Distinctive Anthocyanin Accumulation Responses to Temperature and Natural UV Radiation of Two Field-Grown Vitis vinifera L. Cultivars
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Season Thermal Conditions
| Variable | Period | June | July | August | September | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | Δ | x | Δ | x | Δ | x | Δ | ||
| Tmax (°C) | 2009 | 27.5 | 3.0 | 31.1 | 3.3 | 30.3 | 1.5 | 26.9 | 0.9 |
| 2010 | 24.6 | 0.1 | 29.3 | 1.5 | 28.1 | −0.7 | 25.7 | −0.3 | |
| 2011 | 24.9 | 0.4 | 27.6 | −0.2 | 29.7 | 0.9 | 26.8 | 0.8 | |
| 30 year | 24.5 | 27.8 | 28.8 | 26.0 | |||||
| Tmed (°C) | 2009 | 24.5 | 3.6 | 27.5 | 3.5 | 27.3 | 2.4 | 23.5 | 1.2 |
| 2010 | 22.1 | 1.2 | 26.4 | 2.4 | 25.3 | 0.4 | 22.6 | 0.3 | |
| 2011 | 22.2 | 1.3 | 24.4 | 0.4 | 25.7 | 0.8 | 23.7 | 1.4 | |
| 30 year | 20.9 | 24.0 | 24.9 | 22.3 | |||||
| Tmin (°C) | 2009 | 19.9 | 2.6 | 22.5 | 2.4 | 22.6 | 1.5 | 19.1 | 0.4 |
| 2010 | 17.9 | 0.6 | 22.3 | 2.2 | 20.7 | −0.4 | 18.1 | −0.6 | |
| 2011 | 18.2 | 0.9 | 20.5 | 0.4 | 20.5 | −0.6 | 19.0 | 0.3 | |
| 30 year | 17.3 | 20.1 | 21.1 | 18.7 | |||||
2.2. Light Microclimate into the Fruit Zone
| PAR Attenuation (% Reference PAR) | UV-A (W·m−2) | UV-B (mW·m−2) | |||||
|---|---|---|---|---|---|---|---|
| Distance from the Canopy Centre (cm) | 0–10 | 10–20 | East | West | East | West | |
| Bovale Grande | Control | 90 ± 7.4 | 51 ± 9.6 | 3.0 ± 0.70 | 2.7 ± 0.55 | 143.7 ± 59.2 | 150.0 ± 56.5 |
| Vis + UV-A | 92 ± 2.1 | 84 ± 2.4 | 0.8 ± 0.04 | 0.4 ± 0.04 | 13.1 ± 4.6 | 1.0 ± 0.1 | |
| Vis | 94 ± 3.5 | 81 ± 4.1 | 1.0 ± 0.10 | 0.3 ± 0.11 | 7.2 ± 2.3 | 3.0 ± 2.3 | |
| Cannonau | Control | 90 ± 6.0 | 67 ± 12.3 | 1.5 ± 0.52 | 1.9 ± 0.61 | 130.0 ± 44.9 | 102.3 ± 53.8 |
| Vis + UV-A | 98 ± 0.7 | 88 ± 2.1 | 0.6 ± 0.18 | 0.8 ± 0.13 | 5.2 ± 2.1 | 12.2 ± 8.5 | |
| Vis | 96 ± 1.2 | 88 ± 2.4 | 0.6 ± 0.17 | 0.7 ± 0.11 | 1.7 ± 0.8 | 4.5 ± 1.8 | |
2.3. Berry Skin Temperature
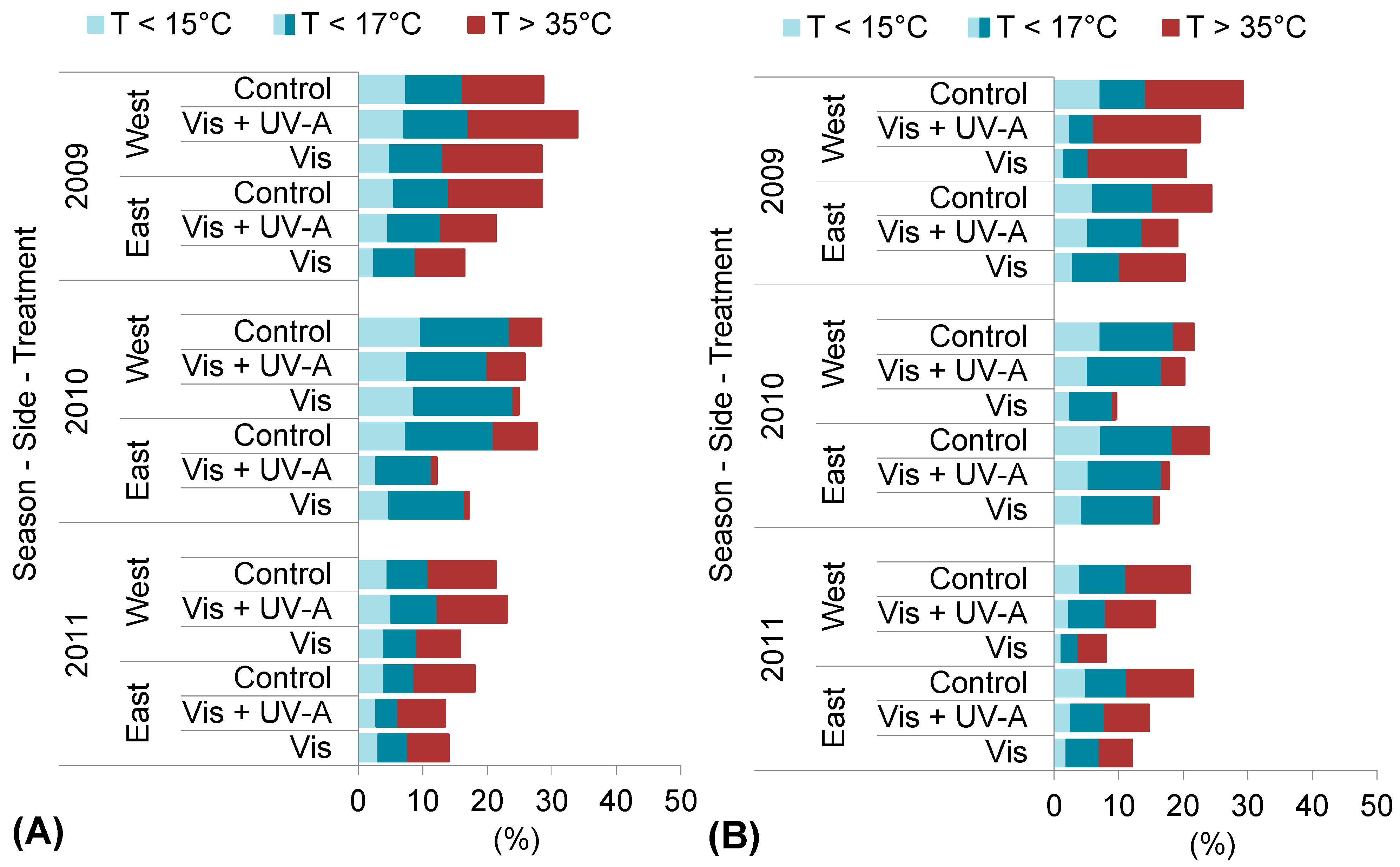
2.4. Berry Skin Anthocyanins
| Bovale Grande | Cannonau | ||||||
|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2009 | 2010 | 2011 | ||
| Treatment | Control | 306.9 | 585.8 a | 654.1 | 119.5 a | 127.0 | 102.1 |
| Vis + UV-A | 298.8 | 450.3 b | 638.4 | 80.9 a,b | 182.1 | 143.2 | |
| Vis | 258.3 | 496.5 b | 650.2 | 55.5 b | 146.4 | 108.6 | |
| Sig. | ns | < 0.05 | ns | <0.05 | ns | ns | |
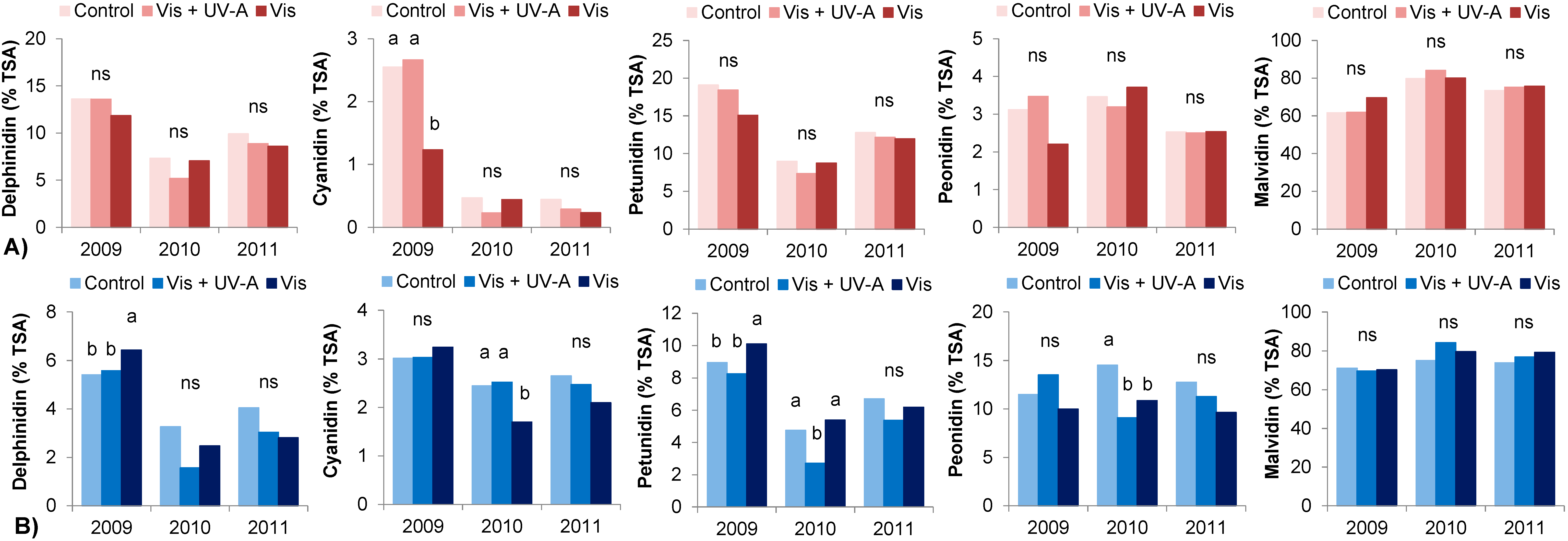

| Bovale Grande | Cannonau | ||||||
|---|---|---|---|---|---|---|---|
| Control | Vis + UV-A | Vis | Control | Vis + UV-A | Vis | ||
| Monoglucosides | 2009 | 56.0 b | 58.2 b | 52.5 b | 77.0 | 76.4 | 63.1 b |
| 2010 | 78.0 a | 77.8 a | 75.5 a | 76.0 | 76.0 | 87.3 a | |
| 2011 | 63.4 b | 65.2 b | 60.2 ab | 73.3 | 78.4 | 80.2 a | |
| Sig. | <0.05 | <0.05 | <0.05 | ns | ns | <0.05 | |
| Acetylglucosides | 2009 | 12.1 a | 10.2 a | 7.5 b | 10.1 a | 9.4 | 14.0 a |
| 2010 | 6.8 b | 6.1 b | 6.5 b | 4.2 b | 4.2 | 3.4 b | |
| 2011 | 10.2 a | 9.4 a | 9.3 a | 6.1 c | 5.9 | 5.1 b | |
| Sig. | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | |
| Coumaroylglucosides | 2009 | 32.0 | 31.6 a | 40.0 a | 12.9 b | 14.2 | 22.9 a |
| 2010 | 15.3 | 16.0 b | 18.1 b | 19.8 a | 19.8 | 9.3 c | |
| 2011 | 25.6 | 24.5 ab | 29.4 ab | 20.6 a | 15.7 | 14.8 b | |
| Sig. | <0.05 | <0.05 | <0.05 | <0.05 | ns | 0.05 | |
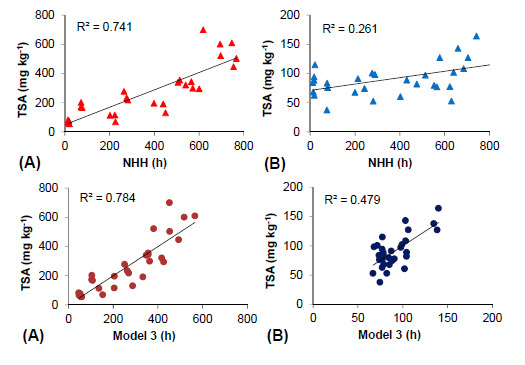
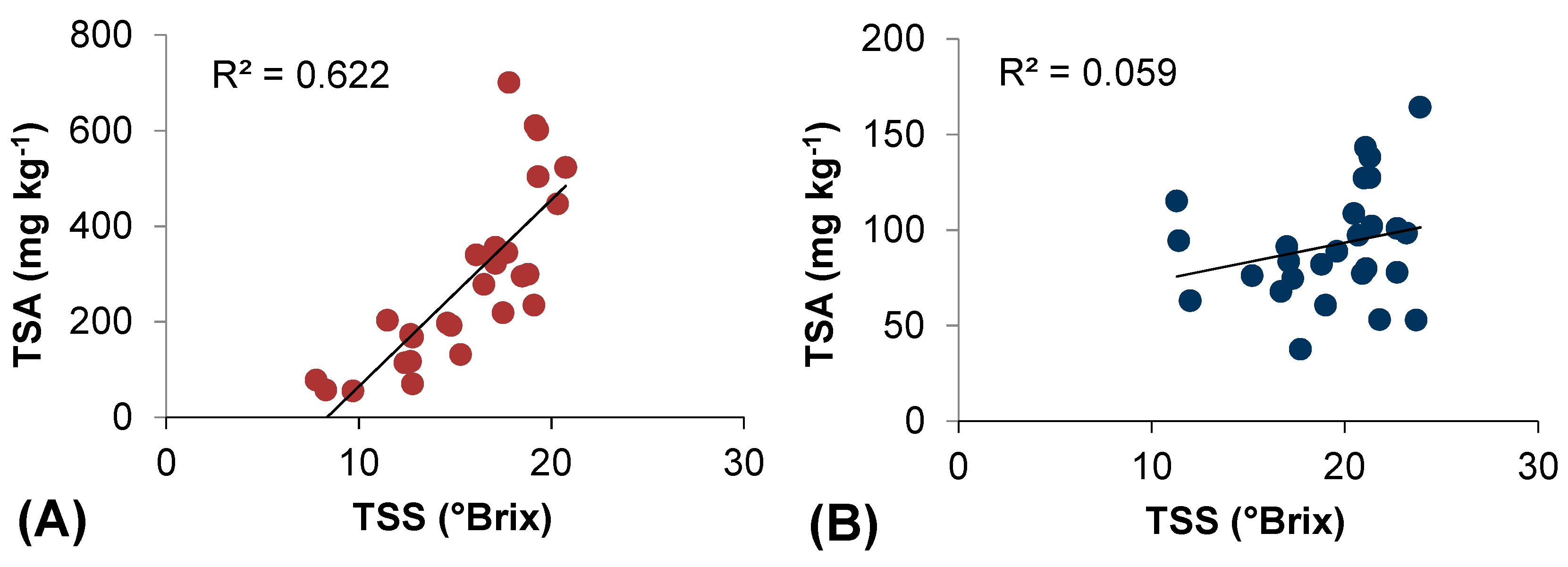
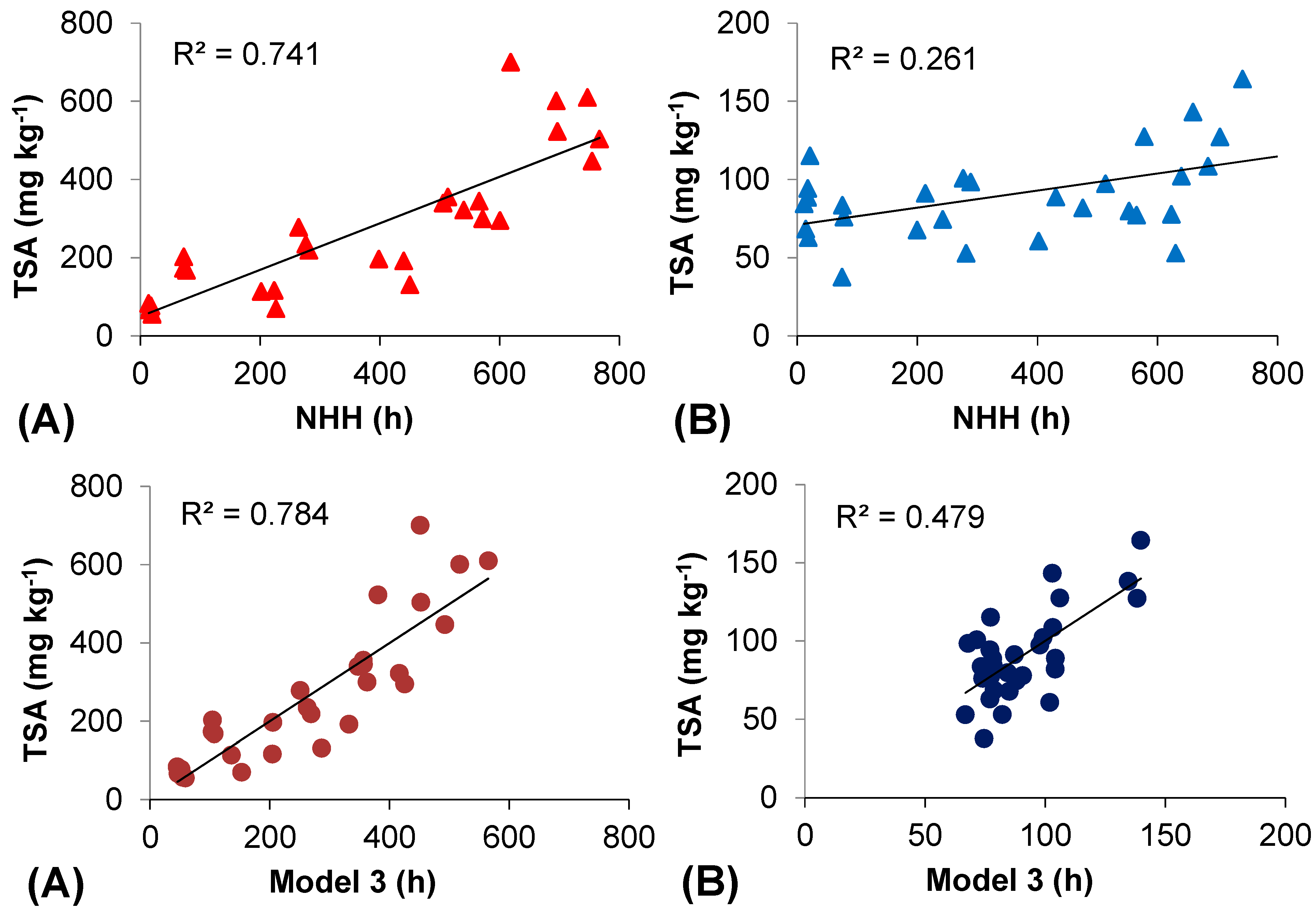
2.5. Thermal Efficiency for Berry Skin Anthocyanin Accumulation
| Model | Predictors | Descriptive Statistics | Model Performance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | df1 | df2 | Regression Sig. | R | R2 | Adj. R2 | Unstandardized Coefficients | Variables Significance | Collinearity Statistics | |||||
| β | Std. Error | T | Sig. | Tolerance | VIF | |||||||||
| 1 | Intercept | 30 | 1 | 28 | 0.0001 | 0.861 | 0.741 | 0.732 | 50.348 | 29.272 | 1.72 | 0.096 | ||
| NHH | 0.594 | 0.066 | 8.845 | 0.000 | 1 | 1 | ||||||||
| 2 | Intercept | 30 | 1 | 27 | 0.0001 | 0.865 | 0.748 | 0.730 | 44.92 | 29.988 | 1.498 | 0.146 | ||
| NHH | 0.552 | 0.081 | 6.79 | 0.000 | 0.671 | 1.489 | ||||||||
| HT > 35 °C | 0.41 | 0.456 | 0.899 | 0.377 | 0.671 | 1.489 | ||||||||
| 3 | Intercept | 30 | 1 | 26 | 0.0001 | 43.206 | 29.741 | 1.524 | 0.140 | |||||
| NHH | 0.808 | 0.153 | 5.538 | 0.000 | 0.187 | 5.359 | ||||||||
| HT > 35 °C | 0.108 | 0.478 | 0.238 | 0.814 | 0.602 | 1.661 | ||||||||
| HT < 17 °C (a) | 0.887 | 0.784 | 0.759 | −1.084 | 0.527 | −2.059 | 0.050 | 0.244 | 4.106 | |||||
| HT < 15 °C (b) | 0.873 | 0.762 | 0.735 | −1.661 | 1.334 | −1.245 | 0.224 | 0.288 | 3.473 | |||||
| Model | Predictors | Descriptive Statistics | Model Performance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | df1 | df2 | Regression Sig. | R | R2 | Adj. R2 | Unstandardized Coefficients | Variables Significance | Collinearity Statistics | |||||
| β | Std. Error | T | Sig. | Tolerance | VIF | |||||||||
| 1 | Intercept | 30 | 1 | 28 | 0.004 | 0.511 | 0.261 | 0.235 | 71.013 | 7.776 | 9.133 | 0.000 | ||
| NHH | 0.055 | 0.017 | 3.145 | 0.004 | 1 | 1 | ||||||||
| 2 | Intercept | 30 | 1 | 27 | 0.001 | 0.649 | 0.421 | 0.378 | 75.066 | 7.168 | 10.473 | 0.000 | ||
| NHH | 0.083 | 0.019 | 4.412 | 0.000 | 0.698 | 1.432 | ||||||||
| HT > 35 °C | −0.271 | 0.099 | −2.727 | 0.011 | 0.698 | 1.432 | ||||||||
| 3 | Intercept | 30 | 1 | 26 | 0.001 | 76.575 | 6.983 | 10.966 | 0.000 | |||||
| NHH | 0.035 | 0.033 | 1.052 | 0.302 | 0.207 | 4.840 | ||||||||
| HT > 35 °C | −0.209 | 0.103 | −2.036 | 0.052 | 0.611 | 1.637 | ||||||||
| HT < 17 °C (a) | 0.692 | 0.479 | 0.419 | 0.235 | 0.138 | 1.706 | 0.100 | 0.271 | 3.695 | |||||
| HT < 15 °C (b) | 0.681 | 0.463 | 0.401 | 0.42 | 0.292 | 1.44 | 0.162 | 0.434 | 2.306 | |||||
3. Experimental Section
3.1. Plant Material and Experimental Site
3.2. Light Microclimate Conditions
3.3. Season Thermal Conditions
3.4. Berry Temperature Monitoring and Determinations
3.5. Berry Composition Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zepp, R.G.; Erickson, D.J., III; Paul, N.D.; Sulzberger, B. Interactive effects of solar UV radiation and climate change on biogeochemical cycling. In The Environmental Effects Assessment Panel Report; United Nations Environment Programme: Nairobi, Kenya, 2006; pp. 135–162. [Google Scholar]
- United Nations Environment Programme (UNEP). Environmental Effects of Ozone Deplection: 2010 Assessment; UNEP: Nairobi, Kenya, 2010; p. 328. [Google Scholar]
- Zepp, R.G.; Erickson, D.J., III; Paul, N.D.; Sulzberger, B. Interactive effects of solar UV radiation and climate change on biogeochemical cycling. Photochem. Photobiol. Sci. 2007, 6, 286–300. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Bornman, J.F.; Ballaré, C.L.; Flint, S.D.; Kulandaivelu, G. Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochem. Photobiol. Sci. 2007, 3, 252–266. [Google Scholar] [CrossRef]
- Sullivan, J.H.; Teramura, A.H. Field study of the interaction between solar ultraviolet-B radiation and drought on photosynthesis and growth in soybean. Plat Physiol. 1990, 2, 141–146. [Google Scholar] [CrossRef]
- Steel, C.C.; Keller, M. Influence of UV-B irradiation on the carotenoid content of Vitis vinifera tissues. Biochem. Soc. Trans. 2000, 28, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Kolb, C.A.; Käser, M.A.; Kopecký, J.; Zotz, G.; Riederer, M.; Pfündel, E.E. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Julkunen-Tiitto, R.; Häggman, H.; Aphalo, P.J.; Lavola, A.; Tegelberg, R.; Veteli, T. Growth and defense in deciduous trees and shrubs under UV-B. Environ. Pollut. 2005, 137, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Ruhland, C.T.; Fogal, M.J.; Buyarski, C.R.; Krna, M.A. Solae ultraviolet-B radiation increases phenolic contents and ferric reducting antioxidant power in Avena sativa. Molecules 2007, 12, 1220–1232. [Google Scholar] [CrossRef] [PubMed]
- Doupis, G.; Chartzoulakis, K.; Beis, A.; Patakas, A. Allometric and biochemical responses of grapevines subjected to drought and enhanced ultraviolet-B radiation. Aust. J. Grape Wine Res. 2011, 17, 36–42. [Google Scholar] [CrossRef]
- Gregan, S.M.; Wargent, J.J.; Liu, L.; Shinkle, J.; Hofmann, R.; Winefield, C.; Trought, M.; Jordan, B. Effects of solar ultraviolet radiation and canopy manipulation on the biochemical composition of Sauvignon Blanc grapes. Aust. J. Grape Wine Res. 2012, 18, 227–238. [Google Scholar] [CrossRef]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA are involved in phenol metabolism of Vitis vinifera L. increasing biosynthesis of berry skin polyphenols. J. Agric. Food Chem. 2011, 59, 4874–4884. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; Downey, M.O.; Iland, P.G.; Bindon, K.; Francis, I.L.; Herderich, M.; Robinson, S.P. Exclusion of sunlight from Shiraz grapes alters wine colour, tannin and sensory properties. Aust. J. Grape Wine Res. 2007, 13, 53–65. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry temperature and solar radiation alter acylation proportion, and concentration of anthocyanin in Merlot grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light quality affects flavonoid biosynthesis in young berries of Cabernet Sauvignon grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N-N.; Mu, L.; Pan, Q-H.; Wang, J.; Reeves, M.J.; Duan, C-Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Smart, R.E. Sunlight interception by vineyards. Am. J. Enol. Vitic. 1973, 24, 141–147. [Google Scholar]
- Smart, R.E.; Robinson, M. Sunlight into wine. In A Handbook for Winegrape Canopy Management; Winetitles: Adelaide, Australia, 1991; p. 88. [Google Scholar]
- Poni, S.; Lakso, A.N.; Intrieri, C.; Rebucci, B.; Filippetti, I. Laser scanning estimation of relative light interception by canopy components in different grapevine training systems. Vitis 1996, 35, 177–182. [Google Scholar]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Mabrouk, H.; Sinoquet, H. Indices of light microclimate and canopy structure determined by 3-D digitising and image analysis and their relationship to grape quality. Aust. J. Grape Wine Res. 1998, 4, 2–13. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Gambetta, G.A.; Matthews, M.A.; Kennedy, J.A. Impact of diurnal temperature variation on grape berry development, proanthocyanidin accumulation, and the expression of flavonoid pathway genes. J. Exp. Bot. 2012, 63, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust. J. Grape Wine Res. 2003, 9, 15–27. [Google Scholar] [CrossRef]
- He., F.; Mu, L.; Yan, G-L.; Liang, N-N.; Pan, Q-H.; Wang, J.; Reeves, M.J.; Duan, G-Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the central San Joaquin Valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar]
- Yamane, T.; Jeong, S.T.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of temperature on anthocyanin biosynthesis in grape berry skins. Am. J. Enol. Vitic. 2006, 57, 54–59. [Google Scholar]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Sauvignon. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Fernandes de Oliveira, A.; Nieddu, G. Effects of natural ultraviolet radiation and high temperature on berry microclimate and anthocyanins in two red grape varieties Aust. J. Grape Wine Res. 2014. submitted. [Google Scholar]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. The effect of bunch shading on berry development and flavonoid accumulation in Shiraz grapes. Aust. J. Grape Wine Res. 2004, 10, 55–73. [Google Scholar] [CrossRef]
- Berli, F.J.; Moreno, D.; Piccoli, P.; Hespanhol-Viana, L.; Silva, M.F.; Bressan-Smith, R.; Cavagnaro, J.B.; Bottini, R. Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ. 2010, 33, 1–10. [Google Scholar] [PubMed]
- Martinez- Lüscher, J.; Sánchez-Díaz, M.; Serge Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B radiation and water deficit interact to alter flavonol and anthocyanin profiles in grapevine berries. Plant Cell Physiol. 2014, 55, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lüscher, J.; Torres, N.; Hilbert, G.; Richard, T.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B radiation modifies the quantitative and qualitative profile of flavonoids and amino acids in grape berries through Transcriptomic Regulation. Phytochemistry 2014, 102, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Bejerano, P.; Diago, M.-P.; Martínez-Abaigar, J.; Martínez-Zapater, J.; Tardáguila, J.; Núnez-Olivera, E. 2014 Solar ultraviolet radiation is necessary to enhance grapevine fruit ripening transcriptional and phenolic responses. Plant Biol. 2014, 14, 183. [Google Scholar]
- Matus, J.T.; Loyola, R.; Vega, A.; Peña-Neira, A.; Bordeu, E.; Arce-Johnson, P.; Alcalde, J.A. Post-veraison sunlight exposure induces MYB-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J. Exp. Bot. 2009, 60, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Nieddu, G.; Chessa, I.; Cocco, G.F.; Nieddu, M.; Deidda, P. Caratterizzazione mediante marcatori RAPD dei vitigni tradizionali della Sardegna. Acta Italus Hortus 2006, 13, 275–280. [Google Scholar]
- Vacca, V.; del Caro, A.; Milella, G.G.; Nieddu, G. Preliminary characterisation of Sardinian red grape cultivars (Vitis vinifera L.) according to their phenolic potential. S. Afr. J. Enol. Vitic. 2009, 30, 93–100. [Google Scholar]
- TEMIS—Tropospheric Emission Monitoring Internet Service. UV station data based on satellite data (SCIAMACHY, GOME-2). Available online: http://www.temis.nl/uvradiation/SCIA/stations_uv.html (accessed on 4 August 2014).
- Aeronautica Militare. Atlante Climatico D’Italia 1971–2000; Centro Nazionale di Meteorologia e Climatologia Aeronautica: Rome, Italy, 2009; Volume III. Available online: http://clima.meteoam.it/AtlanteClim2/pdf/Atlas71-00%20-%20Vol%203.pdf (accessed on 16 August 2014).
- Tutiempo Network, S.L. Tutiempo. Net. Available online: http://www.tutiempo.net/clima/Capo_Frasca/165390.htm (accessed on 16 August 2014).
- Fernandes de Oliveira, A.; Mameli, M.G.; de Pau, L.; Satta, D.; Nieddu, G. Deficit irrigation strategies in Vitis vinifera L. cv. Cannonau under Mediterranean climate. Part I—Physiological responses, growth-yield balance and berry composition. S. Afr. J. Enol. Vitic. 2013, 34, 170–183. [Google Scholar]
- Fernandes de Oliveira, A.; Nieddu, G. Deficit irrigation strategies in Vitis vinifera L. cv. Cannonau under Mediterranean climate. Part II—Cluster microclimate and anthocyanin accumulation patterns. S. Afr. J. Enol. Vitic. 2013, 34, 184–195. [Google Scholar]
- Deidda, P.; Nieddu, G.; Pellizzaro, G.; Pia, G. Dynamics of anthocyanins in “Cannonau” grapevine as related to the environmental conditions. Polyphenols 1995, 94, 207–208. [Google Scholar]
- Rustioni, L.; Rossoni, M.; Cola, G.; Mariani, L.; Failla, O. Microclima termico e luminoso e accumulo di antociani in “Nebbiolo”. Quad. Vitic. Enol. 2006, 28, 137–147. [Google Scholar]
- Cola, G.; Mariani, L.; Parisi, S.; Failla, O. Thermal time and grapevine phenology. Acta Italus Hortus 2012, 3, 31–34. [Google Scholar]
- Murada, G.; Zecca, O.; Cola, G.; Mariani, L.; Failla, O. Maturità fenolica del “Nebbiolo” in Valtellina: effetto dell’annata e del sito. Quad. Vitic. Enol. 2006, 28, 125–136. [Google Scholar]
- OIV recueil des méthodes internationales d’analyse des vins et des moûts; Office International de la vigne et du vin: Paris, France, 1990; p. 368.
- Di Stefano, R.; Cravero, M.C. The grape phenolic determination. Riv. Vit. Ital. 1991, 49, 37–45. [Google Scholar]
- Sample Availability: Samples are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes de Oliveira, A.; Mercenaro, L.; Del Caro, A.; Pretti, L.; Nieddu, G. Distinctive Anthocyanin Accumulation Responses to Temperature and Natural UV Radiation of Two Field-Grown Vitis vinifera L. Cultivars. Molecules 2015, 20, 2061-2080. https://doi.org/10.3390/molecules20022061
Fernandes de Oliveira A, Mercenaro L, Del Caro A, Pretti L, Nieddu G. Distinctive Anthocyanin Accumulation Responses to Temperature and Natural UV Radiation of Two Field-Grown Vitis vinifera L. Cultivars. Molecules. 2015; 20(2):2061-2080. https://doi.org/10.3390/molecules20022061
Chicago/Turabian StyleFernandes de Oliveira, Ana, Luca Mercenaro, Alessandra Del Caro, Luca Pretti, and Giovanni Nieddu. 2015. "Distinctive Anthocyanin Accumulation Responses to Temperature and Natural UV Radiation of Two Field-Grown Vitis vinifera L. Cultivars" Molecules 20, no. 2: 2061-2080. https://doi.org/10.3390/molecules20022061
APA StyleFernandes de Oliveira, A., Mercenaro, L., Del Caro, A., Pretti, L., & Nieddu, G. (2015). Distinctive Anthocyanin Accumulation Responses to Temperature and Natural UV Radiation of Two Field-Grown Vitis vinifera L. Cultivars. Molecules, 20(2), 2061-2080. https://doi.org/10.3390/molecules20022061