Anti-Tuberculous Activity of Treponemycin Produced by a Streptomyces Strain MS-6-6 Isolated from Saudi Arabia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Anti-Tuberculous Activity of Streptomyces Isolates
| Strain Number | Inhibition Zone (mm) | Equivalent Activity to Rifampicin (mg/L) | Cytotoxic Activity (IC50) (µg/mL) |
|---|---|---|---|
| MS-6-6 | 29 | 0.08 | 280 |
| MS-18-4 | 20 | 0.03 | 315 |
| MKS-9-3 | 20 | 0.03 | 275 |
| JS-8-4 | 20 | 0.03 | 315 |
| JS-9-4 | 24 | 0.05 | 265 |
| JS-22-9 | 27 | 0.06 | 240 |
| GS-42-1 | 24 | 0.05 | 320 |
| ES-15-3 | 22 | 0.04 | 260 |
2.2. Identification of Streptomyces Strain MS-6-6
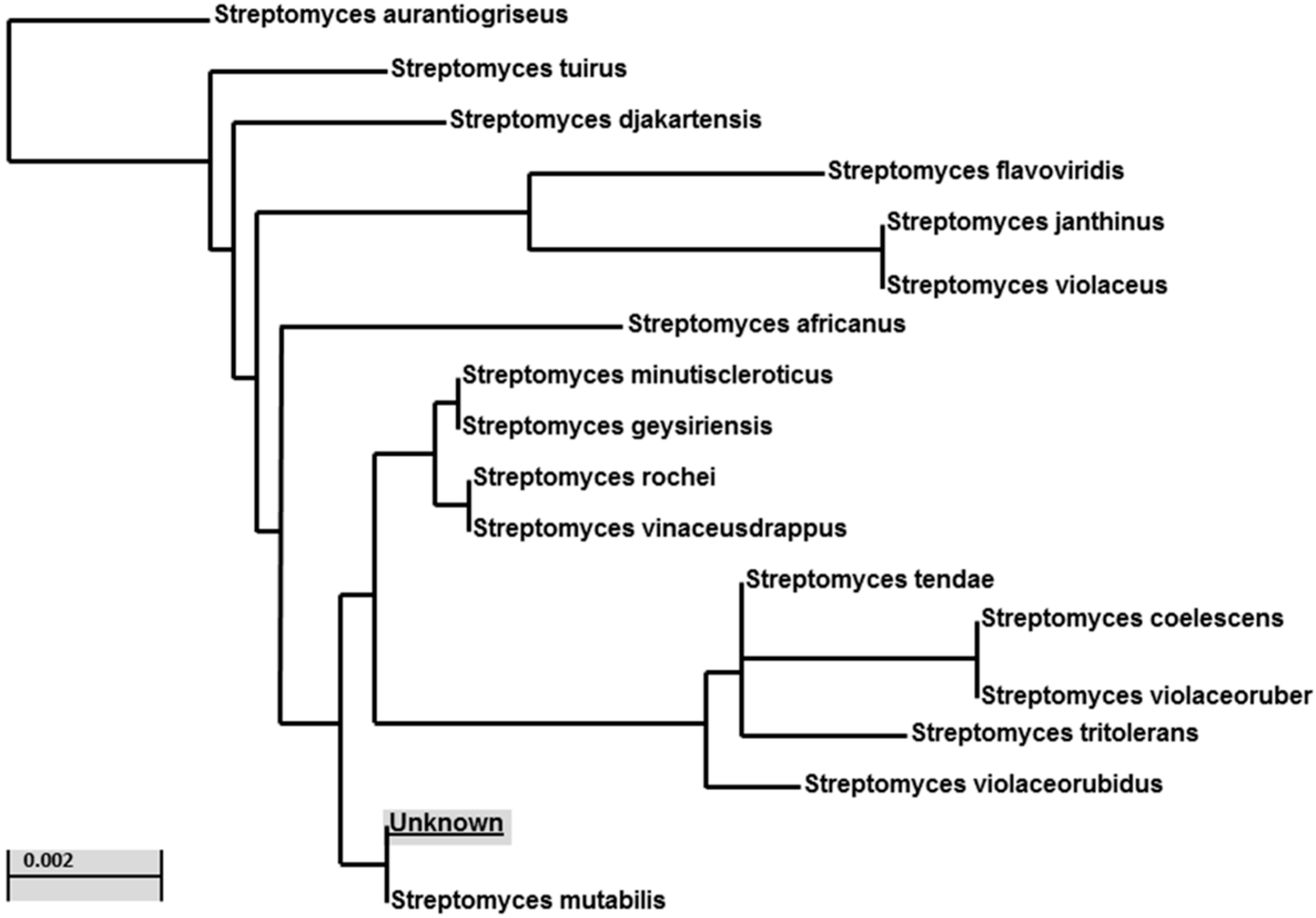
2.3. Isolation and Structure Elucidation of the Bioactive Compound from the Streptomyces Mutabilis
| Position | H | C | COSY | HMBC (H with C) |
|---|---|---|---|---|
| 1 | - | 172.1 | - | - |
| 2 | 2.42, 2.32 m | 39.3(CH2) | 3.86 | 172.1, 69.9, 35.1 |
| 3 | 3.86, m | 69.9(CH) | 2.32, 2.42 | 43, 172.1 |
| 4 | 1.67, m | 35.1(CH) | 3.86, 1.25, 0.84 | 69.9, 39.3, 27.2 |
| 5 | 0.92, 1.25 | 43.1(CH2) | 1.67, 1.57 | 18.23, 47.8, 69.9 |
| 6 | 1.57 | 27.2(CH) | 1.10, 0.97, 1.25 | 26.3, 35.1 |
| 7 | 0.97, 1.10 | 47.8 | 1.62, 1.57 | 37.5, 43.1 |
| 8 | 1.62 | 26.3(CH) | 0.85, 1.03, 1.1 | 65.3, 27.2 |
| 9 | 1.03, 0.76 | 37.5(CH2) | 1.85, 1.62 | 14.9, 73.2, 26.3 |
| 10 | 1.85 | 65.3(CH) | 4.1, 1.03 | 118.1, 26.3 |
| 11 | 4.10, d, J = 9.6 | 73.2(CH) | 1.85 | 116.1, 37.47, 143.9, 14.9 |
| 12 | - | 118.1 | - | - |
| 13 | 6.80, d, J = 10.8 | 143.9(CH) | 6.40 | 118.1, 138.5, 73.2,126.9 |
| 14 | 6.40, m | 126.9(CH) | 6.20, 6.80 | 116.1, 35.9, 143.9 |
| 15 | 6.20, m | 138.5(CH) | 6.40, 2.56 | 143.9, 35.9, 76.6 |
| 16 | 2.56, 2.59, m | 35.9(CH2) | 5.0, 6.20 | 126.9, 138.5, 76.5, 45.9 |
| 17 | 5.0, m | 76.5 (CH) | 2.56, 2.59, 2.70 | 172.1,138.5, 29.6,35.9 |
| 18 | 2.70, t | 45.9 (CH) | 1.30, 1.97, 2.51 | 176.1, 76.5, 47.8, 29.6 |
| 19 | 1.97, 1.30m | 29.6(CH2) | 2.70, 5.0, 2.51 | 76.5, 47.8, 31.1, 25.2 |
| 20 | 1.83, 1.79 | 25.2 (CH2) | 1.30, 2.03, 1.90 | 45.9, 47.8 |
| 21 | 2.03, 1.90 m | 31.1(CH2) | 1.83, 1.79, 2.51 | 176.1, 45.9, 47.8 |
| 22 | 2.51, m | 47.8(CH) | 1.90, 2.03, 2.70 | 176.1, 76.5, 45.9, 29.6, 31.1 |
| CH3 at C4 | 0.84, d, J = 6 | 17.0 | - | 35.1, 43.1, 69.9 |
| CH3 at C6 | 0.80, d, J = 6 | 18.2 | - | 27.2, 47.8, 43.1 |
| CH3 at C8 | 0.85, d, J = 6 | 20.2 | - | 26.3, 37.5, 47.8 |
| CH3 at C10 | 1.04, d, J = 6 | 14.9 | - | 65.3, 73.2 |
| CN | - | 116.1 | - | - |
| COOH | - | 176.1 | - | - |
2.4. Antimicrobial Activity of Treponemycin
| Organisms | MIC (µg/mL) |
|---|---|
| Mycobacterium tuberculosis | 4.17 |
| Staphylococcus epidermidis | 1.7 |
| Streptococcus pyogenes | 2.1 |
| Bacillus subtilis | 2.9 |
| Escherichia coli | 8.3 |
| Clostridium perfringens | 16.7 |
| Brucella melitensis | 16.7 |
| Pseudomonas aeruginosa | 26.7 |
| Proteus mirabilis | 11.3 |
| Candida albicans | 13.3 |
2.5. Improvement of Treponemycin Productivity
2.5.1. Improvement of Antimicrobial Activity
| Ingredients (g/L) | M1 * | M2 | M3 | M4 | M5 | M6 | M7 | M8 |
|---|---|---|---|---|---|---|---|---|
| CSL | - | - | 20.0 | 20.0 | 20.0 | 10.0 | 10.0 | 20.0 |
| Yeast extract | - | 10.0 | 10.0 | - | - | 10.0 | - | - |
| Peptone | - | - | - | 10.0 | - | - | - | - |
| Tryptone | - | 10.0 | - | 10.0 | - | 10.0 | - | |
| Soluble starch | 20.0 | 10.0 | 20.0 | 20.0 | 20.0 | - | - | 10.0 |
| Glucose | - | 10.0 | - | - | - | 20.0 | 10.0 | 10.0 |
| KNO3 | 2.0 | - | - | - | - | - | - | - |
| CaCO3 | 3.0 | - | - | - | - | - | - | - |
| MgSO4 7H2O | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| NaCl | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 5.0 | 5.0 | 5.0 |
| K2HPO4 | 1.0 | - | - | - | - | - | - | - |
| FeSO4 7H2O | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | - | - | - |
| MnCl2 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | - | - | - |
| ZnSO4 7H2O | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | - | - | - |
| Inhibition Zones (mm) | TP Titer | ||||
|---|---|---|---|---|---|
| Media | M. tuberculosis | S. epidermisis. | E. coli | C. albicans. | mg/L |
| M1 | 24 | 18 | 16 | 14 | 0.45 |
| M2 | 17 | 15 | 14 | 12 | 0.38 |
| M3 | 29 | 24 | 21 | 18 | 0.67 |
| M4 | 26 | 21 | 18 | 15 | 0.50 |
| M5 | 28 | 22 | 19 | 16 | 0.68 |
| M6 | 17 | 15 | 14 | 12 | 0.40 |
| M7 | 17 | 15 | 14 | 11 | 0.36 |
| M8 | 22 | 17 | 15 | 13 | 0.45 |
2.5.2. LC-DAD/ESI-MS Analysis of TP and Its Metabolites in Different Culture Media
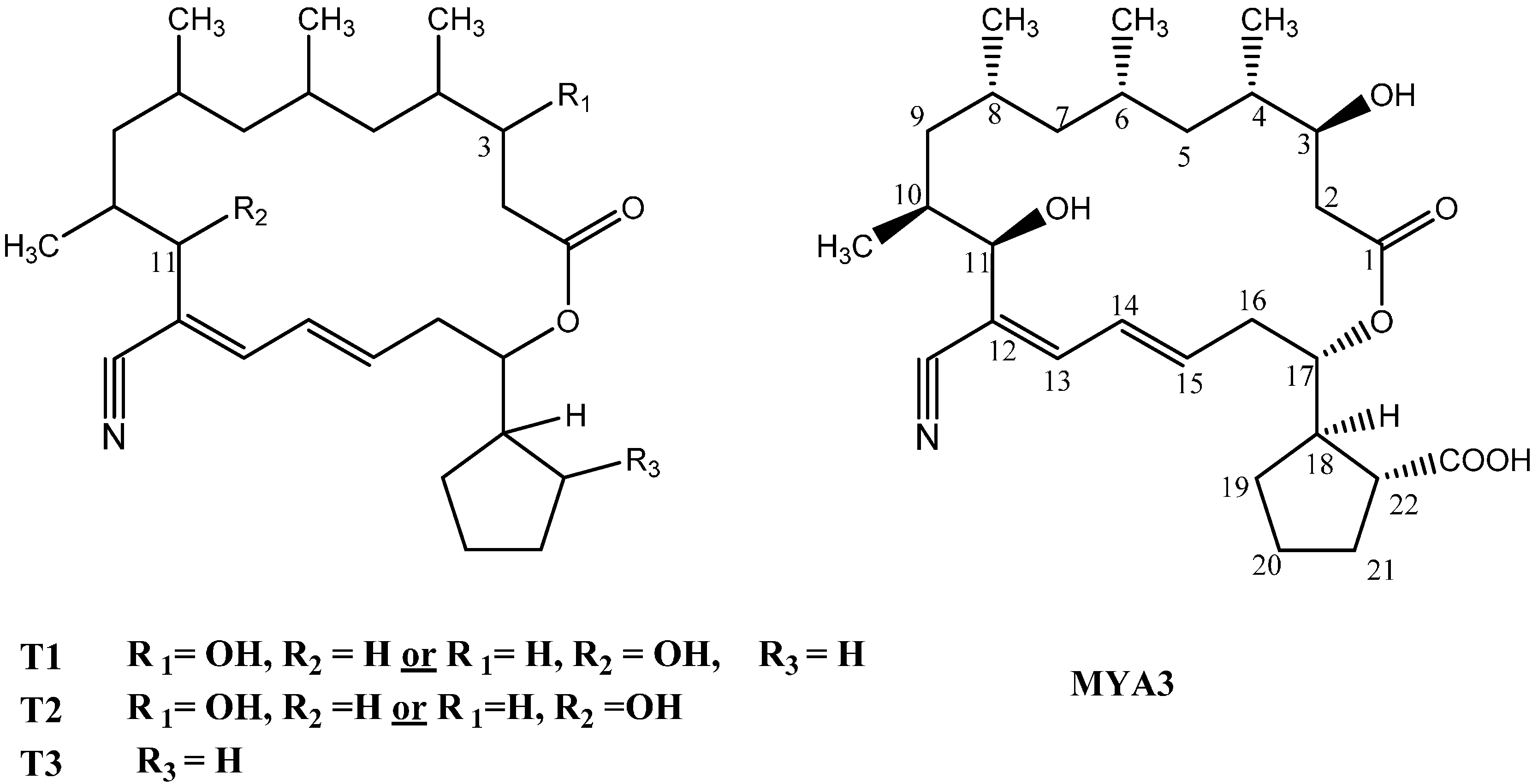
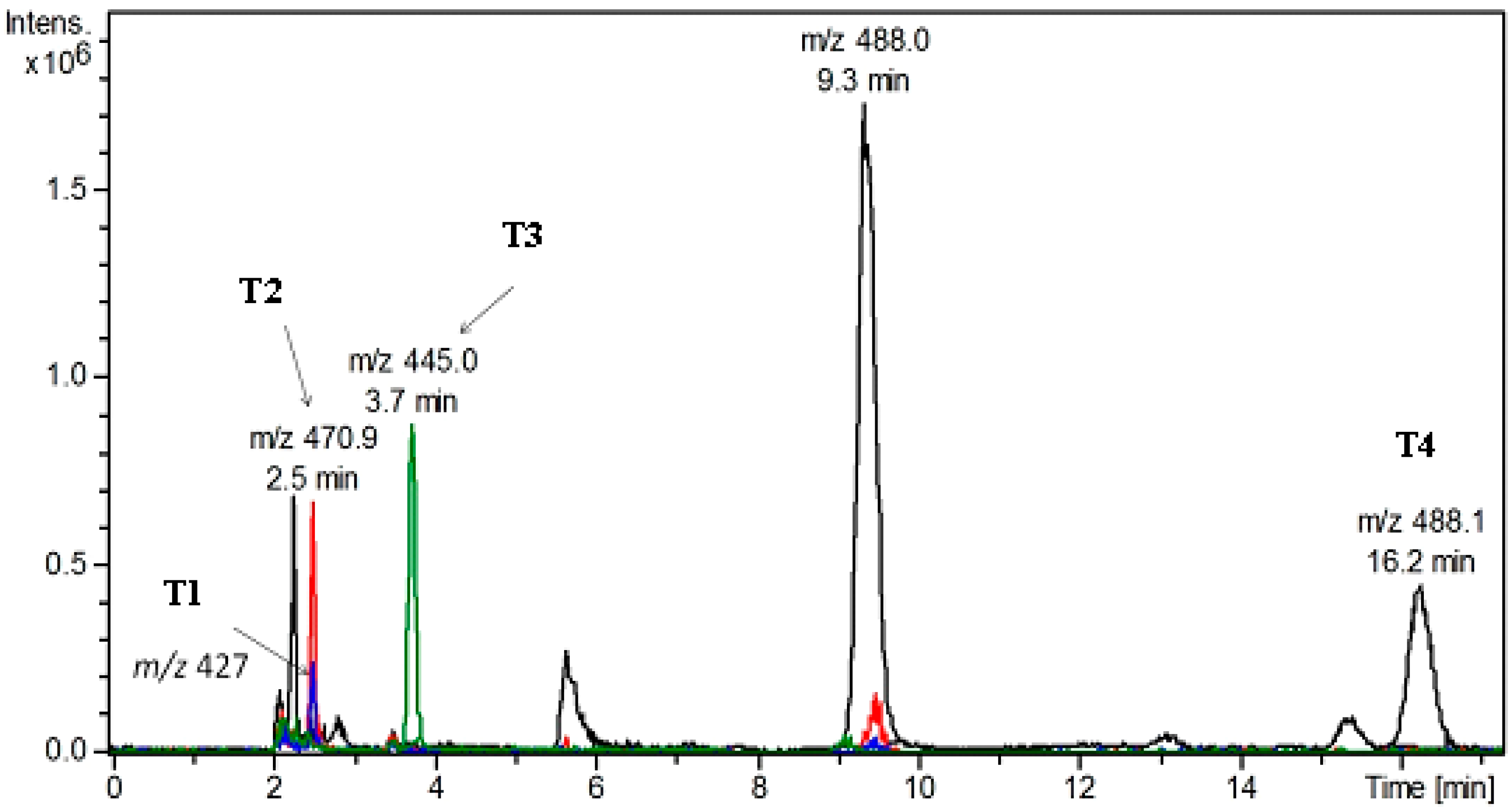
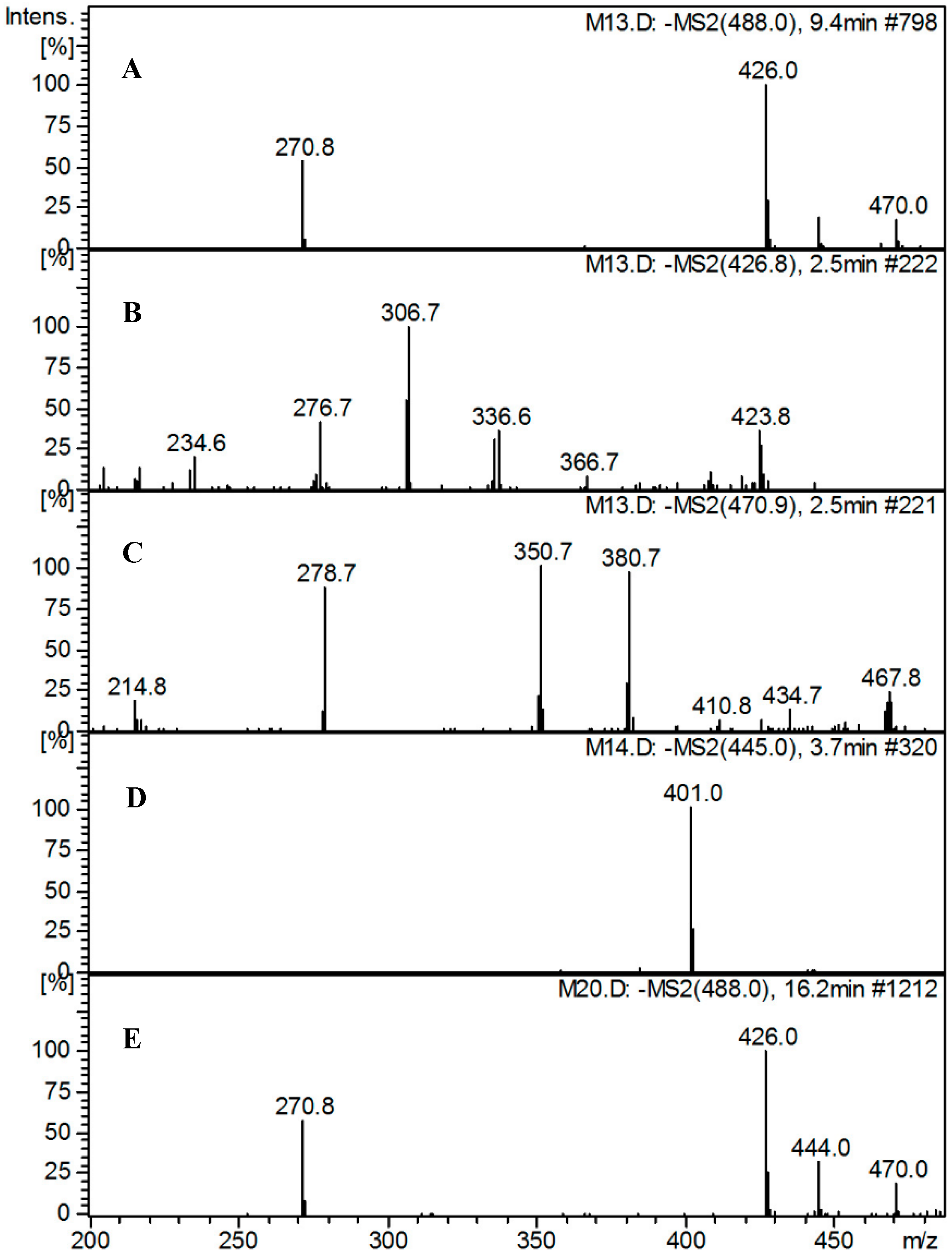
2.5.3. Identification of Metabolites
3. Experimental Section
3.1. Microorganisms
3.2. Isolation of Streptomyces Strains
3.3. Evaluation of Antimicrobial Activity
3.3.1. Fermentation and Preparation of Streptomyces Clear Supernatants
3.3.2. Bacterial and Fungal Susceptibility Testing
3.3.3. Evaluation of Anti-Tuberculous Activity
Preparation of Bacterial Suspension
Anti-Tuberculous Activity by Cup-Diffusion Techniques
Evaluation of Anti-Tuberculous Activity by REMA Plate Assay
3.4. Evaluation of Cytotoxic Activity
3.5. Isolation and Structure Elucidation of the Bioactive Compound
3.5.1. General
3.5.2. Biologically Guided Isolation of Active Compounds
3.6. Improvement of Antimicrobial Productivity
3.7. LC-DAD/MS Analysis of Trepenomycin and Its Metabolites in Different Media
3.7.1. Apparatus and Conditions
3.7.2. Preparation of Streptomyces Extracts from Different Media for LC-DAD/MS Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barkei, J.J.; Kevany, B.M.; Felnagle, E.A.; Thomas, M.G. Investigations into viomycin biosynthesis by using heterologous production in Streptomyces lividans. ChemBiochem 2009, 10, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajoj, S.A. Tuberculosis in Saudi Arabia: Can we change the way we deal with the disease? J. Infect. Public Health 2010, 3, 17–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marinelli, F. Antibiotics and Streptomyces: the future of antibiotic discovery—many options for drug discovery are still available. Microbiol. Today 2009, 36, 21–23. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2006, 23, 26–78. [Google Scholar] [CrossRef] [PubMed]
- Bull, A.T.; Stach, J.E.; Ward, A.C.; Goodfellow, M. Marine actinobacteria: Perspectives, challenges, future directions. Antonie Leeuwenhoek 2005, 87, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kharat, K.R.; Kharat, A.; Hardikar, B. Antimicrobial and cytotoxic activity of Streptomyces sp. from Lonar Lake. Afr. J. Biotechnol. 2009, 8, 6645–6648. [Google Scholar]
- Tanaka, Y.; Kasahara, K.; Hirose, Y.; Murakami, K.; Kugimiya, R.; Ochi, K. Activation and products of the cryptic secondary metabolite biosynthetic gene clusters by rifampin resistance (rpoB) mutations in actinomycetes. J. Biotechnol. 2013, 195, 2959–2970. [Google Scholar]
- Elnakady, Y.A.; Rohde, M.; Sasse, F.; Backes, C.; Keller, A.; Lenhof, H.P.; Weissman, K.J.; Müller, R. Evidence for the mode of action of the highly cytotoxic Streptomyces polyketide kendomycin. ChemBiochem 2007, 8, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Koyama, Y.; Oda, K.; Noda, M.; Matoba, Y.; Sugiyama, M. Molecular cloning and heterologous expression of a biosynthetic gene cluster for the antitubercular agent d-cycloserine produced by Streptomyces lavendulae. Antimicrob. Agents Chemother. 2010, 54, 1132–1139. [Google Scholar] [CrossRef]
- Stanbury, P.; Whitaker, A.; Hall, S. Media for industrial fermentations. In Principles of Fermentation Technology, 2nd ed.; Pergamon: Oxford, UK, 1995; pp. 93–121. [Google Scholar]
- Casida, L.E.J. Industrial Microbiology, 2nd ed.; New Age International Limited: New Delhi, India, 1996; pp. 117–134. [Google Scholar]
- Nagamitsu, T.; Takano, D.; Marumoto, K.; Fukuda, T.; Furuya, K.; Otoguro, K.; Takeda, K.; Kuwajima, I.; Harigaya, Y.; Omura, S. Total synthesis of borrelidin. J. Org. Chem. 2007, 72, 2744–2756. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Khodakaramian, G.; Arakawa, K.; Kinashi, H. Isolation of borrelidin as a phytotoxic compound from a potato pathogenic streptomyces strain. Biosci. Biotechnol. Biochem. 2011, 76, 353–357. [Google Scholar] [CrossRef]
- Maehr, H.; Evans, R.H. Identity of borrelidin with treponemycin. J. Antibiot. 1987, 40, 1455–1456. [Google Scholar] [CrossRef] [PubMed]
- Saisivam, S.; Bhikshapathi, D.; Krishnaveni, J.; Kishan, V. Isolation of borrelidin from Streptomyces californicus—An Indian soil isolate. Indian J. Biotechnol. 2008, 7, 349–355. [Google Scholar]
- Atta, H.M.; El-Sehrawi, M.; Bahobail, A. Antifungal macro-diode production by Streptomyces albidoflavus-143: fermentation, purification and biological activities. J. Am. Sci. 2011, 7, 13–22. [Google Scholar]
- Liu, C.-X.; Zhang, J.; Wang, X.-J.; Qian, P.-T.; Wang, J.-D.; Gao, Y.-M.; Yan, Y.-J.; Zhang, S.-Z.; Xu, P.-F.; Li, W.-B. Antifungal activity of borrelidin produced by a Streptomyces strain isolated from soybean. J. Agric. Food Chem. 2012, 60, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Yan, Y.; Wang, J.; Zhang, B.; Zhang, J.; Xiang, W. Streptomyces heilongjiangensis sp. nov., a novel actinomycete that produces borrelidin isolated from the root surface of soybean [Glycine max (L.) Merr]. Int. J. Syst. Evol. Microbiol. 2013, 63, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Lumb, M.; Macey, P.; Spyvee, J.; Whitmarsh, J.; Wright, R. Isolation of vivomycin and borrelidin, two antibiotics with anti-viral activity, from a species of Streptomyces (C2989). Nature 1965, 206, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Vong, B.G.; Kim, S.H.; Abraham, S.; Theodorakis, E.A. Stereoselective Total Synthesis of (−)-Borrelidin. Angew. Chem. 2004, 116, 4037–4041. [Google Scholar] [CrossRef]
- Gantt, J.S.; Bennett, C.A.; Arfin, S.M. Increased levels of threonyl-tRNA synthetase in a borrelidin-resistant Chinese hamster ovary cell line. Proc. Natl. Acad. Sci. USA 1981, 78, 5367–5370. [Google Scholar] [CrossRef] [PubMed]
- Duffey, M.O.; LeTiran, A.; Morken, J.P. Enantioselective total synthesis of borrelidin. J. Am. Chem. Soc. 2003, 125, 1458–1459. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, H.; Ijaz, S.; Ikram-ul-Haq. Production of antitumor antibiotic by Streptomyces capoamus. Pak. J. Bot. 2012, 44, 445–452. [Google Scholar]
- Anderson, B.; Herlt, A.; Rickards, R.; Robertson, G. Crystal and molecular structures of two isomorphous solvates of the macrolide antibiotic borrelidin: absolute configuration determination by incorporation of a chiral solvent in the crystal lattice. Aust. J. Chem. 1989, 42, 717–730. [Google Scholar] [CrossRef]
- Jaradat, Z.; Dawagreh, A.; Ababneh, Q.; Saadoun, I. Influence of culture conditions on cellulase production by Streptomyces sp.(strain J2). Jordan J. Biol. Sci. 2008, 1, 141–146. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Nineteenth Informational Supplement; Document M100-S19; CLSI: Wayne, PA, USA, 2009. [Google Scholar]
- Ahmed, I.; Jabeen, K.; Inayat, R.; Hasan, R. Susceptibility testing of extensively drug-resistant and pre-extensively drug-resistant Mycobacterium tuberculosis against levofloxacin, linezolid, and amoxicillin-clavulanate. Antimicrob. Agents Chemother. 2013, 57, 2522–2525. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.-C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassien, M.A.; Abdallah, H.M.; El-Halawany, A.M.; Jiman-Fatani, A.A.M. Anti-Tuberculous Activity of Treponemycin Produced by a Streptomyces Strain MS-6-6 Isolated from Saudi Arabia. Molecules 2015, 20, 2576-2590. https://doi.org/10.3390/molecules20022576
Yassien MA, Abdallah HM, El-Halawany AM, Jiman-Fatani AAM. Anti-Tuberculous Activity of Treponemycin Produced by a Streptomyces Strain MS-6-6 Isolated from Saudi Arabia. Molecules. 2015; 20(2):2576-2590. https://doi.org/10.3390/molecules20022576
Chicago/Turabian StyleYassien, Mahmoud A., Hossam M. Abdallah, Ali M. El-Halawany, and Asif A. M. Jiman-Fatani. 2015. "Anti-Tuberculous Activity of Treponemycin Produced by a Streptomyces Strain MS-6-6 Isolated from Saudi Arabia" Molecules 20, no. 2: 2576-2590. https://doi.org/10.3390/molecules20022576
APA StyleYassien, M. A., Abdallah, H. M., El-Halawany, A. M., & Jiman-Fatani, A. A. M. (2015). Anti-Tuberculous Activity of Treponemycin Produced by a Streptomyces Strain MS-6-6 Isolated from Saudi Arabia. Molecules, 20(2), 2576-2590. https://doi.org/10.3390/molecules20022576








