Synthesis and Antifungal Activity of the Derivatives of Novel Pyrazole Carboxamide and Isoxazolol Pyrazole Carboxylate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
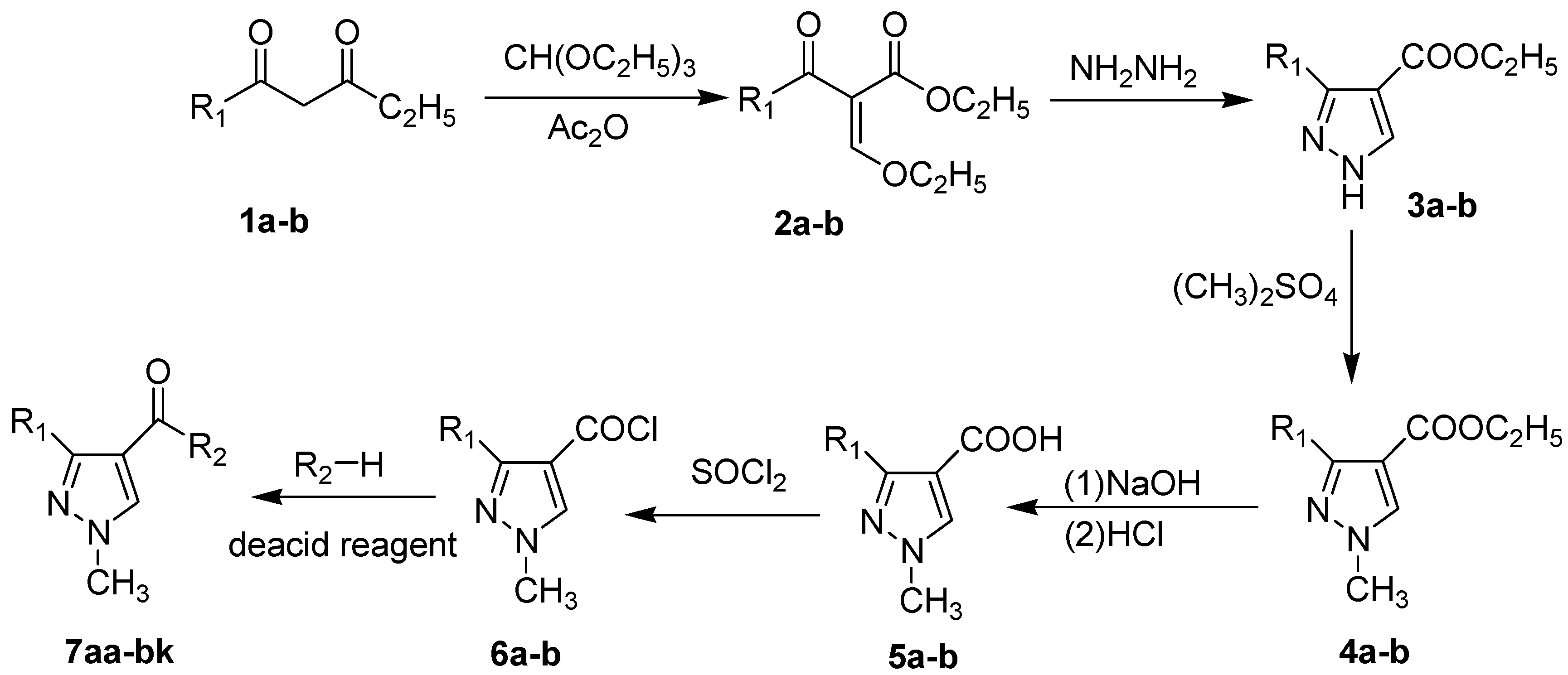
2.2. Antifungal Activity
| Compound | R1 | R2 | EC50 (μg/mL) | |||
|---|---|---|---|---|---|---|
| A. porri | M. coronaria | C. petroselini | R. solani | |||
| 7aa | 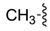 | 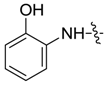 | -- | -- | -- | 31.39 |
| 7ab | 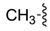 | 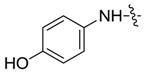 | 80.76 | -- | 38.41 | -- |
| 7ac | 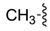 |  | 52.56 | 84.74 | -- | 40.00 |
| 7ad | 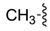 | 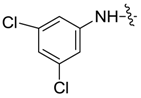 | -- | -- | 6.32 | 18.15 |
| 7ae | 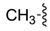 | 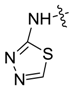 | 65.12 | -- | -- | 14.89 |
| 7af | 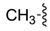 | 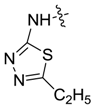 | 63.04 | 7.87 | 35.90 | 5.23 |
| 7ag | 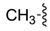 | 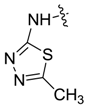 | 54.86 | 76.12 | 51.00 | 16.91 |
| 7ah | 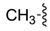 | 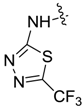 | -- | -- | -- | 69.45 |
| 7ai | 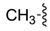 | 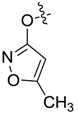 | 2.24 | 3.21 | 10.19 | 0.37 |
| 7ba |  |  | -- | 35.94 | 22.47 | 16.81 |
| 7bb |  | 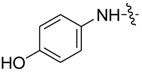 | -- | 74.54 | 27.82 | 19.47 |
| 7bc |  | 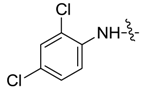 | 10.10 | 14.92 | 5.43 | 3.40 |
| 7bd |  | 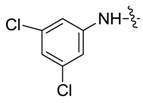 | -- | -- | 74.38 | 27.37 |
| 7be |  | 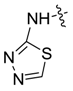 | 23.12 | 13.00 | 13.44 | 8.55 |
| 7bf |  | 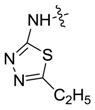 | 72.20 | 61.29 | -- | 81.72 |
| 7bg |  | 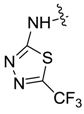 | 11.22 | 7.93 | 27.43 | 4.99 |
| 7bh |  |  | 24.76 | 25.48 | 6.99 | 5.93 |
| 7bi |  | 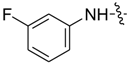 | 21.01 | 9.08 | 32.40 | 7.69 |
| 7bj |  | 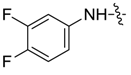 | 11.46 | 15.86 | -- | 8.32 |
| 7bk |  | 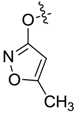 | 35.05 | -- | -- | 28.88 |
| carbendazol | 0.99 | 0.96 | 0.96 | 1.00 | ||
3. Experimental Section
3.1. Chemistry
3.2. General Procedure for the Preparation of 2a–b
3.3. General Procedure for the Preparation of 3a–b
3.4. General Procedure for the Preparation of 5a–b
3.5. General Procedure for the Preparation of 7aa–bk
3.6. Antifungal Bioassays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sumangala, V.; Poojary, B.; Chidananda, N.; Fernandes, J.; Kumari, N.S. Synthesis and antimicrobial activity of 1,2,3-triazoles containing Quinoline moiety. Arch. Pharm. Res. 2010, 33, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Mert, S.; Kasımoǧulları, R.; İça, T.; Çolak, F.; Altun, A.; Ok, S. Synthesis, structure activity relationships, and in vitro antibacterial and antifungal activity evaluations of novel pyrazole arboxylic and dicarboxylic acid derivatives. Eur. J. Med. Chem. 2014, 78, 86–96. [Google Scholar] [CrossRef]
- Gawad, N.M.A.; Georgey, H.H.; Ibrahim, N.A.; Amin, N.H.; Abdelsalam, R.M. Synthesis of novel pyrazole and dihydropyrazoles derivatives as potential anti-inflammatory and analgesic agents. Arch. Pharm. Res. 2012, 35, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.P.; Cai, X.J.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, J.; Kumar, H.V.; Harini, S.T.; Naik, N. Synthesis of benzofuran based 1,3,5-substituted pyrazole derivatives: As a new class of potent antioxidants and antimicrobials-A novel accost to amend biocompatibility. Bioorg. Med. Chem. Lett. 2012, 22, 4773–4777. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.S.; Kadry, H.H.; Abou-Seri, S.M.; Ali, M.M.; Mahmoud, A.E.E. Synthesis and in vitro cytotoxic activity of novel pyrazolo[3,4-d]pyrimidines and related pyrazole hydrazones toward breast adenocarcinoma MCF-7 cell line. Bioorg. Med. Chem. 2011, 19, 6808–6817. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.T.; Chevallet, P.; Kiliç, F.S.; Erol, K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000, 35, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, P.G.; Cacciari, B.; Romagnoli, R.; Spalluto, G.; Moro, S.; Klotz, K.; Leung, E.; Varani, K.; Gessi, S.; Merighi, S.; et al. Pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine derivatives as highly potent and selective human A3 adenosine receptor antagonists: Influence of the Chain at the N8 pyrazole nitrogen. J. Med. Chem. 2000, 43, 4768–4780. [Google Scholar] [CrossRef] [PubMed]
- Lupsor, S.; Aonofriesei, F.; Iovu, M. Antibacterial activity of aminals and hemiaminals of pyrazole and imidazole. Med. Chem. Res. 2012, 21, 3035–3042. [Google Scholar] [CrossRef]
- Palaska, E.; Aytemir, M.; Uzbay, I.T.; Erol, D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur. J. Med. Chem. 2001, 36, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Sauter, H.; Steglich, W.; Anke, T. Strobilurins: Evolution of a new class of active substances. Angew. Chem. Int. Ed. 1999, 38, 1328–1349. [Google Scholar] [CrossRef]
- Shiga, Y.; Okada, I.; Ikeda, Y.; Takizawa, E.; Fukuchi, T. Insecticidal activity of N-(4-aryloxybenzyl)pyrazole-5-carboxamides. J. Pestic. Sci. 2003, 28, 313–314. [Google Scholar] [CrossRef]
- Ohno, R.; Watanabe, A.; Matsukawa, T.; Ueda, T.; Sakurai, H.; Hori, M.; Hirai, K. Synthesis and herbicidal activity of new pyrazole-4-carboxamide derivatives. J. Pestic. Sci. 2004, 29, 15–26. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Li, Y.X.; Li, Y.M.; Yang, X.P.; Mao, M.Z.; Li, Z.M. Design, synthesis and structure-activity of N-glycosyl-1-pyridyl-1H-pyrazole-5-carboxamide as Inhibitors of Calcium channels. Chem. Res. Chin. Univ. 2013, 29, 249–255. [Google Scholar] [CrossRef]
- Lv, H.S.; Wang, L.Y.; Ding, X.L.; Wang, X.H.; Zhao, B.X.; Zuo, H. Synthesis and antifungal activity of novel (1-arylmethyl-3-aryl-1Hpyrazol-5-yl)(4-arylpiperazin-1-yl)methanone derivatives. J. Chem. Res. 2013, 37, 473–475. [Google Scholar] [CrossRef]
- Okada, I.; Okui, S.; Sekine, M.; Takahashi, Y.; Fukuchi, T. Synthesis and acaricidal activity of bicyclic pyrazole-3-carboxamide derivatives. J. Pestic. Sci. 1992, 17, 69–73. [Google Scholar] [CrossRef]
- Lamberth, C.; Dinges, J. Pyrazol carboxamide fungicides inhibiting succinate dehydrogenase. In Bioactivity Heterocylic Compound Classes: Agrochemicals, 1st ed.; Wiley-VCH Verlag GmBH & Co. KGaA: Weinheim, Germany, 2012; pp. 175–194. [Google Scholar]
- Liu, F.; Wang, M.Y.; Teng, X.H.; Zhang, P.Z.; Jiang, L. Synthesis and biological evaluation of novel 2-(substituted isoxazol-4-yl)-5-arylamino-1,3,4-oxadiazoles. Res. Chem. Intermed. 2014, 40, 1575–1581. [Google Scholar] [CrossRef]
- Cernuchova, P.; Vo-Thanh, G.; Milata, V.; Loupy, A. Solvent-free synthesis of quinolone derivatives. Heterocycles 2004, 64, 177–191. [Google Scholar] [CrossRef]
- Nishiwaki, N.; Matsushima, K.; Chatani, M.; Tamura, M.; Ariga, M. New reactivity of nitropyrimidinone: Ring transformation and N-C transfer reactions. Synlett 2004, 15, 703–707. [Google Scholar] [CrossRef]
- Toshio, K.; Kenji, O.; Takao, O. Preparation of 1,3-Dialkylpyrazole-4-Carboxylic Acid Esters. Jpn. Kokai Tokkyo Koho JP 2,000,044,541, 15 February 2000. [Google Scholar]
- Kenji, H.; Bunta, W.; Osamu, K.; Seiichi, K.; Takashi, K.; Junko, S. Preparation of N-(1-Phenyl-1H-pyrazol-4-yl)-1H-pyrazole-4-carboxamide Derivatives as Agrochemical Fungicides. Jpn. Kokai Tokkyo Koho JP 2,010,202,649, 16 September 2010. [Google Scholar]
- Palanki, M.S.S.; Erdman, P.E.; Gayo, F.; Leah, M.; Shevlin, G.I.; Sullivan, R.W.; Suto, M.J.; Goldman, M.E.; Ransone, L.J.; Bennett, B.L.; et al. Inhibitors of NF-κB and AP-1 gene expression: SAR studies on the pyrimidine portion of 2-chloro-4-trifluoromethyl pyrimidine-5-[N-(3',5'-bis(trifluoromethyl)-phenyl) carboxamide]. J. Med. Chem. 2000, 43, 3995–4004. [Google Scholar] [CrossRef]
- Sun, J.L. 3-Trifluoromethyl-4-formyl Pyrazol Compounds. CN Patent 103,554,026A, 5 February 2014. [Google Scholar]
- Sun, J.L. 3-Methyl-4-formyl Pyrazol Compounds. CN Patent 103,524,417A, 22 January 2014. [Google Scholar]
- Xu, G.F.; Song, B.A.; Pinaki, S.B.; Yang, S.; Zhang, P.W.; Jin, L.H.; Xue, W.; Hu, D.Y.; Lu, P. Synthesis and antifungal activity of novel s-substituted-6-fluoro-4-alkyl(aryl) thioquinazoline derivatives. Bioorg. Med. Chem. 2007, 15, 3768–3774. [Google Scholar] [CrossRef] [PubMed]
- Tarun, K.C.; Prem, D.J. Antifungal activity of 4-methyl-6-alkyl-2H-pyran-2-ones. J. Agric. Food Chem. 2006, 54, 2129–2133. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 7aa–bk are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Zhou, Y. Synthesis and Antifungal Activity of the Derivatives of Novel Pyrazole Carboxamide and Isoxazolol Pyrazole Carboxylate. Molecules 2015, 20, 4383-4394. https://doi.org/10.3390/molecules20034383
Sun J, Zhou Y. Synthesis and Antifungal Activity of the Derivatives of Novel Pyrazole Carboxamide and Isoxazolol Pyrazole Carboxylate. Molecules. 2015; 20(3):4383-4394. https://doi.org/10.3390/molecules20034383
Chicago/Turabian StyleSun, Jialong, and Yuanming Zhou. 2015. "Synthesis and Antifungal Activity of the Derivatives of Novel Pyrazole Carboxamide and Isoxazolol Pyrazole Carboxylate" Molecules 20, no. 3: 4383-4394. https://doi.org/10.3390/molecules20034383
APA StyleSun, J., & Zhou, Y. (2015). Synthesis and Antifungal Activity of the Derivatives of Novel Pyrazole Carboxamide and Isoxazolol Pyrazole Carboxylate. Molecules, 20(3), 4383-4394. https://doi.org/10.3390/molecules20034383





