Abstract
Two new flavonolignan glycosides, tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose (4) and tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose (5) were isolated from the roots of Zizania latifolia, together with tricin-7-O-β-d-glucopyranose (1), tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (2), and tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (3). Their structures were identified on the basis of spectroscopic techniques, including HR-ESI/MS, 1D-NMR (1H, 13C, DEPT), 2D-NMR (gCOSY, gHSQC, gHMBC), and IR spectroscopy.
1. Introduction
Zizania latifolia Turcz (wild rice) is cultivated in various regions of Southeastern Asia, including Korea, Japan, and China. Particularly, in China, wild rice grain is used as an important traditional medicine to treat anemia and fever in addition to heart, kidney, and liver disorders. It has been reported that wild rice species possess rich nutrient content [1,2], as well as diverse biological activities such as anti-obesity [3], anti-oxidant [4], anti-inflammatory and anti-allergenic effects [5]. Previous phytochemical studies on Z. latifolia have confirmed the presence of flavonolignan groups along with tricin. In order to identify phytochemical components of Z. latifolia, isolation and structural determination of two new flavonolignan glycosides, tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose (4) and tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose (5), a flavone glycoside, tricin-7-O-β-d-glucopyranose (1), two known flavonolignan glycosides tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (2), and tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (3) were performed. These compounds are of particular interest since flavonolignan glycosides have been reported for the first time from Z. latifolia (Figure 1).
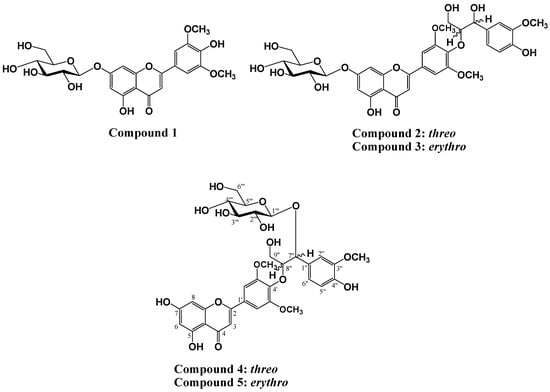
Figure 1.
Chemical structures of isolated compounds 1–5.
2. Results and Discussion
The aerial parts were extracted using 80% MeOH, and the concentrated extracts were successively partitioned using EtOAc, n-BuOH, and H2O. Repeated SiO2, and ODS-A column chromatographic separation of the EtOAc fraction produced a purified flavone glycoside, and four flavonolignans including two new flavonolignan glycosides. These compounds were identified as tricin-7-O-β-d-glucopyranose (1), tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (2), tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (3), tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose (4) and tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7''-O-β-d-gluco-pyranose (5), respectively, based on interpretation of the spectroscopic data including NMR, MS, and IR spectrascopy, and confirmed by comparison of the data with those reported in the literature [6,7,8]. All compounds were isolated for the first time from Z. latifolia.
Compound 4 (a yellow amorphous powder) was developed by TLC, followed by spraying with 10% H2SO4 and heating to produce a dark yellow color. In the 1H-NMR spectrum, three olefin methine proton signals at δ 7.50 (1H, d, J = 1.6 Hz, H-2''), 7.37 (1H, dd, J = 8.0, 1.6 Hz, H-6''), and 7.18 (1H, d, J = 8.0 Hz, H-5'') were observed due to a 1,2,4-trisubstituted benzene ring along with five olefine methine proton signals at δ 7.27 (2H, br. s, H-2', 6'), 6.97 (1H, s, H-3), 6.83 (1H, d, J = 2.0 Hz, H-8), and 6.72 (1H, d, J = 2.0 Hz, H-6) due to a flavone moiety. In the oxygenated region, two oxygenated methylene proton signals at δ 5.89 (1H, d, J = 6.0 Hz, H-7'') and 5.19 (1H, m, H-8''), an oxygenated methylene proton signal at δ 4.75 (1H, dd, J = 12.4, 3.6 Hz, H-9''a) and 4.36 (1H, dd, J = 12.0, 2.8 Hz, H-9''b), and three methoxy proton signals at δ 3.81 (6H, s, H-3', 5'-OCH3) and 3.74 (3H,s, H-3''-OCH3) were observed. There were also oxygenated methine and methylene signals for a monosaccharide moiety observed in the region from δ 4.12 to 4.28, including one hemiacetal proton signal at δ 5.54 (1H, d, J = 8.0 Hz, H-1''') with a 3JH1''/H2'' coupling constant of 8.0 Hz, indicating an anomer hydroxy in β-configuration. The relative configuration of chiral carbons, C-7'' and C-8'', in compound (4) was deduced to be threo type from the coupling constant (J = 6.0 Hz) between the oxygenated methine signals H-7'' (δH 5.89) and H-8'' (δH 5.19). It was reported that the JH-7'',8'' coupling constant of proton resonance in the guaiacylglyceryl moiety can be used to distinguish between the erythro and threo forms, with J values of 4.0–5.0 and 6.0–7.0 Hz, respectively [9,10,11]. The 13C-NMR spectrum showed 33 carbon signals including three methoxy carbons [δ 52.3 (C-3', 5'-OCH3) and 56.6 (C-3''-OCH3)], confirming that compound 4 was a flavonoid with a phenylpropanoid moiety and hexose moiety. In the low-magnetic field region, a conjugated ketone carbon δ 183.9 (C-4), seven oxygenated olefine quaternary carbons [δ 167.3 (C-7), 165.0 (C-2), 164.3 (C-5), 159.7 (C-9), 155.1 (C-3', 5'), 141.3 (C-4')], two olefine quaternary carbons [δ 127.9 (C-1'), 106.3 (C-10)], and five olefin methine carbons [δ 107.0 (C-3), 105.9 (C-2', 6'), 101.3 (C-6), 96.3 (C-8)] signals were observed, indicating the presence of a flavone moiety. In addition, two oxygenated olefine quaternary carbons [δ 149.8 (C-3''), 149.1 (C-4'')], one olefine quaternary carbon δ 133.1 (C-1''), and three olefine methine carbons [δ 122.8 (C-6''), 117.0 (C-5''), 114.0 (C-2'')], two oxygenated methine carbons [δ 87.8 (C-8''), 82.6 (C-7'')] and one oxygenated methylene carbon δ 62.7 (C-9'') signals were observed, indicating the presence of a phenylpropanoid moiety. In the HMBC spectrum, the oxygenated methine proton δH 5.19 (1H, m, H-8'') showed a correlated cross peak with an oxygenated olefine quaternary carbon δC 141.3 (C-4′), indicating that the flavone and phenylpropanoid moieties were linked between δC C-8'' and δC C-4' via an ether linkage, as well as a cross-peak between an anomer proton signal at δH 5.54 (1H, d, J = 8.0 Hz, H-1''') and an oxygenated methine carbon signal at δC 82.6 (C-7''), indicating that glucose was attached to the C-7'' of the phenylpropanoid through a glycosidic likage (Figure 2). Finally, the structure of compound (4) was determined to be tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7''-O-β-d-gluco-pyranose, which is salcolin A 7''-O-β-d-glucopyranose. The molecular weight was determined to be 688.2003 from the pseudomolecular ion peak at m/z 687.1962 [M−H]− in the negative high resolution ESI-MS (calculated for C33H35O16).
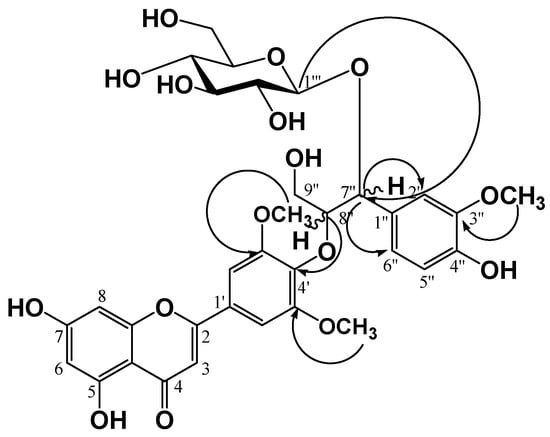
Figure 2.
Key HMBC correlations of compound 4.
Signals in the 1H-NMR (400 MHz, pyridine-d5, δH) and 13C-NMR (100 MHz, pyridine-d5, δC) spectra of compound 5 were almost the same as those of compound 4, with the exception of the relative stereostructure of C-7'' and C-8''. Compound 5 was confirmed to be erythro type from the coupling constant between the oxygenated methine signals of H-7'' and H-8'' (J = 4.8 Hz). The ether linkage (C-8''/C-4') and glycosidic likage (C-1'''/C-7'') were confirmed from the cross peak between the oxygenated methine proton δH 5.16 (1H, m, H-8'') and oxygenated olefine quaternary carbon δC 142.0 (C-4'), as well as the cross-peak between the anomer proton signal at δH 5.35 (1H, d, J = 8.0 Hz, H-1''') and a oxygenated methine carbon signal at δC 81.9 (C-7'') in the HMBC spectrum. Consequently, the structure of compound 5 was determined to be tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose, which is salcolin B 7''-O-β-d-glucopyranose. The molecular weight was determined to be 688.2003 from the pseudomolecular ion peak at m/z 687.1952 [M−H]− in the negative high resolution ESI-MS (calculated for C33H35O16).
3. Experimental
3.1. General
Preparative-liquid chromatography (prep-LC) (LC-Forte/R, YMC, Kyoto, Japan) was carried out using cartridge YMC-DispoPack (ODS-25, 80 g, and 120 g, YMC). Silica gel (230–400 mesh, Merck, Darmastdt, Germany) and Sephadex LH-20 (Merck) were used for column chromatography. Thin–layer Chromatography (TLC) analysis was performed using on a Kiesel gel 60F254 plate (Merck) and a RP-18 F254s plates (Merck), and detection was performed by a UV lamp at 254 and 365 nm and 10% H2SO4 solution by spraying and heating. 1H and 13C-NMR along with 2D-NMR data were obtained on a Bruker AVANCE II 400 (1H-NMR at 400 MHz, 13C-NMR at 100 MHz) spectrometer (Bruker, Rheinstetten, Germany) in pyridine-d5 with TMS as internal standard. ESI/MS was obtained on a AB SCIEX triple TOF 5600 mass spectrometer (AB SCIEX, Concord, ON, Canada). All the reagent grade chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
3.2. Plant Material
Plant sample. Dried aerial parts of Z. latifolia was purchased from store located at Yeongcheon City, Gyeongbuk, Korea. A voucher specimen (KHU-0130301) was deposited at the Laboratory of Natural Products Chemistry, Kyung Hee University, Yongin, Korea.
3.3. Extraction and Isolation
Dried powder (5 kg) was extracted at room temperature with 80% MeOH (45 L). The concentrated extract was partitioned with water (3 L), EtOAc (3 L) and n-BuOH (3 L) successively to produce. EtOAc extract (97 g, ZLE), n-BuOH extract (240 g, ZLB) and H2O extract (57 g, ZLW). The EtOAc extract was applied to a silica gel c.c. (12 × 14 cm), and eluted with CHCl3 : MeOH (50:1 → 30:1 → 20:1 → 12:1 → 10:1 → 7:1 → 5:1 → 3:1 → 1:1, each 3 L) monitoring by thin layer chromatography (TLC) to produce 13 fractions (ZLE-1 to ZLE-13). Fraction ZLE-12 (5.0 g) was combined and subjected to MPLC column chromatography: YMC-DispoPack AT (ODS-25:120 g, particle size: 25 μm). The fractions were analyzed by a UV detector at 254 and 356 nm, and the mobile phase consisted of A: 0.1% formic acid in water, B: 0.1% formic acid in acetonitrile with a flow rate of 10 mL/min. The gradient consisted of 10% B held for 20 min to 30% B at a rate of 0.25%/min, from 30% B to 50% B at a rate of 0.20%/min, from 50% B to 70% B at a rate of 0.25%/min, and from 70% B to 90% B at a rate of 0.30%/min. A total 11 fractions (ZLE-12-1 to ZLE-12-15) were obtained. ZLE-12-10 (642 mg) was subjected to MPLC column chromatography: YMC-DispoPack AT (ODS-25: 80 g, particle size: 25 μm), with A: 0.1% formic acid in water, B: 0.1% formic acid in acetonitrile as the mobile phase at a flow rate of 5 mL/min. The detector was maintained at 254 and 356 nm. The gradient consisted of 20% B to 50% B at a rate of 0.5%/min, and 50% B to 70% B at a rate of 0.6%/min. Compound 1 (32 mg, RT = 6.05 min), compound 2 (35 mg, RT = 7.8 min), compound 3 (42 mg, RT = 9.2 min), compound 4 (22 mg, RT = 12.4 min) and compound 5 (31 mg, RT = 14.6 min) were obtained.
3.4. Spectroscopic Data
Tricin-7-O-β-d-glucopyranose (1). Pale yellow powder (CH3OH); negative ESI/MS m/z 491 [M−H]−; IR (KBr) 3380, 2921, 1730, 1652, 1613, 1491,1358, 1084 cm−1; 1H-NMR (pyridine-d5) δ 7.55 (2H, s, H-2', 6'), 7.17 (1H, d, J = 2.0 Hz, H-8), 7.02 (1H, s, H-3), 6.86 (1H, d, J = 2.0 Hz, H-6), 5.78 (1H, d, J = 7.2 Hz, H-1''), 4.55 (1H, dd, J = 10.4, 1.2 Hz, H-6'''a), 4.38-4.10 (5H, m, H-2''', H-3''', H-4''', H-5''', 6'''b-OCH2), 3.87 (6H, s, OCH3-3', 5'). 13C-NMR (pyridine-d5) δ 182.8 (C-4), 164.9 (C-2), 164.0 (C-7), 162.5 (C-5), 157.8 (C-9), 149.5 (C-3', 5'), 142.2 (C-4'), 121.0 (C-1'), 106.5 (C-10), 105.1 (C-2', 6'), 104.5 (C-3), 101.7 (C-1''), 100.6 (C-6), 95.5 (C-8), 79.2 (C-3''), 78.4 (C-5''), 74.7 (C-2''), 71.0 (C-4''), 62.2 (C-6''), 56.5 (C-3', 5'-OCH3).
Tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (2). Yellow amorphous powder (CH3OH); Negative ESI/MS m/z 687 [M−H]−; IR (KBr) 3369, 2938, 1652, 1611, 1588, 1512, 1495, 1356, 1263, 1159, 1124, 841 cm−1; 1H-NMR (pyridine-d5) δ 7.53 (1H, s, H-2''), 7.37 (1H, dd, J = 8.0, 1.6 Hz, H-6''), 7.31 (2H, s, H-2', 6'), 7.26 (1H, d, J = 8.0 Hz, H-5''), 7.14 (1H, d, J = 2.4 Hz, H-8), 7.01 (1H, s, H-3), 6.85 (1H, d, J = 2.0 Hz, H-6), 5.76 (1H, d, J = 7.2 Hz, H-1'''), 5.70 (1H, d, J = 5.2 Hz, H-7''), 5.08 (1H, q, J = 5.2, 3.2 Hz, H-8''), 4.65 (1H, dd, J = 12.0, 5.2 Hz, H-9''e), 4.54 (1H, dd, J = 12.0, 2.4 Hz, H-6'''a), 4.28 (1H, m, H-9''a), 4.36-4.27 (5H, m, H-2''', H-3''', H-4''', H-5''', 6'''b-OCH2), 3.84 (6H, s, OCH3-3', 5'), 3.74 (3H, s, OCH3-3''). 13C-NMR (pyridine-d5) δ 184.1 (C-4), 165.5 (C-7), 165.4 (C-2), 163.7 (C-5), 159.1 (C-9), 155.3 (C-3', 5'), 149.6 (C-3''), 148.6 (C-4''), 141.9 (C-4'), 135.6 (C-1''), 127.7 (C-1'), 121.8 (C-6''), 117.3 (C-5''), 112.9 (C-2''), 107.8 (C-10), 107.1 (C-3), 106.1 (C-2', 6'), 103.0 (C-1'''), 102.1 (C-6), 96.8 (C-8), 89.4 (C-8''), 80.5 (C-3''′), 79.7 (C-5'''), 76.0 (C-4'''), 75.1 (C-7''), 72.4 (C-2'''), 63.5 (C-6'''), 62.9 (C-9''), 57.7 (C-3', 5'-OCH3), 57.1 (C-3''-OCH3).
Tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (3). Yellow amorphous powder (CH3OH); Negative ESI/MS m/z 687 [M−H]−; IR (KBr) 3364, 2933, 1649, 1607, 1590, 1495, 1457, 1358, 1121, 832 cm−1; 1H-NMR (pyridine-d5) δ 7.57 (1H, s, H-2''), 7.46 (1H, dd, J = 8.0, 1.2 Hz, H-6''), 7.29 (2H, s, H-2', 6'), 7.26 (1H, d, J = 8.0 Hz, H-5''), 7.13 (1H, d, J = 2.0 Hz, H-8), 6.99 (1H, s, H-3), 6.86 (1H, d, J = 2.0 Hz, H-6), 5.77 (1H, d, J = 6.0 Hz, H-7''), 5.76 (1H, d, J = 7.2 Hz, H-1'''), 4.95 (1H, q, H-8''), 4.54 (1H, br. d, J = 10.4 Hz, H-6'''a), 4.43 (1H, dd, J = 11.6, 4.0 Hz, H-9''a), 4.37–4.10 (5H, m, H-2''', H-3''', H-4''', H-5''', 6'''b-OCH2), 4.06 (1H, dd, J = 12.0, 4.0 Hz, H-9''b), 3.82 (6H, s, OCH3-3', 5'), 3.77 (3H, s, OCH3-3''). 13C-NMR (pyridine-d5) δ 183.5 (C-4), 165.0 (C-7), 164.9 (C-2), 163.3 (C-5), 158.6 (C-9), 154.6 (C-3′, 5'), 149.1 (C-3''), 148.2 (C-4''), 141.9 (C-4'), 134.6 (C-1''), 127.2 (C-1'), 121.4 (C-6''), 116.8 (C-5''), 112.6 (C-2''), 107.4 (C-3), 106.6 (C-10), 105.9 (C-2', 6'), 105.7 (C-1'''), 101.3 (C-6), 96.3 (C-8), 87.8 (C-8''), 80.0 (C-3'''), 79.2 (C-5'''), 75.5 (C-4'''), 74.4 (C-7''), 71.9 (C-2'''), 63.9 (C-6'''), 62.5 (C-9''), 57.6 (C-3', 5'-OCH3), 57.1 (C-3''-OCH3).
Tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose (4). Yellow amorphous powder (CH3OH); HRESI/MS m/z 687.1962 [M−H]−; IR (KBr) 3406, 1639, 1614, 1495, 1358, 1121 cm−1; 1H-NMR (pyridine-d5) and 13C-NMR (pyridine-d5), see Table 1.
Tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose (5). Yellow amorphous powder (CH3OH); HRESI/MS m/z 687.1952 [M−H]−; IR (KBr) 3432, 2502, 1685, 1510, 1420, 1399, 1112 cm−1; 1H-NMR (pyridine-d5) and 13C-NMR (pyridine-d5), see Table 1.

Table 1.
1H- (400 MHz) and 13C-NMR (100 MHz) data of compound 4–5 (in pyridine-d5, δ in ppm, J in Hz).
| No. | Compound 4 | Compound 5 | ||
|---|---|---|---|---|
| δC | δH, Coupling Pattern, J in Hz | δC | δH, Coupling Pattern, J in Hz | |
| 2 | 165.0 | 165.2 | ||
| 3 | 107.0 | 6.97, s | 107.2 | 7.00, s |
| 4 | 183.9 | 183.9 | ||
| 5 | 164.3 | 164.4 | ||
| 6 | 101.3 | 6.72, d, 2.0 | 101.4 | 6.74, d, 2.0 |
| 7 | 167.3 | 167.3 | ||
| 8 | 96.3 | 6.83, d, 2.0 | 96.3 | 6.86, d, 2.0 |
| 9 | 159.7 | 159.8 | ||
| 10 | 106.3 | 106.3 | ||
| 1' | 127.9 | 128.2 | ||
| 2' | 105.9 | 7.27, br. s | 106.0 | 7.29, br. s |
| 3' | 155.1 | 155.1 | ||
| 4' | 141.3 | 142.0 | ||
| 5' | 155.1 | 155.1 | ||
| 6' | 105.9 | 7.27, br. s | 106.0 | 7.29, br. s |
| 1'' | 133.1 | 132.1 | ||
| 2'' | 114.0 | 7.50, d, 1.6 | 113.9 | 7.59, d, 1.6 |
| 3'' | 149.4 | 149.4 | ||
| 4'' | 148.8 | 148.9 | ||
| 5'' | 117.0 | 7.18, d, 8.0 | 117.1 | 7.21, d, 8.0 |
| 6'' | 122.8 | 7.37, dd, 8.0, 1.6 | 122.5 | 7.43, dd, 8.0, 1.6 |
| 7'' | 82.6 | 5.89, d, 6.0 | 81.9 | 6.02, d, 4.8 |
| 8'' | 87.8 | 5.19, m | 87.6 | 5.16, m |
| 9''a | 62.7 | 4.75, dd, 12.4, 3.6 | 63.0 | 4.44, dd, 12.0, 4.4 |
| 9''b | 4.36, dd, 12.0, 2.8 | 4.02, dd, 11.6, 5.2 | ||
| 3'-OCH3 | 57.2 | 3.81, s | 57.7 | 3.83, s |
| 5'-OCH3 | 57.2 | 3.81, s | 57.7 | 3.83, s |
| 3''-OCH3 | 56.6 | 3.73, s | 57.1 | 3.67, s |
| 1''' | 105.7 | 5.54, d, 8.0 | 105.6 | 5.35, d, 8.0 |
| 2''' | 75.5 | 4.28–4.12, m | 77.1 | 4.31–3.09, m |
| 3''' | 79.7 | 80.1 | ||
| 4''' | 71.9 | 72.8 | ||
| 5''' | 79.6 | 79.7 | ||
| 6'''a | 63.0 | 4.41, m | 63.8 | 4.45, m |
| 6'''b | 4.20, m | 4.30, m | ||
4. Conclusions
Five compounds were isolated from the aerial parts of Zizania latifolia using a prep-LC instrument equipped with cartridges of various sizes and were identified based on spectroscopic data analysis, including NMR, UV, and ESI-MS. Four flavonolignan glycosides, including two new flavonolignan glycosides, showed structural similarities in being derived from tricin-7-O-β-d-glucopyranose (1), and were shown to be diastereomers of each other. Tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (2) and tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranose (3) were shown to be diastereomers distinguished by threo and erythro configurations, whereas tricin-4'-O-(threo-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose (4) and tricin-4'-O-(erythro-β-guaiacylglyceryl) ether 7''-O-β-d-glucopyranose (5) were shown to be threo and erythro, respectively.
Supplementary Materials
1H-NMR, 13C-NMR, and HR-ESIMS spectra of 4–5 are available as supporting data. Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/04/5616/s1.
Acknowledgments
This work was supported by the Next Generation Bio-Green 21(PJ008020) Project from Rural Development Administration, Republic of Korea, and a grant from Kyung Hee University in 2009 (KHU-20100160), and by a grant from the regional innovation center program of the Ministry of Trade, Industry and Energy at the Skin Biotechnology Center of Kyung Hee University, Korea.
Author Contributions
M.-H.B., N.-I.B., and D.-K.C. designed research; S.-S.L., Y.-S.B., and M.-C.S. performed research and analyzed the data; S.-S.L. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oelke, E.A.; Porter, R.A.; Gramcher, A.W.; Addis, P.B. Wild rice-new interest in old crop. Cereal Foods World 1997, 42, 234–247. [Google Scholar]
- Guo, H.B.; Li, S.M.; Peng, J.; Ke, W.D. Zizania latifolia Trucz. cultivated in China. Genet. Resour. Crop Evol. 2007, 54, 1211–1217. [Google Scholar] [CrossRef]
- Han, S.F.; Zhang, H.; Zhai, C.K. Protective potentials of wild rice (Zizania latifolia (Griseb) Turcz) against obesity and lipotoxicity induced by a high-fat/cholesterol diet in rats. Food Chem. Toxicol. 2012, 50, 2236–2269. [Google Scholar]
- Zhang, H.; Cao, P.; Agellon, L.B.; Zhai, C.K. Wild rice (Zizania latifolia (Griseb) Turcz) improves the serum lipid profile and antioxidant status of rats fed with a high fat/cholesterol diet. Br. J. Nutr. 2009, 102, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Baek, Y.S.; Eun, C.S.; Yu, M.H.; Baek, N.I.; Chung, D.K.; Bang, M.H.; Yang, S.A. Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia. Biosci. Biotechnol. Biochem. 2015, 6, 1–7. [Google Scholar]
- Bouaziz, M.; Veitch, N.C.; Grayer, R.J.; Simmonds, M.S.J.; Damak, M. Flavolignans from Hyparrhenia hirta. Phytochemistry 2002, 60, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zang, Y.; Liu, C.; Liu, J.; Wu, X.; Zang, Y. Separation and purification of tricin from an antioxidant product derived from bamboo leaves. J. Agric. Food Chem. 2007, 55, 10086–10092. [Google Scholar] [CrossRef] [PubMed]
- Jeong, R.H.; Lee, D.Y.; Cho, J.G.; Lee, S.M.; Kang, H.C.; Seo, W.D.; Kang, H.W.; Kim, J.Y.; Baek, N.I. A new flavonolignan from the arial parts of Oryza sativa L. inhibits nitric oxide production in RAW 264.7 macrophage cells. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 865–870. [Google Scholar]
- Nakajima, Y.; Yun, Y.S.; Kunugi, A. Six new flavonolignans from Sasa veitchii (Carr.) Rehder. Tetrahedron 2003, 59, 8011–8015. [Google Scholar] [CrossRef]
- Jung, Y.J.; Park, J.H.; Cho, J.G.; Seo, K.H.; Lee, D.S.; Kim, Y.C.; Kang, H.C.; Song, M.C.; Baek, N.I. Lignan and flavonoids from the stems of Zea mays and their anti-inflammatory and nueroprotective activities. Arch. Pharm. Res. 2014, 37, 1978–2014. [Google Scholar] [CrossRef]
- Jeong, R.H.; Lee, D.Y.; Cho, J.G.; Seo, K.H.; Lee, J.W.; Lee, M.H.; Seo, W.D.; Kang, H.C.; Kim, G.S.; Noh, H.J.; et al. New flavonolignan glucoside from the aerial parts of Oryza Sativa. Chem. Nat. Compd. 2013, 49, 1003–1005. [Google Scholar]
- Sample Availability: Samples of the compounds 1–5 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).