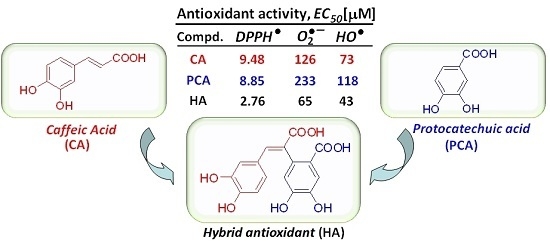
Synthesis and Antioxidant Activity of Polyhydroxylated trans-Restricted 2-Arylcinnamic Acids
Abstract
:1. Introduction
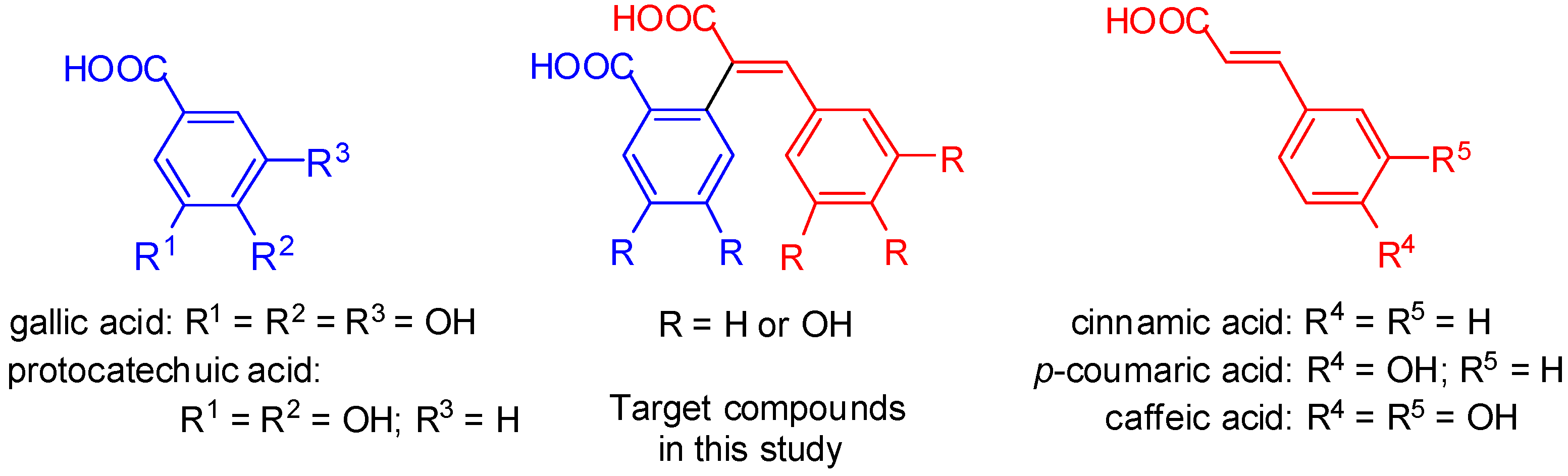
2. Results and Discussion
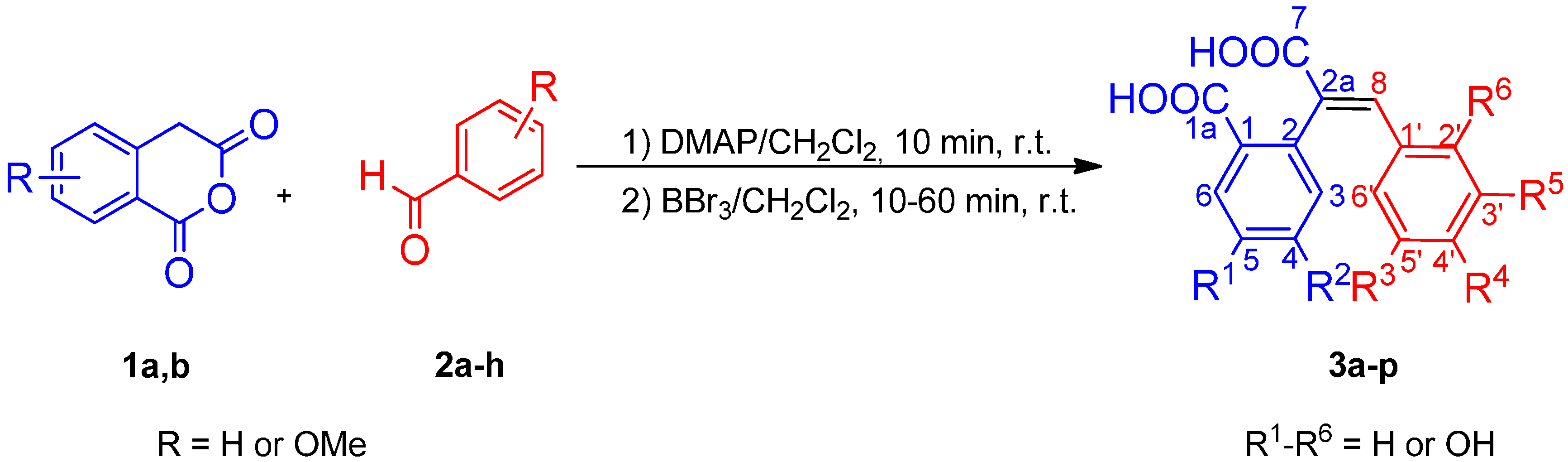
2.1. Chemistry
| Comp. | R1 | R2 | R3 | R4 | R5 | R6 | Total OH | Type of Phenolic Fragments in Rings A/B | Antioxidant Activity a | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH● | O2●▬ (µM) | HO● (µM) | ||||||||||
| EC50 (µM) | TEC50 b (min) | |||||||||||
| 3a | H | H | H | H | H | H | 0 | –/– | na c | nd c | na | 201.0 ± 8.0 |
| 3b | H | H | H | H | H | OH | 1 | –/phenol | na | nd | na | 133.7 ± 16.1 |
| 3c | H | H | H | OH | H | H | 1 | –/phenol | na | nd | na | 134.2 ± 5.6 |
| 3d | H | H | H | OH | H | OH | 2 | –/resorcinol | 41.30 ± 0.35 | 32.25 ± 2 | na | 120.9 ± 9.8 |
| 3e | H | H | OH | H | H | OH | 2 | –/hydroQ c | 6.74 ± 0.30 | 3.5 ± 0.1 | na | 122.9 ± 2.0 |
| 3f | H | H | H | H | OH | OH | 2 | –/catechol | 6.26 ± 0.58 | 4 ± 0.1 | 372.9 ± 8.5 | 108.9 ± 14.0 |
| 3g | H | H | H | OH | OH | H | 2 | –/catechol | 5.81 ± 0.29 | 2.75 ± 0.1 | 109.7 ± 5.3 | 59.4 ± 0.4 |
| 3h | H | H | OH | OH | OH | H | 3 | –/pyrogallol | 4.47 ± 0.07 | 3 ± 0.1 | 11.1 ± 0.1 | 100.6 ± 2.1 |
| 3i | OH | OH | H | H | H | H | 2 | catechol/– | 7.62 ± 0.28 | 31.75 ± 1.5 | 233.6 ± 0.9 | 118.7 ± 2.6 |
| 3j | OH | OH | H | H | H | OH | 3 | catechol/phenol | 8.04 ± 0.32 | 26 ± 0.75 | 238.0 ± 5.4 | 79.1 ± 0.6 |
| 3k | OH | OH | H | OH | H | H | 3 | catechol/phenol | 7.34 ± 0.25 | 33 ± 2 | 230.7 ± 4.9 | 64.5 ± 0.8 |
| 3l | OH | OH | H | OH | H | OH | 4 | catechol/resorcinol | 5.43 ± 0.36 | 26.5 ± 0.75 | 269.2 ± 12.8 | 68.9 ± 0.8 |
| 3m | OH | OH | OH | H | H | OH | 4 | catechol/hydroQ c | 3.33 ± 0.04 | 10 ± 0.5 | nd | 58.8 ± 1.1 |
| 3n | OH | OH | H | H | OH | OH | 4 | catechol/catechol | 3.52 ± 0.05 | 13 ± 0.5 | 137.2 ± 3.9 | 84.6 ± 2.3 |
| 3o | OH | OH | H | OH | OH | H | 4 | catechol/catechol | 2.76 ± 0.12 | 6.5 ± 0.25 | 64.9 ± 1.9 | 42.6 ± 1.8 |
| 3p | OH | OH | OH | OH | OH | H | 5 | catechol/pyrogallol | 2.09 ± 0.11 | 9 ± 0.75 | 11.3 ± 0.5 | 58.6 ± 0.9 |
| Trolox | – | – | – | – | – | – | 1 | phenol | 9.34 ± 0.07 | 5.75 ± 0.25 | na | 109.6 ± 7.8 |
| CA | – | – | H | OH | OH | H | 2 | catechol | 9.48 ± 0.17 | 9 ± 0.75 | 126 ± 10.6 | 73.0 ± 1.8 |
| PCA | OH | OH | – | – | – | – | 2 | catechol | 8.85 ± 0.24 | 28.25 ± 1.25 | 233.5 ± 3.0 | 117.7 ± 1.8 |
| GA | – | – | – | – | – | – | 3 | pyrogallol | 5.32 ± 0.34 | 10 ± 0.5 | 29.1 ± 1.0 | 146.9 ± 4.0 |
| CA:PCA | – | – | – | – | – | – | 2 | catechol + catechol | 13.28 ± 0.54 | 10 ± 0.5 | 143.9 ± 5.7 | 135.7 ± 13.1 |
2.2. In Vitro Antioxidant Capacity Assays
2.2.1. DPPH● Radical Scavenging Assay
2.2.2. O2●▬ Radical Anion Scavenging Activity
2.2.3. HO● Radical Scavenging Activity
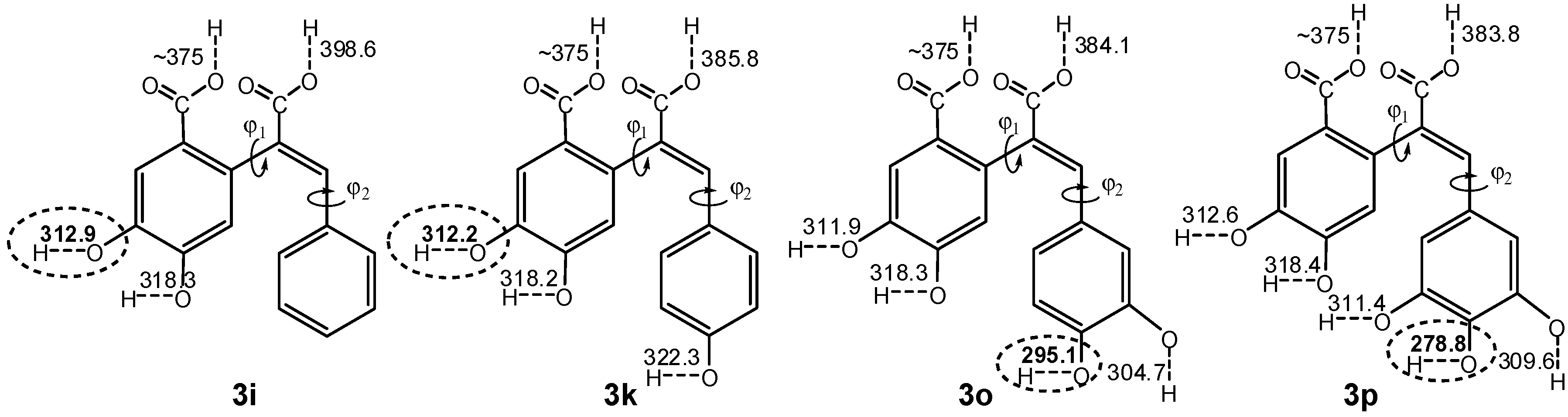
2.3. Quantum Chemistry Computations
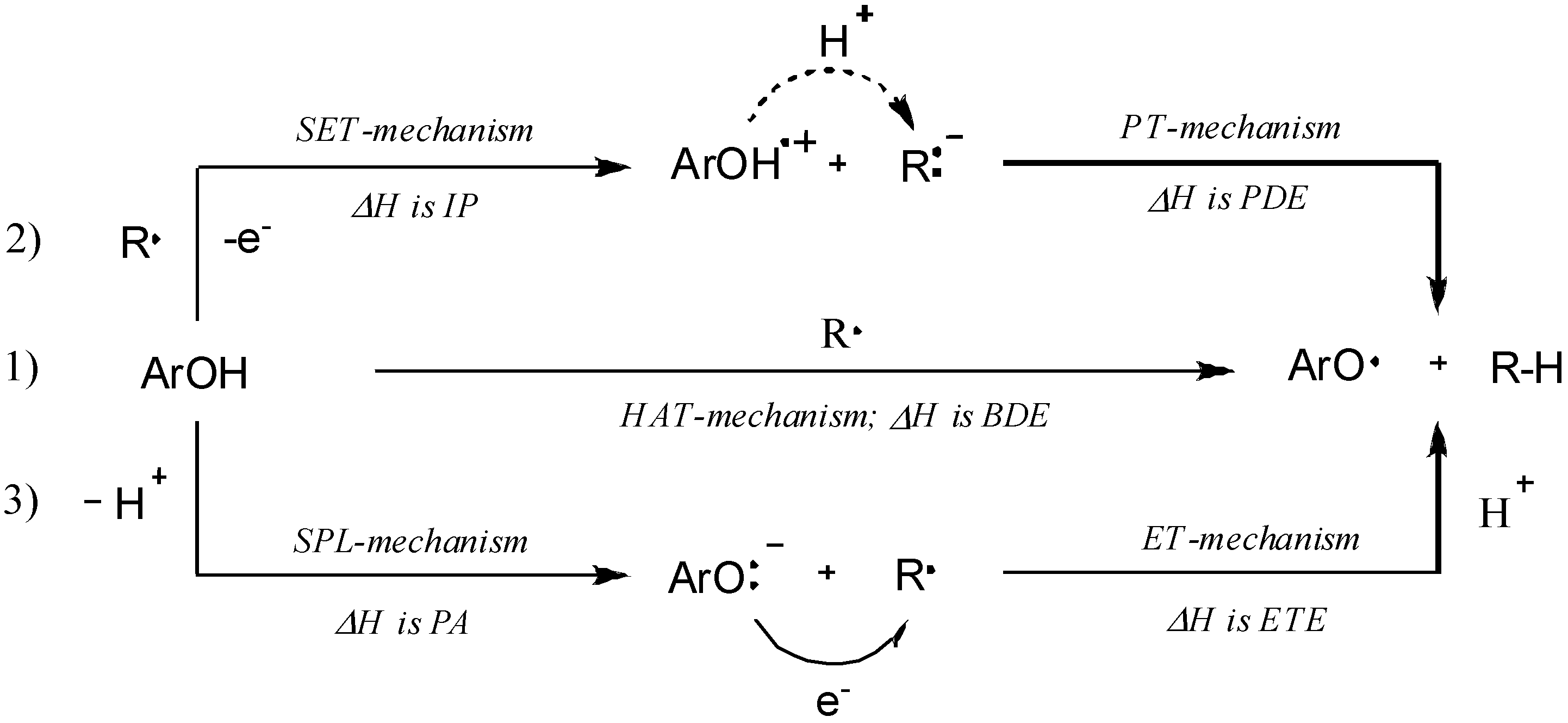
| Compound | BDE a | IP b | PDE c | PA d | ETE e |
|---|---|---|---|---|---|
| 3i | 324.4 | 826.2 | 169.0 | 759.2 | 684.8 |
| 3k | 323.7 | 803.8 | 192.5 | 760.3 | 683.1 |
| 3o | 306.4 | 796.1 | 189.2 | 749.3 | 676.6 |
| 3p | 290.2 | 795.2 | 118.3 | 677.4 | 732.3 |
| Compound | Mulliken Spin Density | Natural Spin Density | ||||
|---|---|---|---|---|---|---|
| Radical Centre | Protocatechuic Fragment | Cinnamic Fragment | Radical Centre | Protocatechuic Fragment | Cinnamic Fragment | |
| 3i | 0.359 | 0.602 | 0.039 | 0.284 | 0.609 | 0.107 |
| 3k | 0.337 | 0.608 | 0.055 | 0.269 | 0.666 | 0.065 |
| 3o | 0.268 | 0.011 | 0.721 | 0.198 | 0.020 | 0.782 |
| 3p | 0.287 | 0.038 | 0.675 | 0.218 | 0.021 | 0.761 |
3. Experimental Section
3.1. General Remarks
3.2. Chemistry
3.2.1. General Procedure for One-Pot Synthesis of Polyhydroxy (E)-2-(1-Carboxy-2-phenylvinyl)-benzoic Acids 3a–p
(E)-2-(1-Carboxy-2-(2-hydroxyphenyl)vinyl)benzoic Acid (3b)
(E)-2-(1-Carboxy-2-(4-hydroxyphenyl)vinyl)benzoic Acid (3c)
(E)-2-(1-Carboxy-2-(2,4-dihydroxyphenyl)vinyl)benzoic Acid (3d)
(E)-2-(1-Carboxy-2-(2,5-dihydroxyphenyl)vinyl)benzoic Acid (3e)
(E)-2-(1-Carboxy-2-(2,3-dihydroxyphenyl)vinyl)benzoic Acid (3f)
(E)-2-(1-Carboxy-2-(3,4-dihydroxyphenyl)vinyl)benzoic Acid (3g)
(E)-2-(1-Carboxy-2-(3,4,5-trihydroxyphenyl)vinyl)benzoic Acid (3h)
(E)-2-(1-Carboxy-2-(2-hydroxyphenyl)vinyl)-4,5-dihydroxybenzoic Acid (3j)
(E)-2-(1-Carboxy-2-(2,4-dihydroxyphenyl)vinyl)-4,5-dihydroxybenzoic Acid (3l)
(E)-2-(1-Carboxy-2-(2,5-dihydroxyphenyl)vinyl)-4,5-dihydroxybenzoic Acid (3m)
(E)-2-(1-Carboxy-2-(2,3-dihydroxyphenyl)vinyl)-4,5-dihydroxybenzoic Acid (3n)
3.2.2. General Procedure for Derivatization of Polyhydroxy (E)-2-(1-Carboxy-2-phenylvinyl)benzoic Acids 3a–p
3.3. In Vitro Antioxidant Capacity Assays
3.3.1. 1,1-Diphenyl-2-picrylhydrazyl Radical Scavenging Assay (DPPH●)
3.3.2. Superoxide Anion Radical Scavenging Assay (O2●▬)
3.3.3. Hydroxyl Radical Scavenging Assay (HO●)
3.4. Statistical Analysis
3.5. Theoretical Approach
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brookes, P.S.; Levonen, A.-L.; Shiva, S.; Sarti, P.; Derley-Usmar, V.M. Introduction to serial reviews: Reactive nitrogen species, tyrosine nitration and cell signaling. Free Radic. Biol. Med. 2002, 33, 755–764. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Yang, Y.; Ming, M.; Liu, B. Mitochondrial ROS generation for regulation of autophagic pathways in cancer. Biochem. Biophys. Res. Commun. 2011, 41, 45–48. [Google Scholar]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Adly, A.A.M. Oxidative stress and disease: An updated review. Res. J. Immunol. 2010, 3, 129–145. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Bohn, T. Exogenous antioxidants – double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Berger, R.G.; Lunkenbein, S.; Ströhle, A.; Hahn, A. Antioxidants in food: Mere myth or magic medicine? Crit. Rev. Food Sci. Nutr. 2012, 52, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Smith, A.; Lin, X.; Yuan, F.; Copes, N.; Delic, V.; Tan, J.; Cao, C.; Shytle, R.D.; Esposito, E.; et al. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol. Aging 2002, 23, 719–735. [Google Scholar] [CrossRef]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [PubMed]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M.P. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef] [PubMed]
- Kamat, C.D.; Gadal, S.; Mhatre, M.; Williamson, K.S.; Pye, Q.N.; Hensley, K. Antioxidants in central nervous system diseases: Preclinical promise and translational challenges. J. Alzheimers Dis. 2008, 15, 473–493. [Google Scholar] [PubMed]
- Zhao, B. Natural antioxidants protect neurons in Alzheimer’s disease and Parkinson’s disease. Neurochem. Res. 2009, 34, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Fresco, P.; Borges, F.; Marques, M.P.; Diniz, C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 2010, 16, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.J.; Lee, K.W. Naturally occurring phytochemicals for the prevention of Alzheimer's disease. J. Neurochem. 2010, 112, 1415–1430. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, P.C. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer’s amyloid-induced mitochondrial dysfunction. J. Alzheimers Dis. 2011, 26, 507–521. [Google Scholar] [PubMed]
- Benfeito, S.; Oliveira, C.; Soares, P.; Fernandes, C.; Silva, T.; Teixeira, J.; Borges, F. Antioxidant therapy: Still in search of the “magic bullet”. Mitochondrion 2013, 13, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Patents 2014, 24, 1257–1270. [Google Scholar] [CrossRef]
- Siquet, C.; Paiva-Martins, F.; Lima, J.L.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy- and trihydroxyphenolic acids—A structure-activity relationship study. Free Radic. Res. 2006, 40, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Garrido, E.M.; Esteves, M.; Quezada, E.; Milhazes, N.; Garrido, J.; Borges, F. New insights into the antioxidant activity of hydroxycinnamic acids: Synthesis and physicochemical characterization of novel halogenated derivatives. Eur. J. Med. Chem. 2009, 44, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Martins, M.; Silva, P.; Garrido, E.M.; Garrido, J.; Firuzi, O.; Miri, R.; Saso, L.; Borges, F. Dietary phenolic acids and derivatives. Evaluation of the antioxidant activity of sinapic acid and its alkyl esters. J. Agric. Food Chem. 2010, 58, 11273–11280. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.; Martins, M.; Barreto, B.; Milhazes, N.; Garrido, E.M.; Silva, P.; Garrido, J.; Borges, F. Structure-property-activity relationship of phenolic acids and derivatives. Protocatechuic acid alkyl esters. J. Agric. Food Chem. 2010, 58, 6986–6993. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.; Siquet, C.; Orru, E.; Garrido, E.M.; Garrido, J.; Milhazes, N.; Podda, G.; Paiva-Martins, F.; Reis, S.; Carvalho, R.A.; et al. Lipophilic phenolic antioxidants: Correlation between antioxidant profile, partition coefficients and redox properties. Bioorg. Med. Chem. 2010, 18, 5816–5825. [Google Scholar] [CrossRef]
- Serafim, T.L.; Carvalho, F.S.; Marques, M.P.; Calheiros, R.; Silva, T.; Garrido, J.; Milhazes, N.; Borges, F.; Roleira, F.; Silva, E.T.; et al. Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chem. Res. Toxicol. 2011, 24, 763–774. [Google Scholar] [CrossRef]
- Menezes, J.C.; Kamat, S.P.; Cavaleiro, J.A.; Gaspar, A.; Garrido, J.; Borges, F. Synthesis and antioxidant activity of long chain alkyl hydroxycinnamates. Eur. J. Med. Chem. 2011, 46, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Silva, T.; Benfeito, S.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Exploring nature profits: Development of novel and potent lipophilic antioxidants based on galloyl-cinnamic hybrids. Eur. J. Med. Chem. 2013, 62, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Miliovsky, M.; Svinyarov, I.; Mitrev, Y.; Evstatieva, Y.; Nikolova, D.; Chochkova, M.; Bogdanov, M.G. A novel one-pot synthesis and preliminary biological activity evaluation of cis-restricted polyhydroxy stilbenes incorporating protocatechuic acid and cinnamic acid fragments. Eur. J. Med. Chem. 2013, 66, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.G.; Palamareva, M. cis/trans-Isochromanones. DMAP induced cycloaddition of homophthalic anhydride and aldehydes. Tetrahedron 2004, 60, 2525–2530. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Mitrev, Y.; Tiritiris, I. New highly diastereoselective Perkin/Michael addition domino reaction between homophthalic anhydride and aromatic aldehydes: A facile approach to blue-fluorescent dibenzo[c,h]chromenones. Eur. J. Org. Chem. 2011, 377, 377–384. [Google Scholar] [CrossRef]
- Svinyarov, I.; Bogdanov, M.G. One-pot synthesis and radical scavenging activity of novel polyhydroxylated 3-arylcoumarins. Eur. J. Med. Chem. 2014, 78, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Vogeli, U.; von Philipsborn, W. Vicinal C,H spin coupling in substituted alkenes. Stereochemical significance and structural effects. Org. Magn. Reson. 1975, 7, 617–627. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Villaño, D.; Garcıa-Parrilla, M.C.; Troncoso, A.M. Antioxidant activity of wines and relation with their polyphenolic composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Saito, S.; Kawabata, J. Effects of electron-withdrawing substituents on DPPH radical scavenging reactions of protocatechuic acid and its analogues in alcoholic solvents. Tetrahedron 2005, 61, 8101–8108. [Google Scholar] [CrossRef]
- Villano, D.; Fernandez-Pachon, M.S.; Moya, M.L.; Troncoso, A.M.; Garcıa-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Lean-Teik, N.; Horng-Huey, K.; Tzy-Ming, L. Potential antioxidants and tyrosinase inhibitors from synthetic polyphenolic deoxybenzoins. Bioorg. Med. Chem. 2009, 17, 4360–4366. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.; Yamaki, K.; Masumizu, T.; Nakai, Y.; Saito, K.; Sano, H.; Tamura, Y. Superoxide anion radical scavenging activities of herbs and pastures in northern Japan determined using electron spin resonance spectrometry. Int. J. Biol. Sci. 2007, 3, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, М.; Rao, N.A.; Yagi, К. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A. A convenient mechanism for the free radical scavenging activity of resveratrol. Int. J. Phytomed. 2011, 3, 459–469. [Google Scholar]
- Baxendale, J.H.; Khan, A.A. The pulse radiolysis оf p-nitrosodimethylaniline in aqueous solution. Int. J. Radiat. Phys. Chem. 1969, 1, 11–24. [Google Scholar] [CrossRef]
- Evans, C.; Scaiano, J.C.; Ingold, K.U. Absolute kinetics of hydrogen abstraction from .alpha.-tocopherol by several reactive species including an alkyl radical. J. Am. Chem. Soc. 1992, 114, 4589–4593. [Google Scholar] [CrossRef]
- Mukai, K.; Uemoto, Y.; Fukuhara, M.; Nagoaka, S.; Ishizu, K. ENDOR study of the cation radicals of vitamin E derivatives. Relation between antioxidant activity and molecular structure. Bull. Chem. Soc. Jpn. 1992, 65, 2016–2020. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Sun, Y.-M.; Wang, X.-L. Substituent effects on O–H bond dissociation enthalpies and ionization potentials of catechols: A DFT study and its implications in the rational design of phenolic antioxidants and elucidation of structure-activity relationships for flavonoid antioxidants. Chem. Eur. J. 2003, 9, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Vafiadis, A.P.; Bakalbassis, E.G. A DFT study on the deprotonation antioxidant mechanistic step of ortho-substituted phenolic cation radicals. Chem. Phys. 2005, 316, 195–204. [Google Scholar] [CrossRef]
- Litwinenko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstraction. 3. Novel kinetics in sequential proton loss electron transfer chemistry. J. Org. Chem. 2005, 70, 8982–8990. [Google Scholar] [CrossRef] [PubMed]
- Musialik, M.; Kuzmicz, R.; Pawlowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Fifen, J.J.; Nsangou, M.; Dhaouadi, Z.; Motapon, O.; Jaidane, N. Solvent effects on the antioxidant activity of 3,4-dihydroxyphenylpyruvic acid: DFT and TD-DFT studies. Comp. Theor. Chem. 2011, 966, 232–243. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Santacruz-Ortega, H.; Balandran-Quintana, R.R. Relationship between structure, properties, and the radical scavenging activity of morin. J. Mol. Struct. 2011, 995, 134–141. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Suzumura, K.; Yasuhara, M.; Narita, H. Superoxide anion scavenging properties of fluvastatin and its metabolites. Chem. Pharm. Bull. 1999, 47, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Kunchandy, E.; Rao, M.N.A. Effect of curcumin on hydroxyl radical generation through Fenton reaction. Int. J. Pharm. 1989, 57, 173–176. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Stephens, P.J.; Delvin, F.J.; Chabalowski, F.J.; Frisch, C.F. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Tomasi, J.; Perisco, M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Klein, E.; Lukes, V.; Ilcin, M. DFT/B3LYP study of tocopherols and chromans antioxidant action energetic. Chem. Phys. 2007, 336, 51–57. [Google Scholar] [CrossRef]
- Rice-Evens, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationship of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1a,b and 3a–p are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miliovsky, M.; Svinyarov, I.; Prokopova, E.; Batovska, D.; Stoyanov, S.; Bogdanov, M.G. Synthesis and Antioxidant Activity of Polyhydroxylated trans-Restricted 2-Arylcinnamic Acids. Molecules 2015, 20, 2555-2575. https://doi.org/10.3390/molecules20022555
Miliovsky M, Svinyarov I, Prokopova E, Batovska D, Stoyanov S, Bogdanov MG. Synthesis and Antioxidant Activity of Polyhydroxylated trans-Restricted 2-Arylcinnamic Acids. Molecules. 2015; 20(2):2555-2575. https://doi.org/10.3390/molecules20022555
Chicago/Turabian StyleMiliovsky, Mitko, Ivan Svinyarov, Elena Prokopova, Daniela Batovska, Simeon Stoyanov, and Milen G. Bogdanov. 2015. "Synthesis and Antioxidant Activity of Polyhydroxylated trans-Restricted 2-Arylcinnamic Acids" Molecules 20, no. 2: 2555-2575. https://doi.org/10.3390/molecules20022555
APA StyleMiliovsky, M., Svinyarov, I., Prokopova, E., Batovska, D., Stoyanov, S., & Bogdanov, M. G. (2015). Synthesis and Antioxidant Activity of Polyhydroxylated trans-Restricted 2-Arylcinnamic Acids. Molecules, 20(2), 2555-2575. https://doi.org/10.3390/molecules20022555