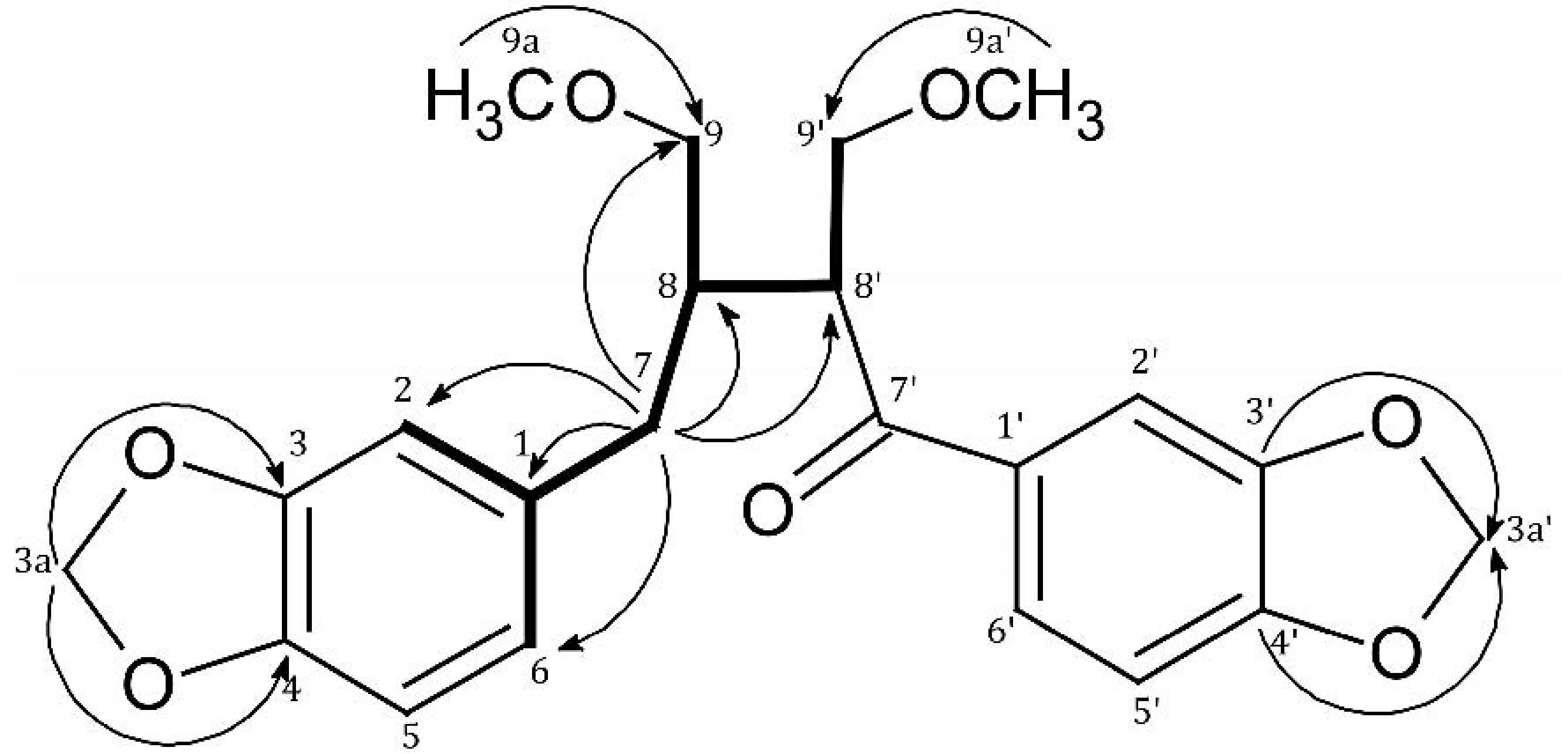
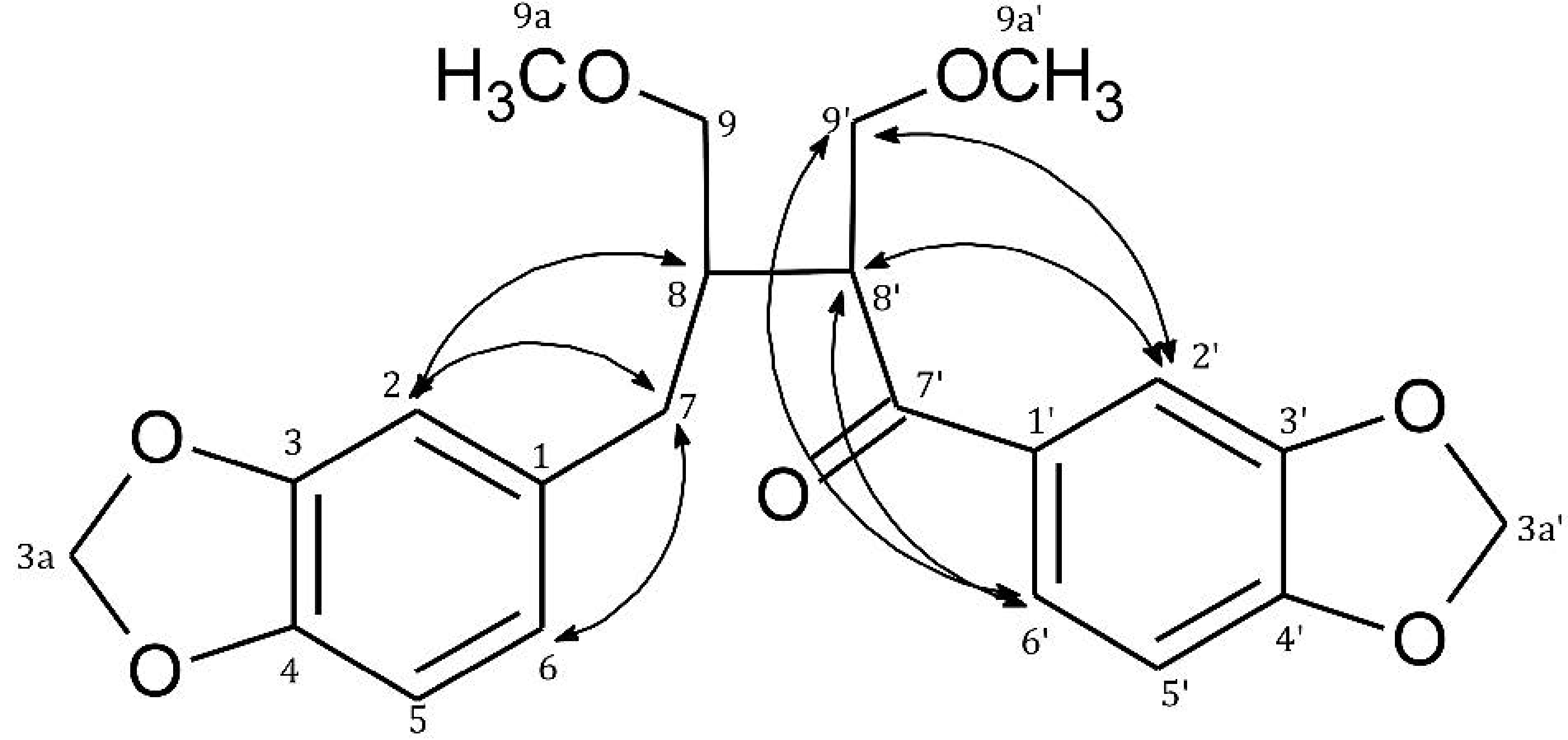
| Position | δH ( J in Hz) | δC (from HSQC) | HMBC | COSY | |
|---|---|---|---|---|---|
| 1 | - | 135.6 | - | - | |
| 2 | 1H, 6.66 (d, 1.5) | 110.8 | C-7,C-6, C-3, C-4 | H-6 | |
| 3 | - | 149.0 | - | - | |
| 4 | - | 147.3 | - | - | |
| 5 | 1H, 6.74 d (7.8) | 108.9 | C-1, C-3, C-4 | H-6 | |
| 6 | 1H, 6.61 dd (7.8, 1.5) | 123.6 | C-2, C-4, C-7 | H-2, H-5 | |
| 7 | 2H, 2.53 m | 36.4 | C-1, C-2, C-6, C-8, C-8', C-9 | H-2, H-6, H-8 | |
| 8 | 1H, 2.15 m | 43.1 | C-1, C-7, C-9, C-7', C-8' | H-7, H-9, H-8' | |
| 9 | 2H, | 3.12 dd (9.7; 4.9) 3.15 dd (9.7; 4.7) | 72.5 | C-7, C-8, C-8', C-9a | H-8 |
| 3a (3,4-OCH2O-) | 6.04 s | 102.5 | C-3, C-4 | - | |
| 9a (9-OCH3) | 3.17 s | 59.2 | C-9 | - | |
| 1' | - | 134.1 | - | - | |
| 2' | 1H, 7.26 d (1.7) | 109.2 | C-3', C-4', C-6', C-7' | H-6’ | |
| 3' | - | 149.6 | - | - | |
| 4' | - | 153.1 | - | - | |
| 5' | 1H, 6.86 d (8.3) | 108.8 | C-1', C-3', C-4' | H-6' | |
| 6' | 1H, 7.42 dd (8.3, 1.7) | 125.9 | C-2', C-4', C-7' | H-2', H-6' | |
| 7' | - | 201.3 | - | - | |
| 8' | 1H, 3.78 ddd (8.8; 4.5; 5.7) | 47.7 | C-7, C-8, C-9, C-7', C-9' | H-8, H-9' | |
| 9' | 2H, | 3.61 dd (8.8; 4.4) 3.72 t (8.8) | 73.1 | C-8, C-8', C-7', C-9a' | H-8' |
| 3a' (3',4'-OCH2O-) | 5.91 s | 103.7 | C-3', C-4' | - | |
| 9a' (9'-OCH3) | 3.20 s | 59.5 | C-9' | - | |
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Chemistry
3.5. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patel, J.R.; Tripathi, P.; Sharma, V.; Chauhan, N.S.; Dixit, V.K. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J. Ethnopharm. 2011, 138, 286–313. [Google Scholar] [CrossRef]
- Joseph, B.; Raj, S.J. An overview: Pharmacognostic properties of Phyllanthus amarus. Int. J. Pharm. 2011, 7, 40–45. [Google Scholar] [CrossRef]
- Calixto, J.B.; Santos, A.R.S.; Cechinel Filho, V.; Yunes, R.A. A review of the plants of the genus Phyllanthus: Their chemistry, pharmacology, and therapeutic potential. Med. Res. Rev. 1998, 18, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Huang, Y.L.; Ou, J.C.; Chen, C.C.; Hsu, F.L.; Chang, C. Screening of 25 compounds isolated from Phyllanthus species for anti-human hepatitis b virus in vitro. Phytother. Res. 2003, 17, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Chirdchupunseree, H.; Pramyothin, P. Protective activity of phyllanthin in ethanol-treated primary culture of rat hepatocytes. J. Ethnopharm. 2010, 128, 172–176. [Google Scholar] [CrossRef]
- Krithika, R.; Mohankumar, R.; Verma, R.J.; Shrivastav, P.S.; Mohamad, I.L.; Gunasekaran, P.; Narasimhan, S. Isolation, characterization and antioxidative effect of phyllanthin against ccl4-induced toxicity in HepG2 cell line. Chem.-Biol. Interact. 2009, 181, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kassuya, C.A.L.; Leite, D.F.P.; de Melo, L.V.; Rehder, V.L.C.; Calixto, J.B. Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus. Planta Med. 2005, 71, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Shanker, K.; Singh, M.; Srivastava, V.; Verma, R.; Gupta, A.; Gupta, M. Simultaneous analysis of six bioactive lignans in Phyllanthus species by reversed phase hyphenated high performance liquid chromatographic technique. Acta Chromatogr. 2011, 23, 321–337. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, M.; Malasoni, R.; Shanker, K.; Verma, R.K.; Gupta, M.M.; Gupta, A.K.; Khanuja, S.P.S. Separation and quantification of lignans in Phyllanthus species by a simple chiral densitometric method. J. Sep. Sci. 2008, 31, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tiwari, N.; Shanker, K.; Verma, R.K.; Gupta, A.K.; Gupta, M.M. Two new lignans from phyllanthus amarus. J. Asian Nat. Prod. Res. 2009, 11, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Al-Qurainy, F.; Ram, M.; Ahmad, S.; Abdin, M.Z. Phyllanthin biosynthesis in Phyllanthus amarus: Schum and Thonn growing at different altitudes. J. Med. Plants Res. 2010, 4, 041–048. [Google Scholar]
- Sharma, A.; Singh, R.T.; Handa, S.S. Estimation of phyllanthin and hypophyllanthin by high performance liquid chromatography in Phyllanthus amarus. Phytochem. Anal. 1993, 4, 226–229. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Bhattacharya, S.; Wenzel-Mathers, M.; Buckwold, V.E. Phyllanthus amarus root clone with significant activity against bovine viral diarrhoea virus—A surrogate model of hepatitis c virus. Curr. Sci. 2003, 84, 529–533. [Google Scholar]
- Bhattacharyya, R.; Bhattacharya, S. Development of a potent in vitro source of Phyllanthus amarus roots with pronounced activity against surface antigen of the hepatitis b virus. In Vitro Cell. Dev.-Plant 2004, 40, 504–508. [Google Scholar] [CrossRef]
- Abhyankar, G.; Suprasanna, P.; Pandey, B.N.; Mishra, K.P.; Rao, K.V.; Reddy, V.D. Hairy root extract of Phyllanthus amarus induces apoptotic cell death in human breast cancer cells. Innov. Food Sci. Emerg. 2010, 11, 526–532. [Google Scholar] [CrossRef]
- Chang, C.C.; Lien, Y.C.; Liu, K.C.S.C.; Lee, S.S. Lignans from Phyllanthus urinaria. Phytochemistry 2003, 63, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Elfahmi; Batterman, S.; Koulman, A.; Hackl, T.; Bos, R.; Kayser, O.; Woerdenbag, H.J.; Quax, W.J. Lignans from cell suspension cultures of Phyllanthus niruri, an indonesian medicinal plant. J. Nat. Prod. 2006, 69, 55–58. [Google Scholar]
- Abhyankar, G.; Rao, K.V.; Reddy, V.D. Genomic and metabolomic fingerprinting of Phyllanthus amarus (Schumm & Thonn) hairy root clones. Ann. Phytomed. 2013, 2, 74–88. [Google Scholar]
- Somanabandhu, A.; Nitayangkura, S.; Mahidol, C.; Ruchirawat, S.; Likhitwitayawuid, K.; Shieh, H.L.; Chai, H.; Pezzuto, J.M.; Cordell, G.A. 1H- and 13C-NMR assignments of phyllanthin and hypophyllanthin: Lignans that enhance cytotoxic responses with cultured multidrug-resistant cells. J. Nat. Prod. 1993, 56, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.F.P.; Kassuya, C.A.L.; Mazzuco, T.L.; Silvestre, A.; de Melo, L.V.; Rehder, V.L.G.; Rumjanek, V.M.; Calixto, J.B. The cytotoxic effect and the multidrug resistance reversing action of lignans from Phyllanthus amarus. Planta Med. 2006, 72, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.L.; Loh, S.I.; Sattar, M.A.; Muhammad, T.S.T.; Sulaiman, S.F. Cytotoxic, caspase-3 induction and in vivo hepatoprotective effects of phyllanthin, a major constituent of Phyllanthus niruri. J. Funct. Foods 2015, 14, 236–243. [Google Scholar] [CrossRef]
- Giridharan, P.; Somasundaram, S.T.; Perumal, K.; Vishwakarma, R.A.; Karthikeyan, N.P.; Velmurugan, R.; Balakrishnan, A. Novel substituted methylenedioxy lignan suppresses proliferation of cancer cells by inhibiting telomerase and activation of c-myc and caspases leading to apoptosis. Br. J. Cancer 2002, 87, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Dougherty, A.; Caldwell, C.G.; Meyers, R.O.; Timmermann, B.N.; Dorr, R.T. Cytotoxic lignans from Larrea tridentata. Phytochemistry 2005, 66, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Yamauchi, S.; Kondo, A.; Ohno, F.; Tominaga, S.; Nakashima, Y.; Kishida, T.; Akiyama, K.; Maruyama, M. First stereoselective synthesis of meso-secoisolariciresinol and comparison of its biological activity with (+) and (−)-secoisolariciresinol. Biosci. Biotechnol. Biochem. 2007, 71, 2962–2968. [Google Scholar] [CrossRef] [PubMed]
- Wukirsari, T.; Nishiwaki, H.; Nishi, K.; Sugahara, T.; Akiyama, K.; Kishida, T.; Yamauchi, S. Cytotoxic activity of butane type of 1,7-seco-2,7'-cyclolignanes and apoptosis induction by caspase 9 and 3. Bioorg. Med. Chem. Lett. 2014, 24, 4231–4235. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, T.A.; Ramalho, R.M.; Rodrigues, C.M.P.; Ferreira, M.-J.U. Dibenzylbutane- and butyrolactone-type lignans as apoptosis inducers in human hepatoma Huh-7 cells. Phytother. Res. 2012, 26, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compound 1 is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparzak, B.; Krauze-Baranowska, M.; Kawiak, A.; Sowiński, P. Cytotoxic Lignan from the Non-Transformed Root Culture of Phyllanthus amarus. Molecules 2015, 20, 7915-7924. https://doi.org/10.3390/molecules20057915
Sparzak B, Krauze-Baranowska M, Kawiak A, Sowiński P. Cytotoxic Lignan from the Non-Transformed Root Culture of Phyllanthus amarus. Molecules. 2015; 20(5):7915-7924. https://doi.org/10.3390/molecules20057915
Chicago/Turabian StyleSparzak, Barbara, Mirosława Krauze-Baranowska, Anna Kawiak, and Paweł Sowiński. 2015. "Cytotoxic Lignan from the Non-Transformed Root Culture of Phyllanthus amarus" Molecules 20, no. 5: 7915-7924. https://doi.org/10.3390/molecules20057915
APA StyleSparzak, B., Krauze-Baranowska, M., Kawiak, A., & Sowiński, P. (2015). Cytotoxic Lignan from the Non-Transformed Root Culture of Phyllanthus amarus. Molecules, 20(5), 7915-7924. https://doi.org/10.3390/molecules20057915







