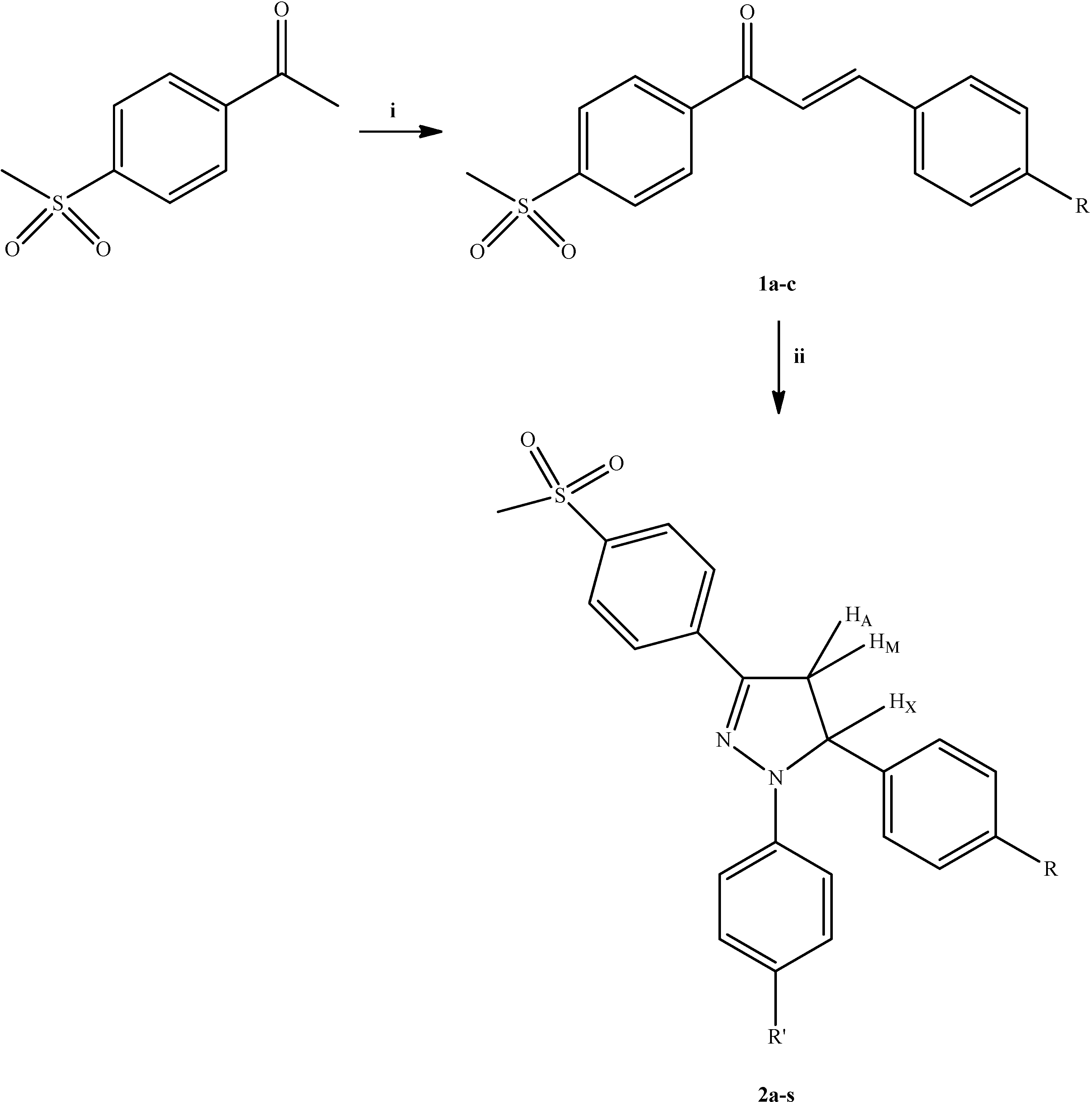
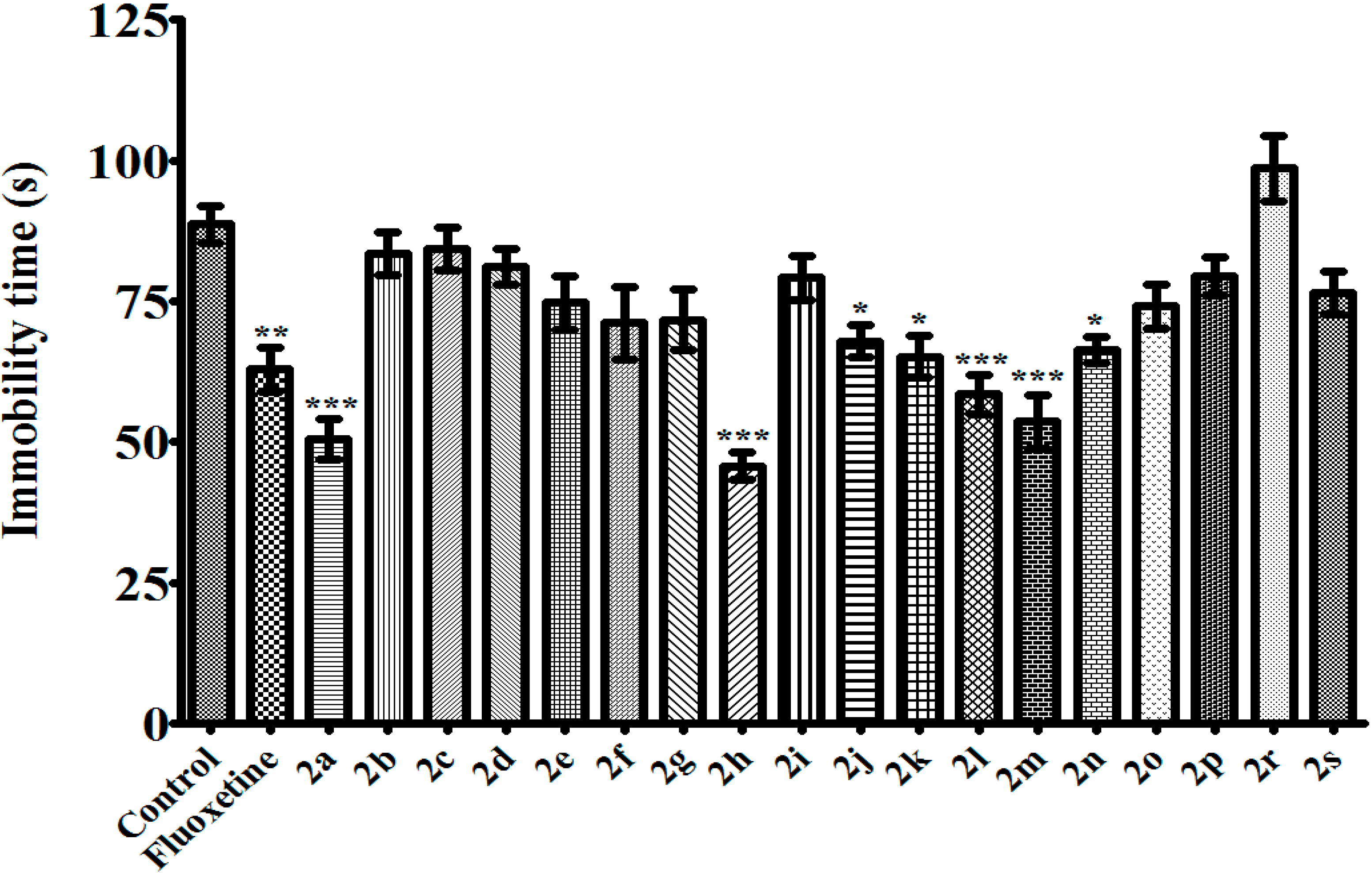
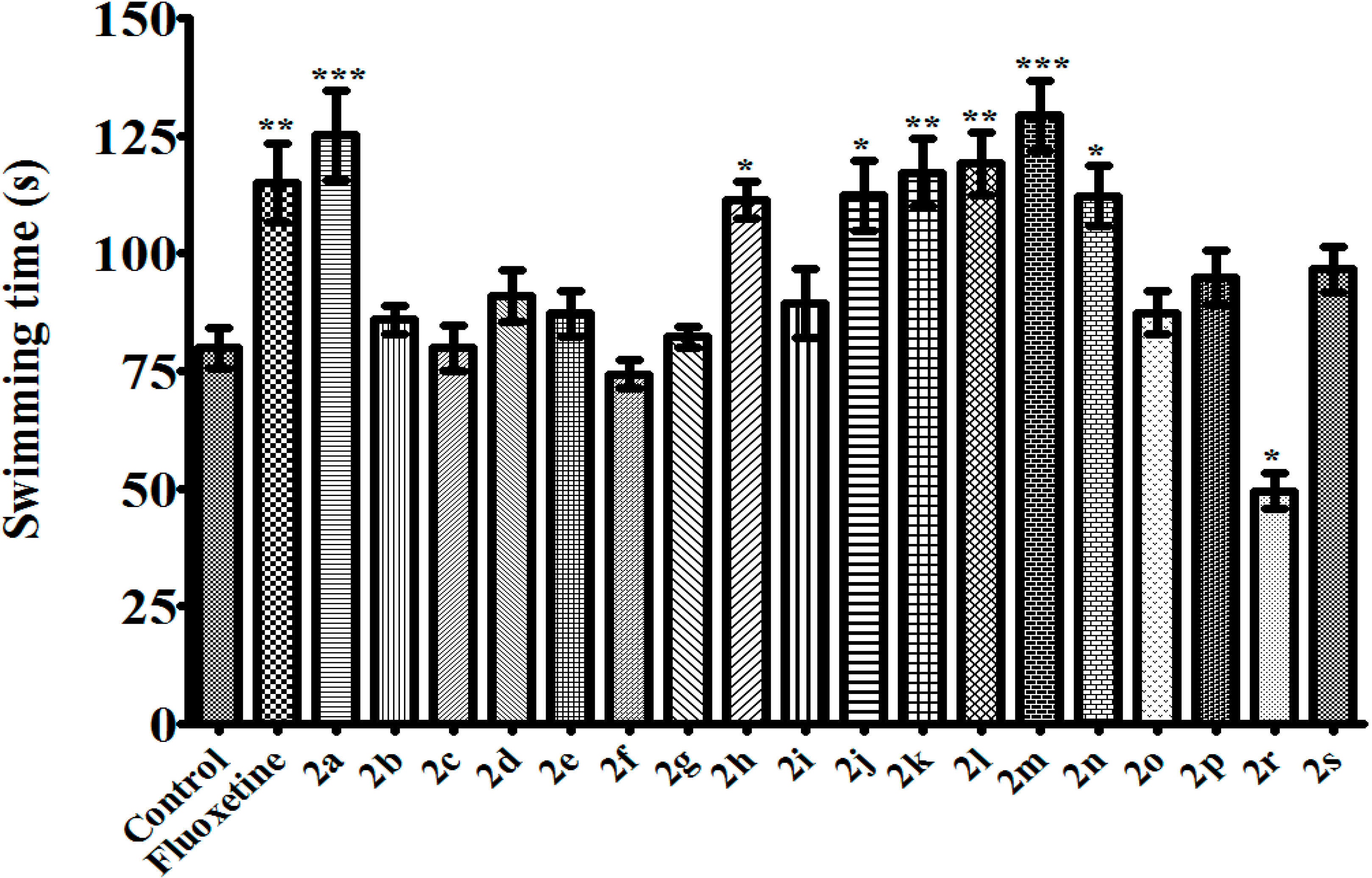
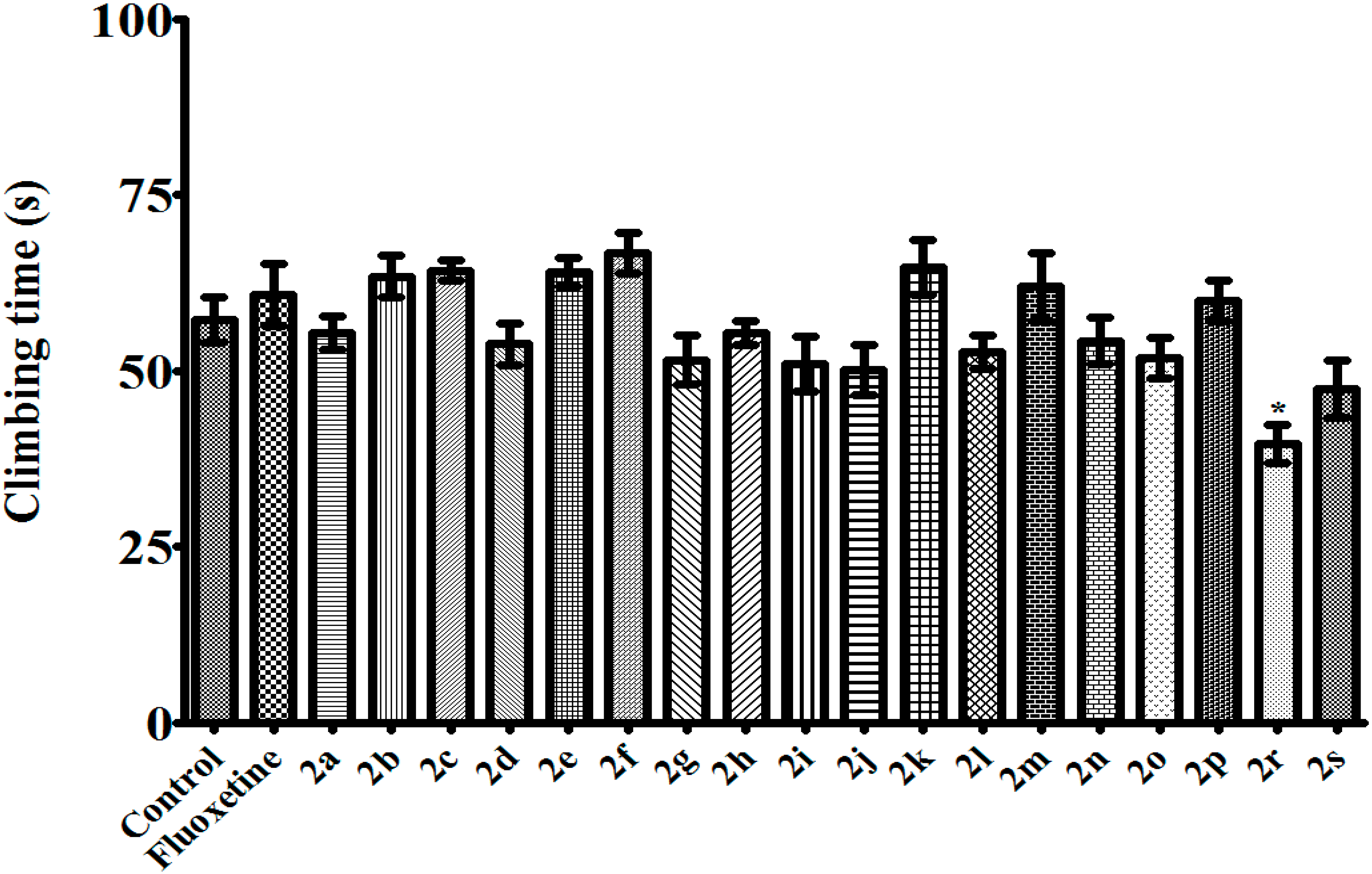
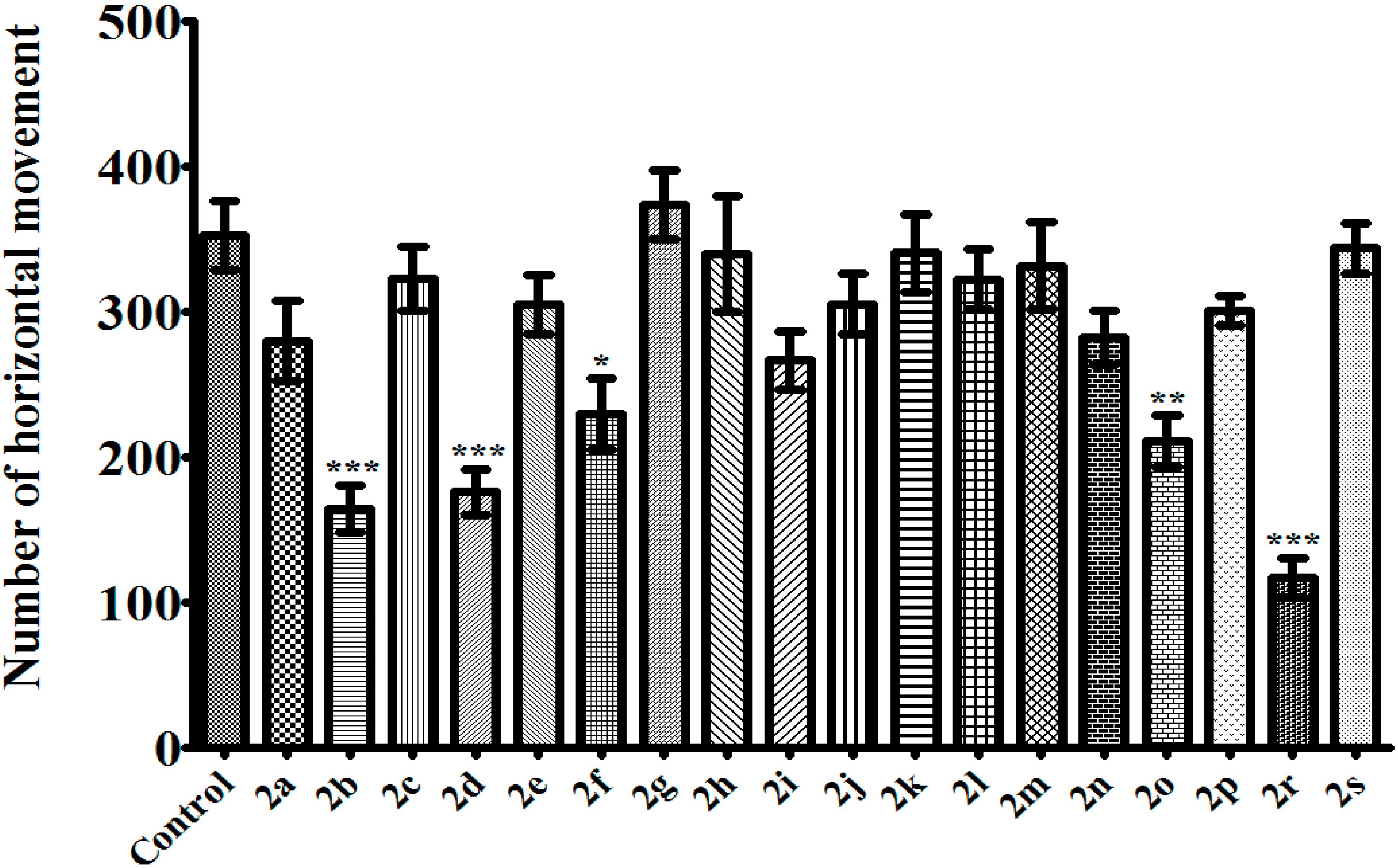
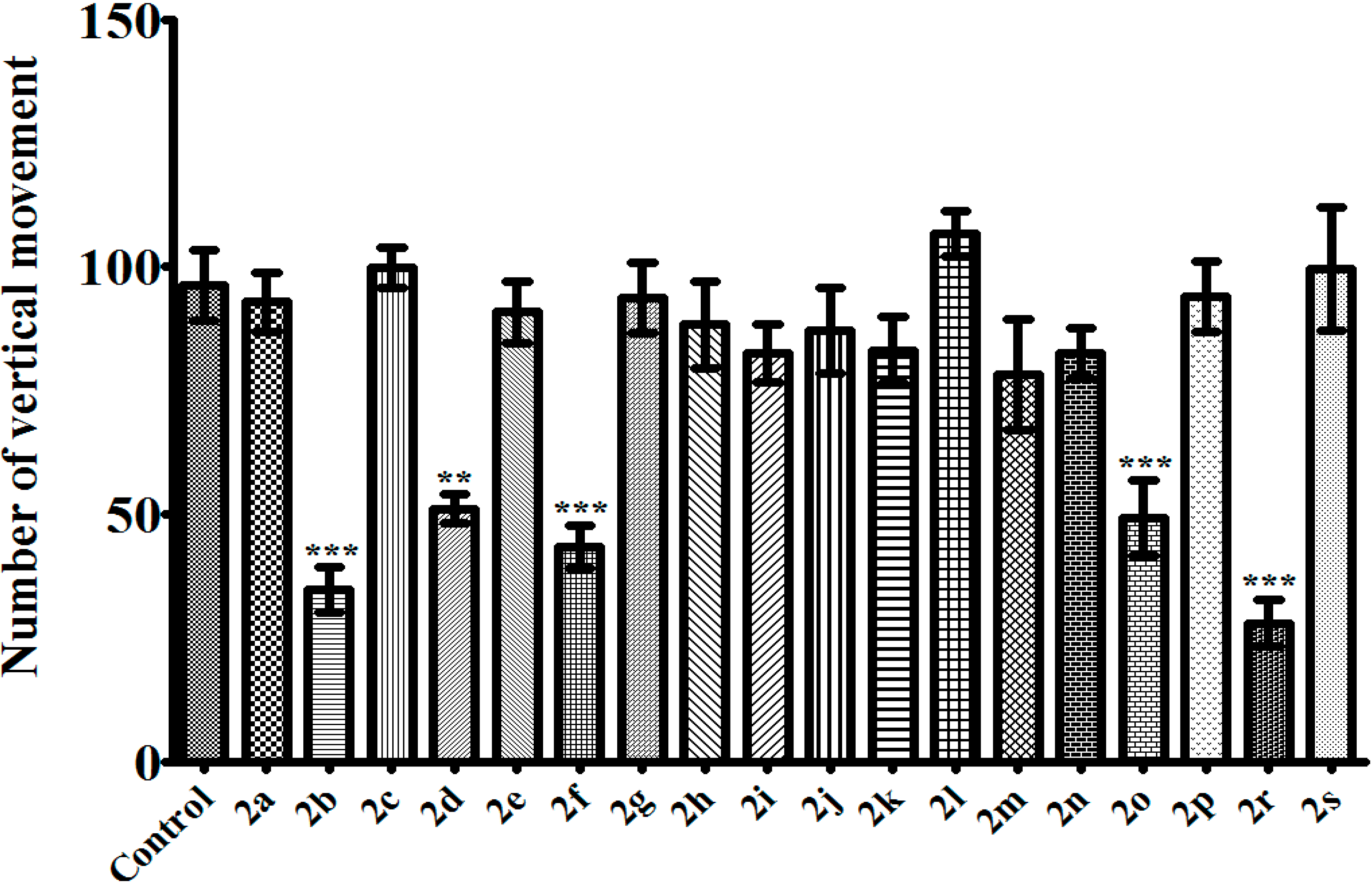
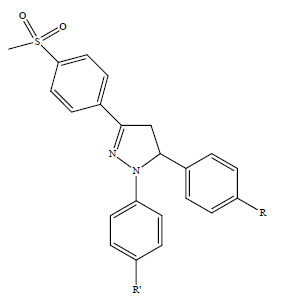
3.2.2. 1,5-Diaryl-3-[4-(methylsulfonyl)phenyl]-4,5-dihydro-1H-pyrazoles 2a–s
A mixture of the appropriate chalcone 1a–c (10.0 mmol) and phenylhydrazine hydrochloride derivative (20.0 mmol) in the presence of acetic acid (50 mL) was refluxed for 8 h, then poured into crushed ice. The precipitate was separated by filtration, washed with water and crystallized from methanol.
1-(4-Chlorophenyl)-5-(4-fluorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2a): Yield: 93%; m.p. 193 °C. IR (KBr) νmax (cm−1): 3012.60 (Aromatic C-H), 2918.10 (Aliphatic C-H), 1585.38, 1490.87 (C=N and C=C), 1307.65, 1149.50, 1087.78 (SO2 and C-N), 835.12 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.19 (1H, dd, JAM = 17.5 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.96 (1H, dd, JMA = 17.5 Hz, JMX = 12.0 Hz, C4-HM pyrazoline), 5.65 (1H, dd, JMX = 12.5 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 7.04 (2H, d, J = 9.0 Hz, aromatic protons), 7.15–7.19 (2H, m, aromatic protons), 7.23 (2H, d, J = 9.0 Hz, aromatic protons), 7.29–7.32 (2H, m, aromatic protons), 7.94–8.02 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.52 (CH3), 43.51 (CH2), 62.59 (CH), 114.74 (2CH), 115.78 (CH), 115.95 (CH), 126.28 (C), 127.33 (2CH), 127.98 (CH), 128.77 (2CH), 131.53 (CH), 136.72 (CH), 137.77 (CH), 140.12 (C), 142.19 (C), 144.05 (C), 146.46 (C), 152.05 (C), 160.46 (C). Anal. Calcd. for C22H18ClFN2O2S: C, 61.61; H, 4.23; N, 6.53; Found: C, 61.60; H, 4.25; N, 6.52. MS (ESI) (m/z): [M+1]+ 429.
1,5-Bis(4-fluorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2b): Yield: 85%; m.p. 221 °C. IR (KBr) νmax (cm−1): 1508.23 (C=N), 1305.72, 1228.57, 1151.42 (SO2 and C-N), 835.12 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.18 (1H, dd, JAM = 18.0 Hz, JAX = 7.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.94 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.61 (1H, dd, JMX = 12.5 Hz, JAX = 6.5 Hz, C5-HX pyrazoline), 7.04 (4H, d, J = 6.5 Hz, aromatic protons), 7.15–7.19 (2H, m, aromatic protons), 7.31–7.34 (2H, m, aromatic protons), 7.93–7.97 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.54 (CH3), 43.53 (CH2), 63.13 (CH), 114.54 (CH), 114.60 (CH), 115.46 (CH), 115.76 (CH), 115.93 (CH), 126.14 (2CH), 127.33 (2CH), 128.00 (CH), 128.07 (CH), 136.92 (CH), 138.01 (C), 139.92 (C), 140.25 (C), 145.75 (C), 155.34 (C), 160.45 (C), 162.39 (C). Anal. Calcd. for C22H18F2N2O2S: C, 64.06; H, 4.40; N, 6.79; Found: C, 64.05; H, 4.39; N, 6.80. MS (ESI) (m/z): [M+1]+ 413.
1-(4-Bromophenyl)-5-(4-fluorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2c): Yield: 77%; m.p. 169 °C. IR (KBr) νmax (cm−1): 3014.53 (Aromatic C-H), 1583.45, 1488.94 (C=N and C=C), 1307.65, 1151.42, 1085.85 (SO2 and C-N), 835.12 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.19 (1H, dd, JAM = 17.5 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.95 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.65 (1H, dd, JMX = 12.5 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 6.99 (2H, d, J = 9.0 Hz, aromatic protons), 7.15–7.19 (2H, m, aromatic protons), 7.29–7.32 (2H, m, aromatic protons), 7.33–7.36 (2H, m, aromatic protons), 7.94–7.98 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.52 (CH3), 43.51 (CH2), 62.49 (CH), 115.22 (2CH), 115.80 (CH), 115.97 (CH), 125.98 (2CH), 127.35 (2CH), 127.97 (CH), 129.36 (C), 131.62 (2CH), 136.71 (CH), 137.73 (C), 140.14 (C), 142.52 (C), 146.56 (C), 160.47 (C), 162.41 (C). Anal. Calcd. for C22H18BrFN2O2S: C, 55.82; H, 3.83; N, 5.92. Found: C, 55.80; H, 3.81; N, 5.92. MS (ESI) (m/z): [M+1]+ 474.
1-(4-Methoxyphenyl)-5-(4-fluorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2d): Yield: 60%; m.p. 170 °C. IR (KBr) νmax (cm−1): 1510.16 (C=N), 1321.15, 1245.93, 1153.35, 1099.35 (SO2, C-N and C-O), 811.98 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.13 (1H, dd, JAM = 17.5 Hz, JAX = 7.0 Hz, C4-HA pyrazoline), 3.22 (3H, s, SO2CH3), 3.65 (3H, s, OCH3), 3.89 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.54 (1H, dd, JMX = 12.5 Hz, JAX = 7.0 Hz, C5-HX pyrazoline), 6.80 (2H, d, J = 7.0 Hz, aromatic protons), 6.99 (2H, d, J = 7.0 Hz, aromatic protons), 7.14–7.18 (2H, m, aromatic protons), 7.31–7.34 (2H, m, aromatic protons), 7.90–7.94 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.35 (CH3), 43.56 (CH2), 55.16 (CH3), 63.54 (CH), 114.41 (2CH), 114.78 (2CH), 115.67 (CH), 115.84 (CH), 125.85 (2CH), 127.32 (2CH), 128.05 (CH), 128.11 (CH), 137.19 (C), 138.31 (C), 139.51 (C), 144.53 (C), 153.18 (C), 160.41 (C), 162.34 (C). Anal. Calcd. for C23H21FN2O3S: C, 65.08; H, 4.99; N, 6.60. Found: C, 65.10; H, 4.97; N, 6.59. MS (ESI) (m/z): [M+1]+ 425.
1-(4-Methylphenyl)-5-(4-fluorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2e): Yield: 76%; m.p. 186 °C. IR (KBr) νmax (cm−1): 3028.03 (Aromatic C-H), 2920.03 (Aliphatic C-H), 1585.38, 1510.16 (C=N and C=C), 1305.72, 1224.71, 1151.42, 1087.78 (SO2 and C-N), 835.12, 781.12 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 2.17 (3H, s, CH3), 3.14 (1H, dd, JAM = 17.5 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.22 (3H, s, SO2CH3), 3.91 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.60 (1H, dd, JMX = 12.0 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 6.95–7.00 (4H, m, aromatic protons), 7.13–7.18 (2H, m, aromatic protons), 7.28–7.35 (2H, m, aromatic protons), 7.91–7.94 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 20.08 (CH3), 43.20 (CH3), 43.56 (CH2), 62.88 (CH), 113.45 (2CH), 115.68 (CH), 115.85 (CH), 115.91 (CH), 125.97 (2CH), 127.38 (2CH), 128.00 (CH), 128.22 (CH), 129.41 (CH), 131.55 (C), 137.11 (C), 139.66 (C), 144.07 (C), 144.89 (C), 160.39 (C), 162.33 (C). Anal. Calcd. for C23H21FN2O2S: C, 67.63; H, 5.18; N, 6.86. Found: C, 67.65; H, 5.17; N, 6.85. MS (ESI) (m/z): [M+1]+ 409.
1-(4-Methoxyphenyl)-5-(4-chlorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2f): Yield: 76%; m.p. 152 °C. IR (KBr) νmax (cm−1): 2925.81 (Aliphatic C-H asymmetric), 2833.24 (Aliphatic C-H symmetric), 1508.23 (C=N), 1307.65, 1242.07, 1151.42, 1087.78 (SO2, C-N and C-O), 825.48 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.14 (1H, dd, JAM = 18.0 Hz, JAX = 7.5 Hz, C4-HA pyrazoline), 3.22 (3H, s, SO2CH3), 3.65 (3H, s, OCH3), 3.90 (1H, dd, JMA = 17.0 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.55 (1H, dd, JMX = 12.5 Hz, JAX = 7.0 Hz, C5-HX pyrazoline), 6.80 (2H, d, J = 9.0 Hz, aromatic protons), 6.98 (2H, d, J = 9.0 Hz, aromatic protons), 7.30 (2H, d, J = 8.5 Hz, aromatic protons), 7.39 (2H, d, J = 8.5 Hz, aromatic protons), 7.90–7.94 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.73 (CH3), 44.06 (CH2), 55.66 (CH3), 64.03 (CH), 114.95 (2CH), 115.25 (2CH), 126.37 (2CH), 127.82 (2CH), 128.49 (2CH), 129.46 (2CH), 131.76 (C), 138.10 (C), 140.05 (C), 141.59 (C), 145.09 (C), 153.70 (C), 160.40 (C). Anal. Calcd. for C23H21ClN2O3S: C, 62.65; H, 4.80; N, 6.35. Found: C, 62.63; H, 4.78; N, 6.34. MS (ESI) (m/z): [M+1]+ 441.
1-(4-Fluorophenyl)-5-(4-chlorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2g): Yield: 87%; m.p. 203 °C. IR (KBr) νmax (cm−1): 2974.03 (Aliphatic C-H asymmetric), 2885.31 (Aliphatic C-H symmetric), 1577.66, 1504.37 (C=N and C=C), 1305.72, 1230.50, 1151.42, 1087.78 (SO2 and C-N), 825.48 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.18 (1H, dd, JAM = 17.5 Hz, JAX = 6.5 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.95 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.61 (1H, dd, JMX = 12.5 Hz, JAX = 6.5 Hz, C5-HX pyrazoline), 7.02–7.07 (4H, m, aromatic protons), 7.30 (2H, d, J = 7.0 Hz, aromatic protons), 7.40 (2H, d, J = 6.5 Hz, aromatic protons), 7.93–7.96 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.42 (CH3), 43.52 (CH2), 63.12 (CH), 114.51 (CH), 114.57 (CH), 115.50 (CH), 115.68 (CH), 126.16 (2CH), 127.34 (2CH), 127.93 (2CH), 129.04 (2CH), 132.13 (C), 136.85 (C), 139.95 (C), 140.78 (C), 145.81 (C), 155.36 (C), 160.41 (C). Anal. Calcd. for C22H18ClFN2O2S: C, 61.61; H, 4.23; N, 6.53. Found: C, 61.60; H, 4.22; N, 6.52. MS (ESI) (m/z): [M+1]+ 429
1-(4-Methylphenyl)-5-(4-chlorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2h): Yield: 83%; m.p. 230 °C. IR (KBr) νmax (cm−1): 2970.17 (Aliphatic C-H asymmetric), 2883.38 (Aliphatic C-H symmetric), 1510.16 (C=N), 1305.72, 1151.42, 1087.78 (SO2 and C-N), 825.48 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 2.17 (3H, s, CH3), 3.15 (1H, dd, JAM = 17.5 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.22 (3H, s, SO2CH3), 3.92 (1H, dd, JMA = 18.0 Hz, JMX = 12.0 Hz, C4-HM pyrazoline), 5.60 (1H, dd, JMX = 12.5 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 6.95 (2H, d, J = 7.0 Hz, aromatic protons), 6.99 (2H, d, J = 8.0 Hz, aromatic protons), 7.29 (2H, d, J = 7.0 Hz, aromatic protons), 7.39 (2H, d, J = 8.5 Hz, aromatic protons), 7.93 (4H, s, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 20.07 (CH3), 42.15 (CH3), 43.55 (CH2), 62.87 (CH), 113.43 (2CH), 125.99 (2CH), 127.33 (2CH), 127.87 (2CH), 128.27 (CH), 128.96 (CH), 129.43 (CH), 132.00 (CH), 137.05 (C), 139.70 (C), 141.07 (C), 141.13 (2C), 144.96 (C), 160.43 (C). Anal. Calcd. for C23H21ClN2O2S: C, 65.01; H, 4.98; N, 6.59. Found: C, 65.00; H, 4.99; N, 6.58. MS (ESI) (m/z): [M+1]+ 425.
1-(4-Bromophenyl)-5-(4-chlorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2i): Yield: 83%; m.p. 204 °C. IR (KBr) νmax (cm−1): 2918.10 (Aliphatic C-H), 1583.45, 1487.01 (C=N and C=C), 1307.65, 1151.42, 1087.78 (SO2 and C-N), 819.69 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.20 (1H, dd, JAM = 18.0 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.96 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.65 (1H, dd, JMX = 12.5 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 6.99 (2H, d, J = 9.0 Hz, aromatic protons), 7.28 (2H, d, J = 8.5 Hz, aromatic protons), 7.35 (2H, d, J = 9.5 Hz, aromatic protons), 7.41 (2H, d, J = 8.5 Hz, aromatic protons), 7.94–7.98 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.90 (CH3), 44.01 (CH2), 63.00 (CH), 115.70 (2CH), 126.84 (2CH), 127.86 (2CH), 128.33 (2CH), 129.59 (2CH), 132.16 (2CH), 132.69 (C), 137.16 (C), 140.68 (C), 141.00 (C), 142.97 (C), 147.13 (C), 160.45 (C). Anal. Calcd. For C22H18BrClN2O2S: C, 53.95; H, 3.70; N, 5.72. Found: C, 53.93; H, 3.69; N, 5.70. MS (ESI) (m/z): [M+1]+ 490.
1,5-Bis(4-chlorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2j): Yield: 73%; m.p. 209 °C. IR (KBr) νmax (cm−1): 2918.10 (Aliphatic C-H), 1583.45, 1488.94 (C=N and C=C), 1307.65, 1151.42, 1087.78 (SO2 and C-N), 821.62 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.20 (1H, dd, JAM = 17.5 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.96 (1H, dd, JMA = 18.0 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.65 (1H, dd, JMX = 12.5 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 7.03 (2H, d, J = 9.0 Hz, aromatic protons), 7.23 (2H, d, J = 9.0 Hz, aromatic protons), 7.29 (2H, d, J = 8.5 Hz, aromatic protons), 7.41 (2H, d, J = 8.5 Hz, aromatic protons), 7.94–7.98 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.91 (CH3), 44.01 (CH2), 63.09 (CH), 115.23 (2CH), 126.82 (2CH), 127.86 (2CH), 128.34 (2CH), 129.32 (2CH), 129.90 (2CH), 132.69 (C), 137.18 (C), 140.66 (C), 141.05 (C), 142.64 (C), 147.04 (C), 160.48 (C). Anal. Calcd. for C22H18Cl2N2O2S: C, 59.33; H, 4.07; N, 6.29. Found: C, 59.32; H, 4.05; N, 6.30. MS (ESI) (m/z): [M+1]+ 446.
1-(4-Methoxyphenyl)-5-(4-bromophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2k): Yield: 68%; m.p. 145 °C. IR (KBr) νmax (cm−1): 2927.74 (Aliphatic C-H asymmetric), 2827.45 (Aliphatic C-H symmetric), 1510.16, 1417.58 (C=N and C=C), 1313.43, 1242.07, 1151.42, 1087.78 (SO2, C-N and C-O), 813.90 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 6.97 (d, J = 3.6 Hz, 1H), 7.11 (d, J = 3.2 Hz, 1H), 7.43–7.49 (m, 3H), 7.83–7.89 (m, 3H), 7.93–7.95 (m, 2H), 8.13–8.14 (m, 1H), 12.37 (brs, 1H). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.17 (CH3), 43.56 (CH2), 55.16 (CH3), 63.56 (CH), 114.45 (2CH), 114.73 (2CH), 120.56 (C), 125.87 (2CH), 127.33 (2CH), 128.33 (2CH), 131.88 (2CH), 137.12 (C), 137.57 (C), 139.54 (C), 141.52 (C), 144.59 (C), 153.19 (C). Anal. Calcd. for C23H21BrN2O3S: C, 56.91; H, 4.36; N, 5.77. Found: C, 56.90; H, 4.35; N, 5.76. MS (ESI) (m/z): [M+1]+ 486.
1-(4-Fluorophenyl)-5-(4-bromophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2l): Yield: 75%; m.p. 196 °C. IR (KBr) νmax (cm−1): 3031.89 (Aromatic C-H), 2918.10 (Aliphatic C-H), 1577.66, 1506.30, 1406.01 (C=N and C=C), 1307.65, 1218.93, 1151.42, 1010.63 (SO2 and C-N), 825.48 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.17 (1H, dd, JAM = 18.0 Hz, JAX = 7.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.95 (1H, dd, JMA = 16.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.59 (1H, dd, JMX = 11.0 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 7.04 (4H, s, aromatic protons), 7.24–7.26 (2H, m, aromatic protons), 7.53–7.55 (2H, m, aromatic protons), 7.95 (4H, s, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.36 (CH3), 43.52 (CH2), 63.17 (CH), 114.57 (CH), 115.50 (CH), 115.68 (2CH), 120.66 (CH), 126.16 (2CH), 127.34 (2CH), 128.26 (2CH), 131.96 (2C), 136.85 (CH), 139.96 (C), 140.17 (C), 141.20 (C), 145.82 (C), 155.37 (C). Anal. Calcd. for C22H18BrFN2O2S: C, 55.82; H, 3.83; N, 5.92. Found: C, 55.82; H, 3.81; N, 5.93. MS (ESI) (m/z): [M+1]+ 474.
1-(4-Methylphenyl)-5-(4-bromophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2m): Yield: 87%; m.p. 227 °C. IR (KBr) νmax (cm−1): 3016.46 (Aromatic C-H), 2918.10 (Aliphatic C-H), 1585.38, 1508.23, 1407.94 (C=N and C=C), 1294.15, 1242.07, 1151.42, 1085.85, 1010.63 (SO2 and C-N), 821.62 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 2.17 (3H, s, CH3), 3.15 (1H, dd, JAM = 17.5 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.22 (3H, s, SO2CH3), 3.92 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.59 (1H, dd, JMX = 12.5 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 6.94 (2H, d, J = 8.5 Hz), 6.99 (2H, d, J = 8.0 Hz), 7.22 (2H, d, J = 8.5 Hz, aromatic protons), 7.53 (2H, d, J = 8.5 Hz, aromatic protons), 7.93 (4H, s, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 20.58 (CH3), 42.59 (CH3), 44.05 (CH2), 63.42 (CH), 113.92 (2CH), 121.02 (C), 126.50 (2CH), 127.83 (2CH), 128.73 (2CH), 128.77 (2CH), 129.94 (2CH), 132.38 (C), 137.54 (C), 140.20 (C), 141.62 (C), 142.00 (C), 145.47 (C). Anal. Calcd. for C23H21BrN2O2S: C, 58.85; H, 4.51; N, 5.97. Found: C, 58.85; H, 4.50; N, 5.95. MS (ESI) (m/z): [M+1]+ 470.
1,5-Bis(4-bromophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2n): Yield: 94%; m.p. 225 °C. IR (KBr) νmax (cm−1): 3012.60 (Aromatic C-H), 2918.10 (Aliphatic C-H), 1583.45, 1487.01, 1409.87 (C=N and C=C), 1307.65, 1151.42, 1085.85, 1010.63 (SO2 and C-N), 819.69 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.20 (1H, dd, JAM = 18.0 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.96 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.63 (1H, dd, JMX = 12.0 Hz, JAX = 5.5 Hz, C5-HX pyrazoline), 6.98 (2H, d, J = 8.5 Hz), 7.21 (2H, d, J = 8.0 Hz, aromatic protons), 7.35 (2H, d, J = 9.0 Hz, aromatic protons), 7.54 (2H, d, J = 8.5 Hz, aromatic protons), 7.93–7.97 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.34 (CH3), 43.51 (CH2), 62.54 (CH), 110.74 (2CH), 115.19 (C), 120.72 (C), 126.33 (2CH), 127.35 (2CH), 128.15 (2CH), 131.99 (2CH), 136.64 (2CH), 140.17 (C), 140.91 (C), 142.45 (C), 146.62 (C), 151.70 (C). Anal. Calcd. for C22H18Br2N2O2S: C, 49.46; H, 3.40; N, 5.24. Found: C, 49.44; H, 3.41; N, 5.25. MS (ESI) (m/z): [M+1]+ 535.
1-(4-Chlorophenyl)-5-(4-bromophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2o): Yield: 95%; m.p. 222 °C. IR (KBr) νmax (cm−1): 3045.39 (Aromatic C-H), 2918.10 (Aliphatic C-H), 1583.45, 1488.94 (C=N and C=C), 1307.65, 1151.42, 1085.85 (SO2 and C-N), 819.69 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.20 (1H, dd, JAM = 18.0 Hz, JAX = 6.0 Hz, C4-HApyrazoline), 3.23 (3H, s, SO2CH3), 3.96 (1H, dd, JMA = 18.0 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.63 (1H, dd, JMX = 12.5 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 7.03 (2H, d, J = 9.0 Hz, aromatic protons), 7.22–7.24 (4H, m, aromatic protons), 7.54 (2H, d, J = 8.5 Hz, aromatic protons), 7.94–7.98 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.35 (CH3), 43.51 (CH2), 62.64 (CH), 114.71 (2CH), 120.71 (C), 123.06 (CH), 126.31 (CH), 127.35 (2CH), 128.17 (2CH), 128.82 (2CH), 131.99 (2CH), 136.66 (C), 140.15 (2C), 140.96 (C), 142.12 (C), 146.53 (C). Anal. Calcd. for C22H18BrClN2O2S: C, 53.95; H, 3.70; N, 5.72. Found: C, 53.93; H, 3.69; N, 5.74. MS (ESI) (m/z): [M+1]+ 490.
1-Phenyl-5-(4-bromophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2p): Yield: 86%; m.p. 205 °C. IR (KBr) νmax (cm−1): 3020.32 (Aromatic C-H), 2921.96 (Aliphatic C-H), 1595.02, 1485.09, 1407.94 (C=N and C=C), 1303.79, 1151.42, 1010.63 (SO2 and C-N), 821.62, 750.26 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.17 (1H, dd, JAM = 17.5 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.94 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.62 (1H, dd, JMX = 12.0 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 6.77 (1H, m, aromatic protons), 7.04 (2H, d, J = 8.0 Hz, aromatic protons), 7.17–7.20 (2H, m, aromatic protons), 7.24 (2H, d, J = 8.5 Hz, aromatic protons), 7.53 (2H, d, J = 8.5 Hz, aromatic protons), 7.93–7.97 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.19 (CH3), 43.54 (CH2), 62.71 (CH), 113.28 (2CH), 119.47 (C), 120.57 (CH), 126.14 (2CH), 127.34 (2CH), 128.18 (2CH), 129.00 (2CH), 131.93 (2CH), 136.92 (C), 139.90 (C), 141.45 (C), 143.28 (C), 145.60 (C). Anal. Calcd. for C22H19BrN2O2S: C, 58.03; H, 4.21; N, 6.15. Found: C, 58.01; H, 4.20; N, 6.17. MS (ESI) (m/z): [M+1]+ 456.
1-Phenyl-5-(4-chlorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2r): Yield: 81%; m.p. 212 °C. IR (KBr) νmax (cm−1): 3020.32 (Aromatic C-H), 2921.96 (Aliphatic C-H), 1596.95, 1492.80, 1411.80 (C=N and C=C), 1303.79, 1151.42, 1087.78 (SO2 and C-N), 823.55, 750.26 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.17 (1H, dd, JAM = 17.5 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.94 (1H, dd, JMA = 18.0 Hz, JMX = 13.0 Hz, C4-HM pyrazoline), 5.63 (1H, dd, JMX = 12.0 Hz, JAX = 6.0 Hz, C5-HX pyrazoline), 6.76–6.79 (1H, m, aromatic protons), 7.04 (2H, d, J = 8.0 Hz, aromatic protons), 7.17–7.20 (2H, m, aromatic protons), 7.30 (2H, d, J = 8.5 Hz, aromatic protons), 7.40 (2H, d, J = 8.5 Hz, aromatic protons), 7.95–7.97 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.24 (CH3), 43.53 (CH2), 62.67 (CH), 113.29 (2CH), 119.46 (CH), 126.13 (2CH), 127.33 (2CH), 127.82 (2CH), 129.00 (3CH), 132.05 (CH), 136.93 (C), 139.89 (C), 141.01 (2C), 143.29 (C), 145.57 (C). Anal. Calcd. for C22H19ClN2O2S: C, 64.30; H, 4.66; N, 6.82. Found: C, 64.31; H, 4.65; N, 6.80. MS (ESI) (m/z): [M+1]+ 411.
1-Phenyl-5-(4-fluorophenyl)-3-(4-(methylsulfonyl)phenyl)-4,5-dihydro-1H-pyrazole (2s): Yield: 78%; m.p. 219 °C. IR (KBr) νmax (cm−1): 3020.32 (Aromatic C-H), 2921.96 (Aliphatic C-H), 1595.02, 1492.80 (C=N and C=C), 1380.94, 1299.93, 1224.71, 1149.50, 1087.78 (SO2 and C-N), 837.05, 748.33 (C-H out of plane deformation). 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.16 (1H, dd, JAM = 17.5 Hz, JAX = 6.0 Hz, C4-HA pyrazoline), 3.23 (3H, s, SO2CH3), 3.93 (1H, dd, JMA = 17.5 Hz, JMX = 12.5 Hz, C4-HM pyrazoline), 5.62 (1H, dd, JMX = 12.5 Hz, JAX = 6.5 Hz, C5-HX pyrazoline), 6.75–6.78 (1H, m, aromatic protons), 7.06 (2H, d, J = 8.0 Hz, aromatic protons), 7.15–7.20 (4H, m, aromatic protons), 7.31–7.34 (2H, m, aromatic protons), 7.94–7.96 (4H, m, 4-methylsulfonylphenyl protons). 13C-NMR (125 MHz, DMSO-d6) δ (ppm): 42.37 (CH3), 43.55 (CH2), 62.68 (CH), 113.32 (2CH), 115.72 (CH), 115.89 (CH), 119.42 (CH), 126.11 (2CH), 127.34 (2CH), 127.96 (CH), 128.96 (2CH), 137.00 (CH), 138.22 (C), 139.85 (C), 143.36 (C), 145.51 (C), 160.41 (C), 162.35 (C). Anal. Calcd. for C22H19FN2O2S: C, 66.99; H, 4.85; N, 7.10. Found: C, 66.98; H, 4.82; N, 7.12. MS (ESI) (m/z): [M+1]+ 395.