Enrichment and Purification of Polyphenol Extract from Sphallerocarpus gracilis Stems and Leaves and in Vitro Evaluation of DNA Damage-Protective Activity and Inhibitory Effects of α-Amylase and α-Glucosidase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Static Adsorption and Desorption
2.1.1. Selection of Optimal Resin
| Resin | Surface Area (m2/g) | Average Pore Diameter (Å) | Polarity | Moisture Content (%) | Adsorption Capacity (mg/g) | Desorption Ratio (%) |
|---|---|---|---|---|---|---|
| X-5 | 500–600 | 290–300 | Non-polar | 60.1 | 51.26 ± 1.45 a | 84.80 ± 2.44 a |
| D4020 | 540–580 | 100–105 | Non-polar | 75.2 | 43.23 ± 2.46 bc | 78.09 ± 3.44 ab |
| D101 | 400–520 | 200–300 | Non-polar | 65.8 | 43.88 ± 2.34 b | 80.55 ± 3.47 a |
| AB-8 | 480–520 | 130–140 | Polar | 65.4 | 43.99 ± 1.97 b | 81.65 ± 3.02 a |
| LS-46D | 400–500 | 150–250 | Polar | 59.3 | 40.67 ± 2.81 c | 84.74 ± 2.65 a |
| LS-305 | 400–500 | 150–250 | Polar | 70.9 | 49.62 ± 2.72 a | 79.71 ± 3.67 ab |
| NKA-9 | 250–290 | 155–165 | Polar | 61.4 | 48.65 ± 2.49 a | 80.45 ± 4.29 a |
| NKA-2 | 160–200 | 145–155 | Polar | 72.7 | 43.05 ± 3.15 bc | 71.88 ± 1.35 b |
2.1.2. Static Adsorption and Desorption Kinetics on X-5

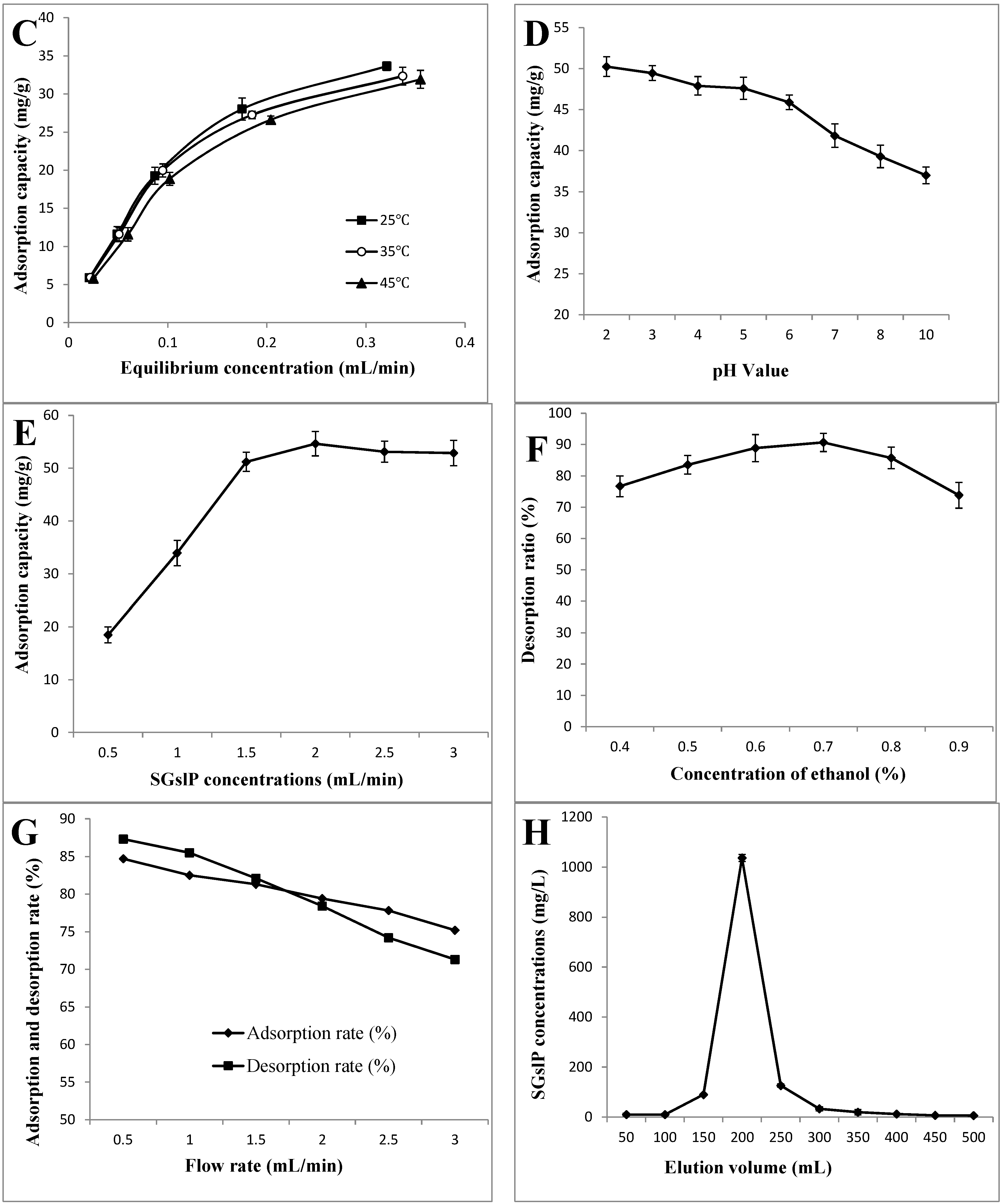
2.1.3. Adsorption Isotherms
| Temperature | Parameter | 25 °C | 35 °C | 45 °C |
|---|---|---|---|---|
| Langmuir equation | Linear equation | Ce/Qe = 0.0198Ce + 0.0030 | Ce/Qe = 0.0213Ce + 0.0031 | Ce/Qe = 0.0216Ce + 0.0035 |
| Q0 (mg/g) | 50.51 | 46.95 | 46.30 | |
| Kad (mL/mg) | 6.59 | 6.87 | 6.97 | |
| R2 | 0.9912 | 0.9906 | 0.9939 | |
| Freundlich equation | Linear equation | LnQe = 0.6529lnCe + 4.3998 | LnQe = 0.6529lnCe + 4.3998 | LnQe = 0.6529lnCe + 4.3998 |
| Kf (mg/g) | 81.43 | 74.79 | 71.77 | |
| 1/n | 0.6529 | 0.6347 | 0.6555 | |
| R2 | 0.9700 | 0.9641 | 0.9707 |
2.2. Effects of Different Parameters on the Adsorption Capacity and the Desorption Ratio of X-5 Resin
2.2.1. pH Values
2.2.2. Sample Concentrations
2.2.3. Ethanol Concentrations
2.3. Dynamic Adsorption and Desorption
2.3.1. Effect of Flow and Elution Speed on the Adsorption Capacity and Desorption Ratio of the X-5 Resin
2.3.2. Dynamic Desorption Curve on the X-5 Resin
2.3.3. Qualitative and Quantitative Analysis of the PSGslP
| Parameter | Peak | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Rt (min) | 30.66 | 39.44 | 41.79 | 48.87 |
| MW | 448 | 490 | 490 | 490 |
| MS (m/z) | 447 [M − H]− | 489 [M − H]− | 489 [M − H]− | 489 [M − H]− |
| MS2 (m/z) | 285 | 285 | 285 | 285 |
| Identified compound | Luteolin-7-glucoside | Acacetin-7-acetyglycoside | Acacetin-7-acetyglycoside | Acacetin-7-acetyglycoside |
| Content a (mg L7G/g DW) | 3.16 ± 0.25 | 0.21 ± 0.02 | 0.11 ± 0.01 | 0.67 ± 0.03 |
2.3.4. DNA Damage-Protective Activity of the PSGslP
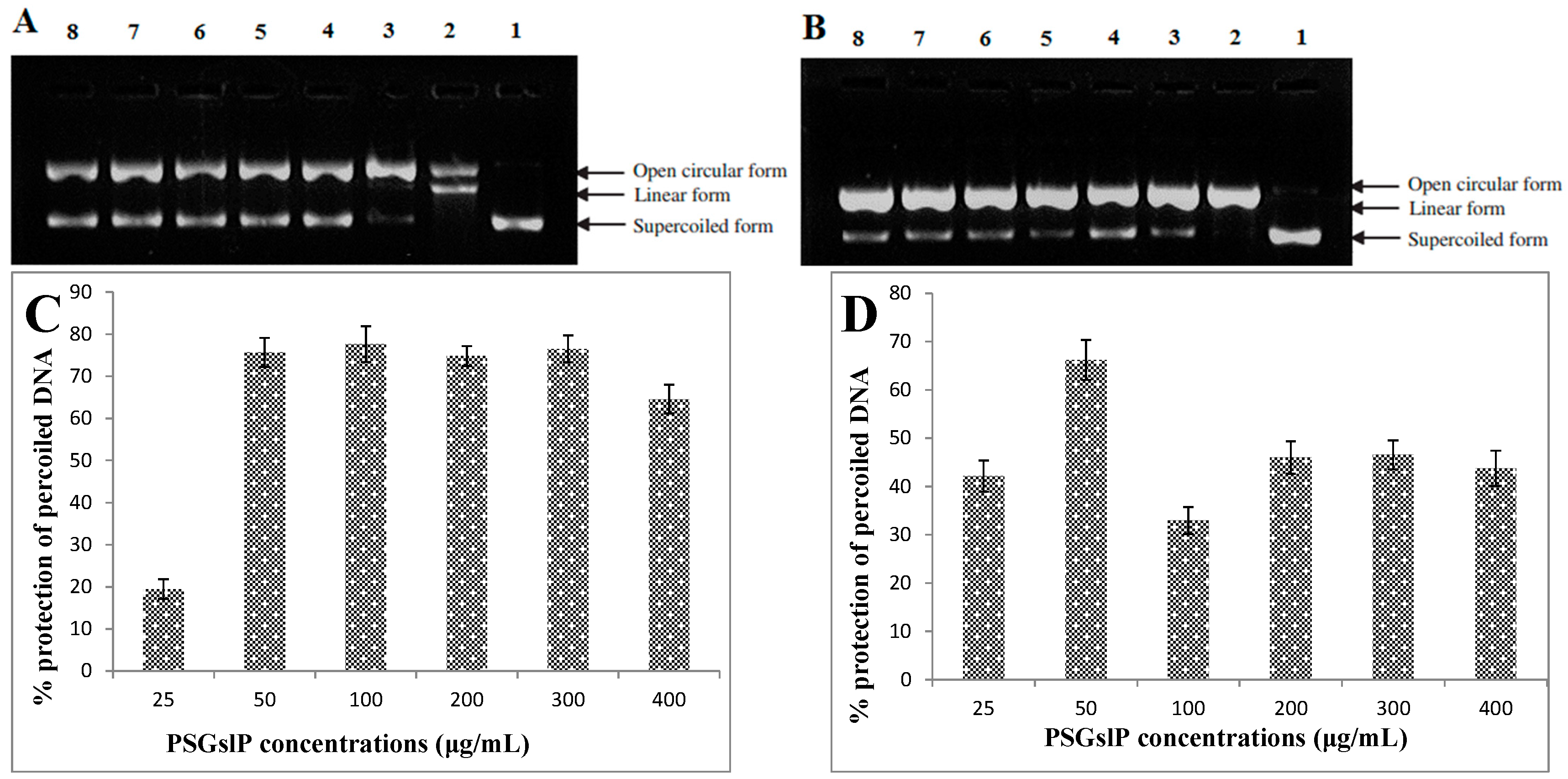
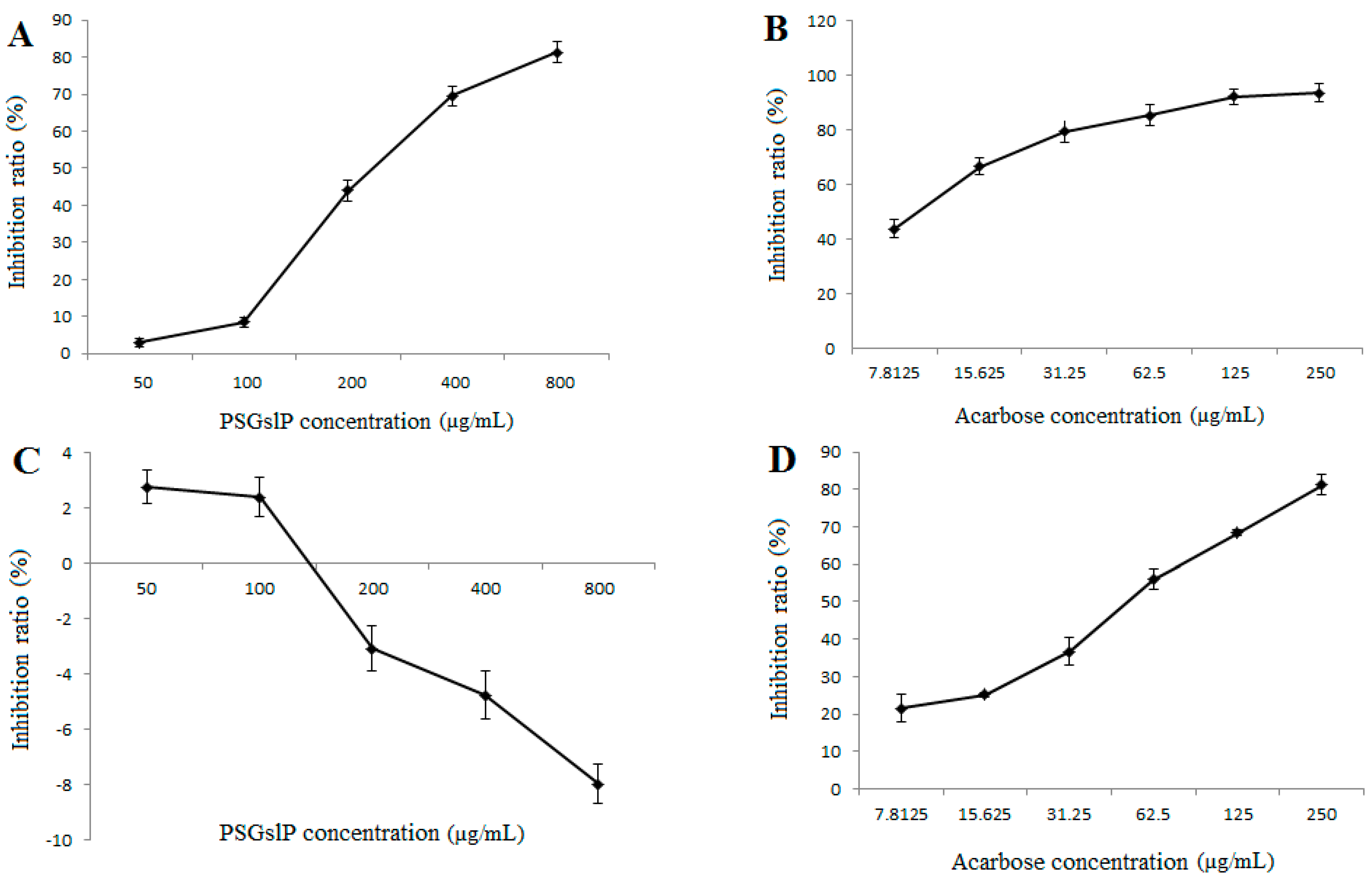
2.3.5. Inhibitory Effects of α-Amylase and α-Glucosidase
3. Experimental Section
3.1. Reagents
3.2. Adsorbents
3.3. Plant Material and Preparation of SGslP Extracts
3.4. Determination of the Total Polyphenol Content (TPC)
3.5. Static Adsorption and Desorption Test
3.5.1. Resin Selection
3.5.2. Adsorption and Desorption Kinetics
3.5.3. Adsorption Isotherms
3.5.4. Effects of Different Parameters on the Adsorption Capacity and Desorption Ratio of the X-5 Resin
3.6. Dynamic Adsorption and Desorption
3.6.1. Effects of Flow Rate on Dynamic Adsorption and Desorption
3.6.2. Dynamic Desorption Curve on the X-5 Resin
3.7. HPLC-MS/MS Analysis of the PSGslPs
3.8. Evaluation of the DNA Damage-Protective Activities of the PSGslP
3.8.1. Determination of Peroxyl Radical-Induced DNA Damage-Protective Activity
3.8.2. Determination of Hydroxyl Radical-Induced DNA Damage-Protective Activity
3.9. Evaluation of the Inhibitory Effects of α-Amylase and α-Glucosidase in Vitro
3.9.1. α-Amylase Inhibition Assay
3.9.2. α-Glucosidase Inhibition Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix

References
- Gao, C.Y.; Lu, Y.H.; Tian, C.R.; Xu, J.G.; Guo, X.P.; Zhou, R. Main nutrients, phenolics, antioxidant activity, DNA damage protective effect and microstructure of Sphallerocarpus gracilis root at different harvest time. Food Chem. 2011, 127, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.R.; Pe, D.; Liu, J.X.; Di, D. Chemical constituents from Sphallerocarpus gracilis. Biochem. Syst. Ecol. 2012, 40, 1–3. [Google Scholar] [CrossRef]
- Gao, C.Y.; Tian, C.R.; Lu, Y.H.; Xu, J.G.; Luo, J.Y.; Guo, X.P. Essential oil composition and antimicrobial activity of Sphallerocarpus gracilis seeds against selected food-related bacteria. Food Control 2011, 22, 508–516. [Google Scholar] [CrossRef]
- Gao, C.Y.; Tian, C.R.; Zhou, R.; Zhang, R.G.; Lu, Y.H. Phenolic composition, DNA damage protective activity and hepatoprotective effect of free phenolic extract from Sphallerocarpus gracilis seeds. Int. Immunopharmacol. 2014, 20, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.T.; Tian, C.R.; Li, L.Z.; Quan, M.P.; Zhang, J.; Zhao, P. Response surface methodology for optimization extraction process of Sphallerocarpus gracilis stem leaves polyphenol and determination of antioxidant activity. J. Food Sci. Biotechnol. 2013, 32, 1218–1216. [Google Scholar]
- Kim, K.H.; Moon, E.; Choi, S.U.; Kim, S.Y.; Lee, K.R. Polyphenols from the bark of Rhus verniciflua and their biological evaluation on antitumor and anti-inflammatory activities. Phytochemistry 2013, 92, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Buk, G.H.; Yun, J.P.; Yong, S.P.; Jong, H.B.; Ja, Y.C.; Kun, P. Anticancer and antioxidant effects of extracts from different parts of indigo plant. Ind. Crop Prod. 2014, 56, 9–16. [Google Scholar]
- Kendall, M.J.; Nuttall, S.L. Anti-oxidant therapy for the treatment of coronary artery disease. Expert Opin. Investig. Drug 1999, 8, 1763–1784. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; Van, E.M.; Wielinga, P.Y. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Budzisz, E.; Rotsztejn, H. The inhibitory effects of polyphenols on skin UV immunesuppression. Adv. Exp. Med. Biol. 2013, 67, 358–362. [Google Scholar]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Petko, D.; Maria, K.; Milan, C.; Antonin, L.; Ondrej, V.; Plamena, N. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014, 157, 37–44. [Google Scholar]
- Li, L.Y.; Xu, J.L.; Mu, Y.; Han, L.; Liu, R.H.; Cai, Y.P. Chemical characterization and anti-hyperglycaemic effects of polyphenol enriched longan (Dimocarpus longan Lour.) pericarp extracts. J. Funct. Foods. 2015, 13, 314–322. [Google Scholar] [CrossRef]
- Jang, G.Y.; Kim, H.Y.; Lee, S.H.; Kang, Y.; Hwang, I.G.; Woo, K.S. Effects of heat treatment and extraction method on antioxidant activity of several medicinal plants. J. Korean Soc. Food Sci. Nutr. 2012, 41, 914–920. [Google Scholar] [CrossRef]
- Sun, L.J.; Guo, Y.R.; Fu, C.C.; Li, J.J.; Li, Z. Simultaneous separation and purification of total polyphenols, chlorogenic acid and phlorizin from thinned young apples. Food Chem. 2013, 136, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.H.; Mu, T.H.; Sun, H.N. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.P.; Zhang, Q.H.; Zhang, D.Y.; Shi, Y.Y.; Jiang, C.X.; Shi, X.J. Preliminary separation and purification of resveratrol from extract of peanut (Arachis hypogaea) sprouts by macroporous adsorption resins. Food Chem. 2014, 145, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Sheng, Z.L.; Han, Q.; Zhao, Y.L.; Cheng, G.D.; Li, Y.H. Enrichment and purification of total flavonoids from Flos Populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J. Chromatogr. B 2014, 945–946, 68–74. [Google Scholar]
- Kim, J.; Yoon, M.; Yang, H.; Jo, J.; Han, D.; Jeon, Y.J. Enrichment and purification of marine polyphenol phlorotannins using macroporous adsorption resins. Food Chem. 2014, 162, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Baskaralingam, P.; Pulikesi, M.; Elango, D.; Ramamurthi, V.; Sivanesan, S. Adsorption of acid dye onto organobentonite. J. Hazard Mater. 2006, 128, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Iqbal, M.; Holl, W.H. Kinetics, equilibrium and mechanism of Cd2+ removal from aqueous solution by mungbean husk. J. Hazard Mater. 2009, 168, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Shi, L.L.; Fan, L.T.; Ding, Y.F.; Zhao, S.; Liu, Y.J. Optimization of extraction and enrichment of phenolics from pomegranate (Punica granatum L.) leaves. Ind. Crop Prod. 2013, 42, 587–594. [Google Scholar] [CrossRef]
- Yin, L.H.; Xu, Y.W.; Qi, Y.; Han, X.; Xu, L.N.; Peng, J.Y. A green and efficient protocol for industrial-scale preparation of dioscin from Dioscorea nipponica Makino by two-step macroporous resin column chromatography. Chem. Eng. J. 2010, 165, 281–289. [Google Scholar] [CrossRef]
- Jia, G.T.; Lu, X.Y. Enrichment and purification of madecassoside and asiaticoside from Centella asiatica extracts with macroporous resins. J. Chromatogr. A 2008, 1193, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Tang, J.; Wang, H.X.; Tao, G.J.; Gu, X.H.; Hu, L.M. Preparative purification of salidroside from Rhodiola rosea by two-step adsorption chromatography on resins. J. Sep. Sci. 2009, 32, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.T.; Sun, X.Y.; Tian, C.R.; Zheng, Y.J.; Zheng, C.P.; Zhan, J.C. Chemical composition and hepatoprotective effects of polyphenols extracted from the stems and leaves of Sphallerocarpus gracilis. J. Funct. Foods 2015, 18, 673–683. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J. Agric. Food Chem. 2007, 55, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Jeong, J.B.; Park, J.H.; Lee, H.K.; Ju, S.Y.; Hong, S.C.; Lee, J.R. Protective effect of the extracts from Cnidium officinale against oxidative damage induced by hydrogen peroxide via antioxidant effect. Food Chem. Toxicol. 2009, 47, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ahire, M.; Patil, S.; Jabgunde, A.; Bhat, D.M.; Joshi, B.N. Antidiabetic activity of Gnidia glauca and Dioscorea bulbifera: Potent amylase and glucosidase inhibitors. Evid. Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Wang, M.H.; Rhee, H.I. A novel α-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004, 339, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Sun, X.; Tian, C.; Luo, J.; Zheng, C.; Zhan, J. Enrichment and Purification of Polyphenol Extract from Sphallerocarpus gracilis Stems and Leaves and in Vitro Evaluation of DNA Damage-Protective Activity and Inhibitory Effects of α-Amylase and α-Glucosidase. Molecules 2015, 20, 21442-21457. https://doi.org/10.3390/molecules201219780
Ma T, Sun X, Tian C, Luo J, Zheng C, Zhan J. Enrichment and Purification of Polyphenol Extract from Sphallerocarpus gracilis Stems and Leaves and in Vitro Evaluation of DNA Damage-Protective Activity and Inhibitory Effects of α-Amylase and α-Glucosidase. Molecules. 2015; 20(12):21442-21457. https://doi.org/10.3390/molecules201219780
Chicago/Turabian StyleMa, Tingting, Xiangyu Sun, Chengrui Tian, Jiyang Luo, Cuiping Zheng, and Jicheng Zhan. 2015. "Enrichment and Purification of Polyphenol Extract from Sphallerocarpus gracilis Stems and Leaves and in Vitro Evaluation of DNA Damage-Protective Activity and Inhibitory Effects of α-Amylase and α-Glucosidase" Molecules 20, no. 12: 21442-21457. https://doi.org/10.3390/molecules201219780






