Use of Modified Phenolic Thyme Extracts (Thymus vulgaris) with Reduced Polyphenol Oxidase Substrates as Anthocyanin Color and Stability Enhancing Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Modified Extracts by Anionic Exchange Chromatography Pre-Treatment
| Extract 1 | Total Soluble Phenolics (mol/Kg) 2 |
|---|---|
| UNP | 0.812 ± 0.006 3 |
| WAE | 0.614 ± 0.007 |
| SAE | 0.700 ± 0.001 |
2.2. PPO Activity in the Presence of Modified Thyme Extracts
| PPO Substrate | Total Soluble Phenolics (mM) 1 | Initial Formation Rate (μmol/min) 2,4 |
|---|---|---|
| UNP | 95.4 | 0.151 ± 0.003 b 3 |
| WAE | 57.4 | 0.171 ± 0.004 b |
| SAE | 61.4 | 0.041 ± 0.002 c |
| Catechin (CAT) | 44.4 | 0.568 ± 0.025 a |
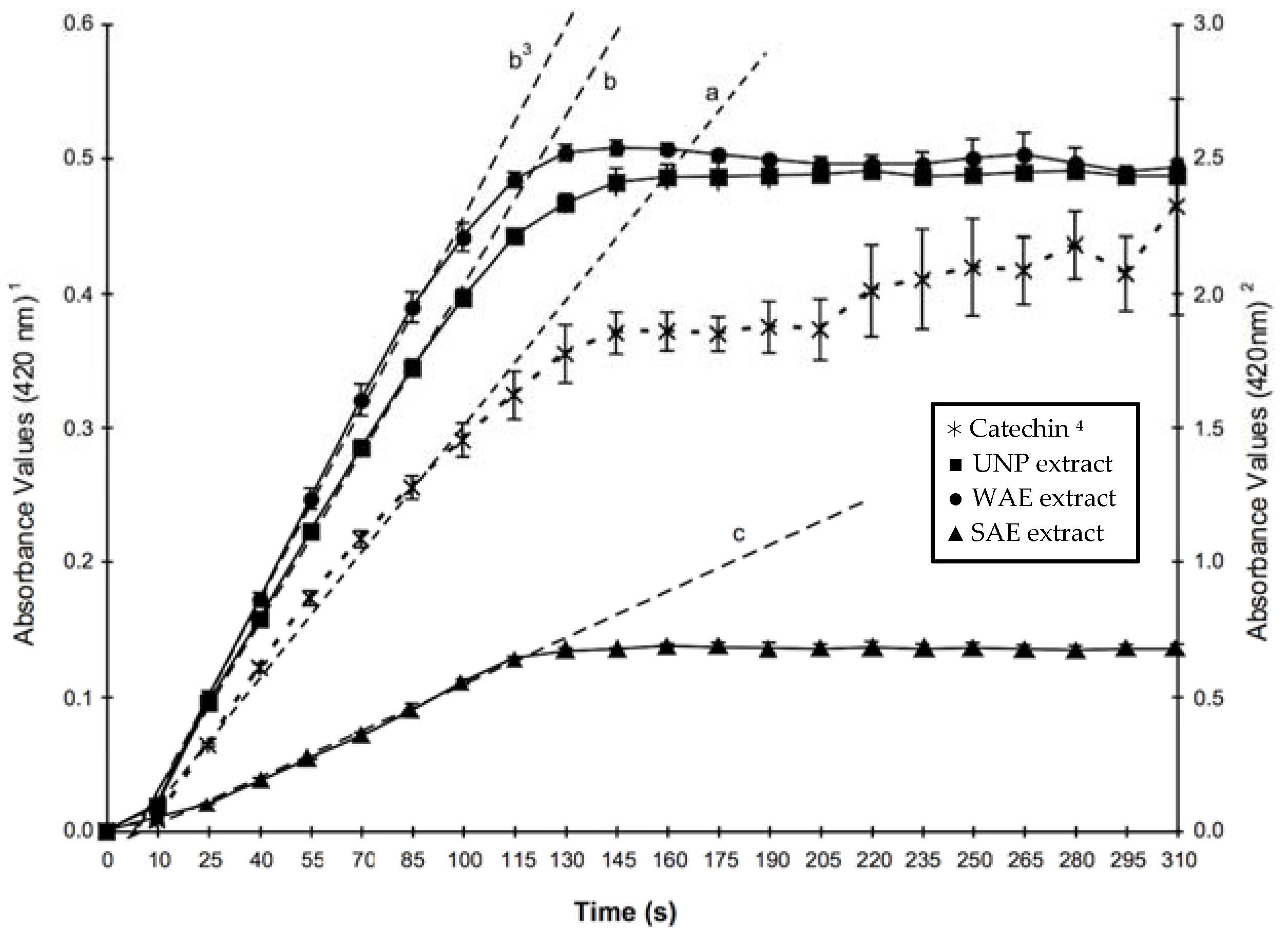
2.3. Copigmentation Using Modified Thyme Extracts
| Thyme Extract | Molar Ratio Copigment/Pigment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | |||||||
| Batochromic shift (nm) 2 | UNP | 0.0 | C 1 | 8.0 | B a 1 | 13.0 | A a | 13.3 | A b | 14.0 | A b |
| WAE | 0.0 | D | 8.0 | C a | 11.3 | B a | 13.7 | A ab | 12.7 | AB b | |
| SAE | 0.0 | D | 9.3 | C a | 12.0 | B a | 15.3 | A a | 16.7 | A a | |
| Hiperchromic shift (%) 3 | UNP | 0.0 | C | 47.0 | A ab | 50.2 | A b | 30.4 | B c | 33.6 | B b |
| WAE | 0.0 | C | 32.5 | B b | 48.8 | AB b | 53.7 | A b | 40.2 | AB b | |
| SAE | 0.0 | E | 56.8 | D a | 73.0 | C a | 98.5 | B a | 116.8 | A a | |
| Lightness (L*) | UNP | 39.1 | A | 30.5 | B a | 28.1 | C a | 26.1 | D a | 25.1 | E a |
| WAE | 37.5 | A | 28.7 | B b | 25.9 | C b | 22.0 | D c | 21.7 | D c | |
| SAE | 38.6 | A | 28.7 | B b | 25.9 | C b | 24.2 | D b | 23.6 | E b | |
| Chroma Value (C*) | UNP | 48.9 | A | 39.8 | B a | 34.8 | C a | 26.2 | D a | 22.0 | E a |
| WAE | 48.8 | A | 36.2 | B b | 28.8 | C c | 20.0 | D b | 19.4 | D b | |
| SAE | 50.4 | A | 40.5 | B a | 33.1 | C b | 26.4 | D a | 22.2 | E a | |
| Hue Angle (h*) | UNP | 41.2 | A | 25.7 | B b | 22.7 | C b | 20.8 | D b | 19.7 | E c |
| WAE | 41.6 | A | 24.6 | B c | 21.5 | C c | 20.8 | D b | 20.3 | D b | |
| SAE | 43.0 | A | 27.3 | B a | 24.2 | C a | 21.8 | D a | 21.2 | E a | |
2.4. Anthocyanins Total Monomer, Polymer Color and Color Density
| Thyme Extract | Molar Ratio Copigment/Pigment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | |||||||
| Monomeric Anthocyanins 2 (mg/L) | UNP | 85.28 | A 1 | 78.72 | B b 1 | 73.67 | C b | 62.27 | D b | 60.89 | D b |
| WAE | 94.47 | A | 92.11 | A a | 83.71 | B a | 80.11 | BC a | 79.12 | C a | |
| SAE | 94.76 | A | 82.04 | B ab | 80.77 | B a | 79.56 | B a | 81.86 | B a | |
| Color Density | UNP | 1.91 | E | 3.10 | D c | 4.36 | C b | 5.63 | B a | 7.12 | A a |
| WAE | 2.14 | D | 3.49 | C a | 5.13 | B a | 5.79 | B a | 6.83 | A a | |
| SAE | 1.79 | E | 3.22 | D b | 4.41 | C b | 5.84 | B a | 7.15 | A a | |
| Polymeric Color (%) | UNP | 18.64 | E | 32.81 | D a | 38.34 | C a | 49.18 | B a | 55.51 | A a |
| WAE | 17.08 | D | 30.88 | C a | 41.62 | B a | 42.71 | AB b | 48.37 | A b | |
| SAE | 26.40 | C | 31.90 | C a | 39.72 | B a | 45.90 | A ab | 45.77 | A b | |
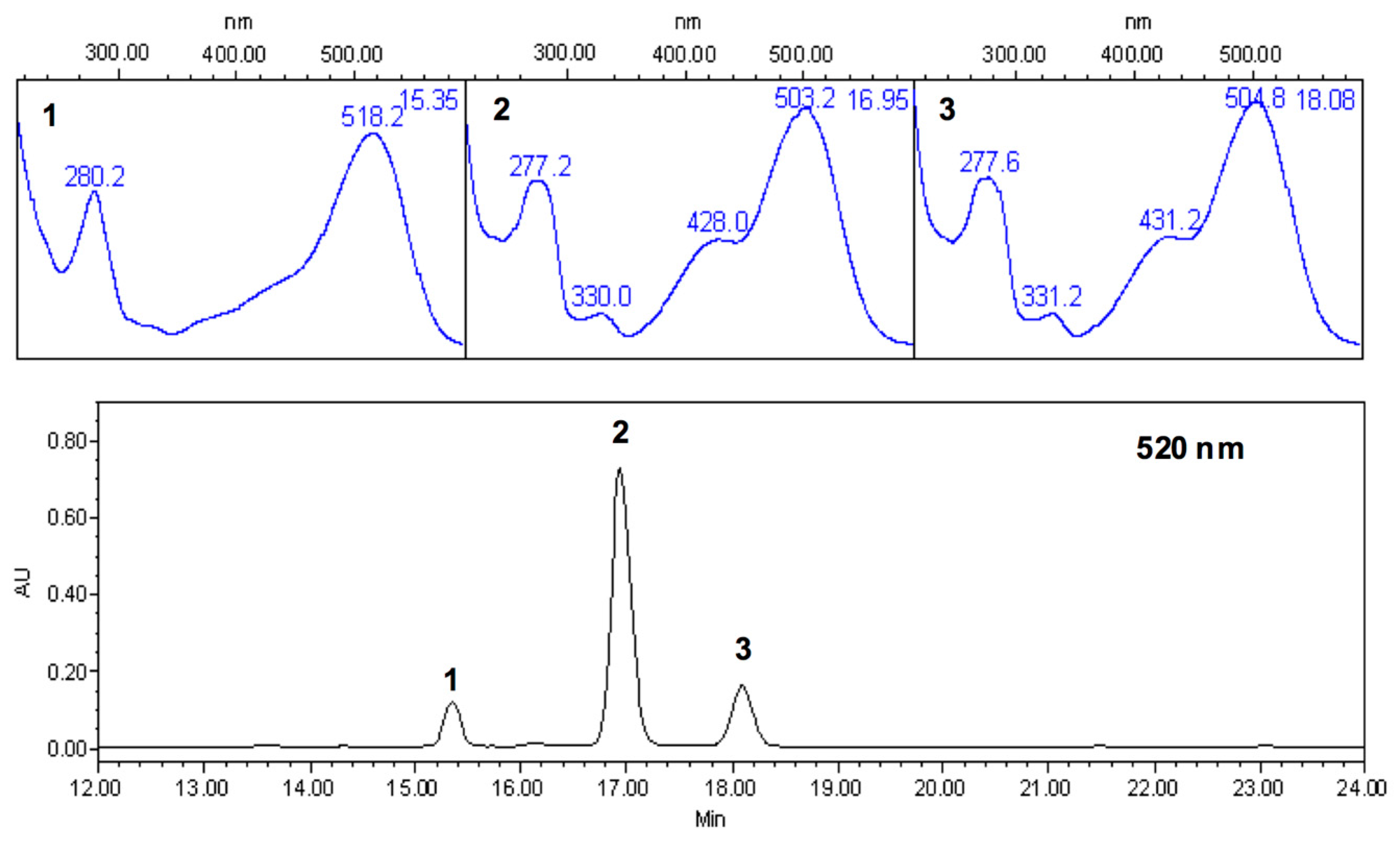
2.5. Characterization of Thyme Extracts by HPLC-PDA
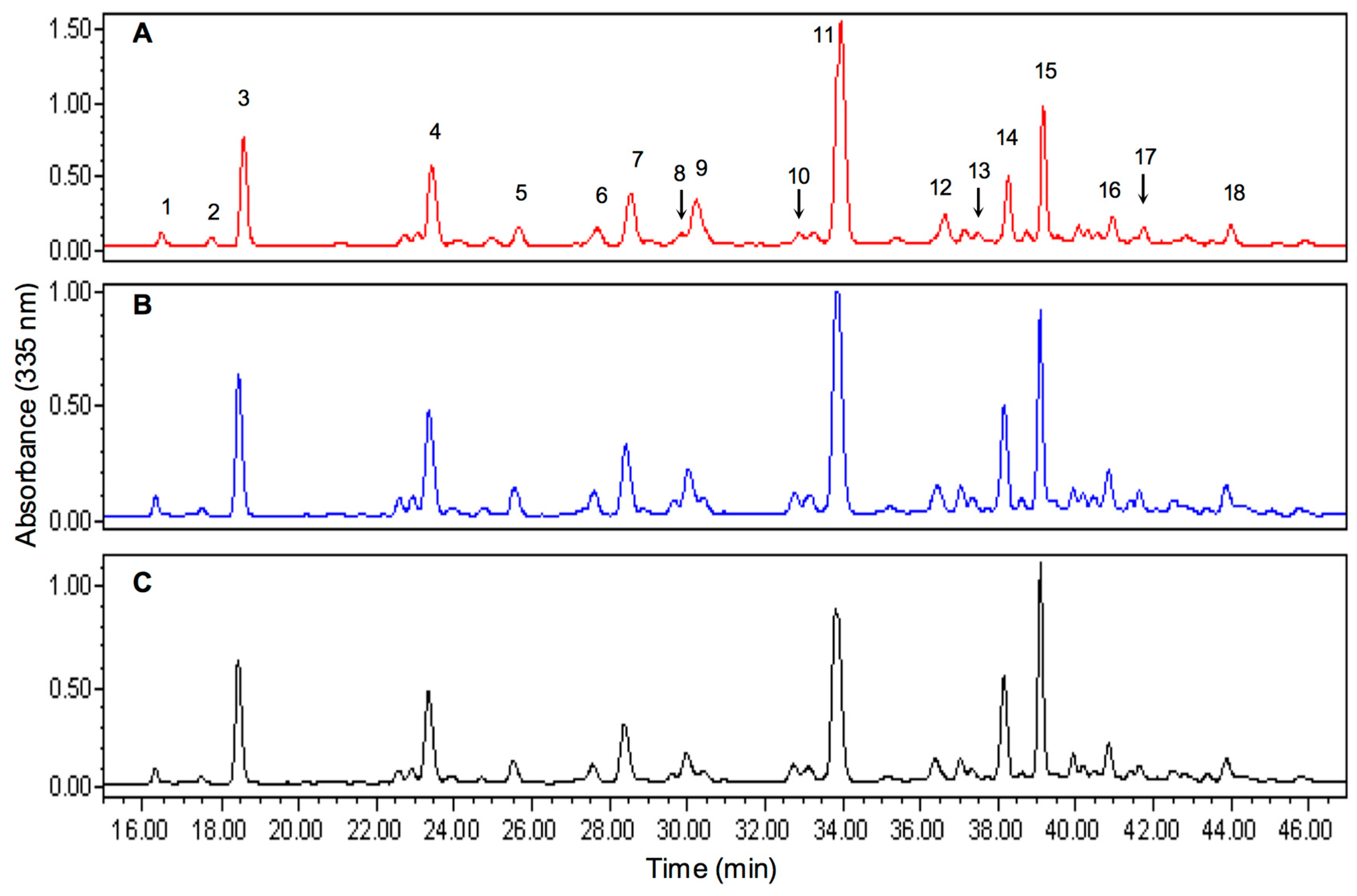
| Peak (Retention Time, min) 1 | λmax (nm) Absorption Bands 2 | Chemical Identity | Identification Method 3 |
|---|---|---|---|
| 1 (16.5) | 268.7, 349.5 | Kaempferol-3-glucoside or probable 3,7-O-glycoside | A, B |
| 2 (17.72) | 297.1, 325.1 | Coelution | A |
| 3 (18.57) | 216.8, 273.4, 335.2, | Apigenin-8-C-glucoside | A, B |
| 4 (23.44) | 216.8, 282.9, 330.4s | Naringenin derivative | A, B |
| 5 (25,64) | 268.7, 335.2 | Glycosilated apigenin (ring B) | A, B |
| 6 (27.67) | 268.7, 340.0 | Apigenin | A, B |
| 7 (28.54) | 268.7, 349.5 | Luteolin derivative (ring A) | A, B |
| 8 (29.83) | 282.9, 335.2 | Possible Flavone | |
| 9 (30.44) | 282.9, 335.2 | Hesperetin | A, C |
| 10 (32.9) | 282.9, 340.0 | Probable di-glycoside of peak 17 | |
| 11 (33.93) | 330.4 | Rosmarinic acid derivative | A, B |
| 12 (36.62) | 287.6, 325.7 | Unknown, probable coelution | A, B, C |
| 13 (37.48) | 287.6 | Naringenin | |
| 14 (38.26) | 254.5, 349.5 | Luteolin | A, B, C |
| 15 (39.17) | 330.4 | Rosmarinic acid | A, B, C |
| 16 (40.96) | 282.9, 340.0 | Glycoside of peak 17 | |
| 17 (41.52) | 282.9, 340.0 | Possible Flavone | |
| 18 (43.97) | 287.6, 344.7 | Possible Flavone |
| Peak (Retention Time, min) | Chemical Identity | Concentration in ppm 1 | ||
|---|---|---|---|---|
| UNP | WAE | SAE | ||
| 5 (25,64) | Glycosylated apigenin (ring B) | 126.2 a 2 | 99.0 b | 101.7 b |
| 6 (27.67) | Apigenin | 157.8 a | 114.9 b | 94.4 b |
| 7 (28.54) | Luteolin derivative (ring A) | 329.8 a | 267.5 b | 274.5 b |
| 9 (30.44) | Hesperetin | 512.9 a | 427.9 b | 441.4 a,b |
| 10 (32.9) | Probable diglycoside of peak 17 | 118.9 a | 67.8 b | 89.0 b |
| 11 (33.93) | Rosmarinic acid derivative | 764.1 a | 480.2 b | 481.0 b |
| 13 (37.48) | Naringenin | 319.8 a | 217.7 c | 254.2 b |
| 14 (38.26) | Luteolin | 341.2 a,b | 318.2 b | 360.1 a |
| 15 (39.17) | Rosmarinic acid | 422.0 a | 365.5 b | 442.5 a |
3. Experimental Section
3.1. Materials
3.2. Preparation of Water-Soluble Extracts from Thyme
3.3. Copigmentation Assays
3.4. Polyphenol Oxidase Assay
3.5. Chromatographic Profile of Thyme Phenolic Extracts
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bobinaitė, R.; Viškelis, P.; Šarkinas, A.; Venskutonis, P.R. Phytochemical composition, antioxidant and antimicrobial properties of raspberry fruit, pulp, and marc extracts. CyTA-J. Food 2013, 11, 334–342. [Google Scholar] [CrossRef]
- Jackman, R.L.; Smith, J.L. Anthocyanins and Betalains. In Natural Food Colorants, 2nd ed.; Hendry, G.A.F., Houghton, J.D., Eds.; Blackie and Son Ltd.: London, UK, 1996; pp. 245–280. [Google Scholar]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra-and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Markakis, P. Stability of anthocyanins in foods. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 163–180. [Google Scholar]
- Junqueira-Gonçalves, M.P.; Yáñez, L.; Morales, C.; Navarro, M.; Contreras, R.A.; Zúñiga, G.E. Isolation and Characterization of Phenolic Compounds and Anthocyanins from Murta (Ugni molinae Turcz.) Fruits. Assessment of Antioxidant and Antibacterial Activity. Molecules 2015, 20, 5698–5713. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- González-Montilla, F.M.; Chávez-Santoscoy, R.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Isolation and identification of phase II enzyme inductors obtained from black Shawaya sorghum [Sorghum bicolor (L.) Moench] bran. J. Cereal Sci. 2012, 55, 126–131. [Google Scholar] [CrossRef]
- Lamikanra, O.; Watson, M.A. Effects of Ascorbic Acid on Peroxidase and Polyphenoloxidase Activities in Fresh—Cut Cantaloupe Melon. J. Food Sci. 2001, 66, 1283–1286. [Google Scholar]
- Serradell, M.D.L.A.; Rozenfeld, P.A.; Martínez, G.A.; Civello, P.M.; Chaves, A.R.; Anon, M.C. Polyphenoloxidase activity from strawberry fruit (Fragaria ananassa, Duch., cv Selva): Characterisation and partial purification. J. Sci. Food Agric. 2000, 80, 1421–1427. [Google Scholar] [CrossRef]
- Baranac, J.M.; Petranovic, N.A.; Dimitric-Markovic, J.M. Spectrophotometric study of anthocyan copigmentation reactions. J. Agric. Food Chem. 1996, 44, 1333–1336. [Google Scholar] [CrossRef]
- Figueiredo, P.; Toki, K. Anthocyanin-aluminium and-gallium complexes in aqueous solution. J. Chem. Soc. Perkin Trans. 1997, 2, 355–362. [Google Scholar]
- Darias-Martín, J.; Carrillo, M.; Díaz, E.; Boulton, R.B. Enhancement of red wine colour by pre-fermentation addition of copigments. Food Chem. 2001, 73, 217–220. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.T.; Pratt, D.E. Apple polyphenol oxidase activity in relation to various phenolic compounds. J. Food Sci. 1967, 32, 479–483. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Del Pozo-Insfran, D.; Follo-Martinez, A.; Talcott, S.T.; Brenes, C.H. Stability of copigmented anthocyanins and ascorbic acid in muscadine grape juice processed by high hydrostatic pressure. J. Food Sci. 2007, 72, S247–S253. [Google Scholar] [CrossRef] [PubMed]
- Talcott, S.T.; Brenes, C.H.; Pires, D.M.; del Pozo-Insfran, D. Phytochemical stability and color retention of copigmented and processed muscadine grape juice. J. Agric. Food Chem. 2003, 51, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Bakowska, A.; Kucharska, A.Z.; Oszmiański, J. The effects of heating, UV irradiation, and storage on stability of the anthocyanin—Polyphenol copigment complex. Food Chem. 2003, 81, 349–355. [Google Scholar] [CrossRef]
- Stauffer, C.E. Enzyme Assays for Food Scientists; Van Nostrand Reinhold: New York, NY, USA, 1989. [Google Scholar]
- Jones, A.M.P.; Saxena, P.K. Inhibition of Phenylpropanoid Biosynthesis in Artemisia annua L.: A Novel Approach to Reduce Oxidative Browning in Plant Tissue Culture. PLoS ONE 2013, 8, e76802. [Google Scholar] [CrossRef] [PubMed]
- Guillén, D.A.; Merello, F.; Barroso, C.G.; Pérez-Bustamante, J.A. Solid-phase extraction for sample preparation, in the HPLC analysis of polyphenolic compounds in “Fino” sherry wine. J. Agric. Food Chem. 1997, 45, 403–406. [Google Scholar] [CrossRef]
- Cardona, J.A.; Lee, J.H.; Talcott, S.T. Color and polyphenolic stability in extracts produced from muscadine grape (Vitis rotundifolia) pomace. J. Agric. Food Chem. 2009, 57, 8421–8425. [Google Scholar] [CrossRef] [PubMed]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Petelinc, T.; Polak, T.; Demšar, L.; Jamnik, P. Fractionation of phenolic compounds extracted from propolis and their activity in the yeast Saccharomyces cerevisiae. PLoS ONE 2013, 8, e56104. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.T.; Bank, V.R.; Lenoble, R.; Richheimer, S.L. Pigment Composition Containing Anthocyanins Stabilized by Plant Extracts. U.S. Patent No. 5,908,650, 1 June 1999. [Google Scholar]
- Pascual-Teresa, S.; Moreno, D.A.; García-Viguera, C. Flavanols and Anthocyanins in Cardiovascular Health: A Review of Current Evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rababah, T.M.; Banat, F.; Rababah, A.; Ereifej, K.; Yang, W. Optimization of Extraction Conditions of Total Phenolics, Antioxidant Activities, and Anthocyanin of Oregano, Thyme, Terebinth, and Pomegranate. J. Food Sci. 2010, 75, C626–C632. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Castellanos-Dohnal, G.; Caballero-Mata, P.; Hernández-Brenes, C. Cambios bioquímicos durante el almacenamiento de puré de aguacate adicionado con antioxidantes naturales y procesado con alta presión hidrostática. CyTA-J. Food 2013, 11, 379–391. [Google Scholar] [CrossRef]
- González-Cebrino, F.; Durán, R.; Delgado-Adámez, J.; Contador, R.; Ramírez, R. Changes after high-pressure processing on physicochemical parameters, bioactive compounds, and polyphenol oxidase activity of red flesh and peel plum purée. Innov. Food Sci. Emerg. 2013, 20, 34–41. [Google Scholar] [CrossRef]
- García-Viguera, C.; Bridle, P. Influence of structure on colour stability of anthocyanins and flavylium salts with ascorbic acid. Food Chem. 1999, 64, 21–26. [Google Scholar] [CrossRef]
- Markovic, D.; Petranovic, N.A.; Baranac, J.M. A spectrophotometric study of the copigmentation of malvin with caffeic and ferulic acids. J. Agric. Food Chem. 2000, 48, 5530–5536. [Google Scholar] [CrossRef] [PubMed]
- Gauche, C.; Malagoli, E.D.S.; Bordignon-Luiz, M.T. Effect of pH on the copigmentation of anthocyanins from Cabernet Sauvignon grape extracts with organic acids. Sci. Agric. 2010, 67, 41–46. [Google Scholar] [CrossRef]
- Davies, A.J.; Mazza, G. Copigmentation of simple and acylated anthocyanins with colorless phenolic compounds. J. Agric. Food Chem. 1993, 41, 716–720. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Brenes, C.H.; Talcott, S.T. Phytochemical composition and pigment stability of Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2004, 52, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Gonnet, J.F. Colour effects of co-pigmentation of anthocyanins revisited—2. A colorimetric look at the solutions of cyanin co-pigmented byrutin using the CIELAB scale. Food Chem. 1999, 66, 387–394. [Google Scholar] [CrossRef]
- Escribano-Bailón, T.; Dangles, O.; Brouillard, R. Coupling reactions between flavylium ions and catechin. Phytochemistry 1996, 41, 1583–1592. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Anthocyanins and Phenolic Acids of Hybrid and Native Blue Maize (Zea mays L.) Extracts and Their Antiproliferative Activity in Mammary (MCF7), Liver (HepG2), Colon (Caco2 and HT29) and Prostate (PC3) Cancer Cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R. Techniques of Flavonoid Identification; Academic Press: London, UK, 1982. [Google Scholar]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Cisneros-Zeballos, L. Correlations of antioxidant activity against phenolic content revisited: A new approach in data analysis for food and medicinal plants. J. Food Sci. 2009, 74, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol antioxidant quantity and quality in foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef] [PubMed]
- Wrolstad, R.E. Color and Pigment Analyses in Fruit Products; Agricultural Communications Oregon State University: Corvallis, OR, USA, 1976. [Google Scholar]
- Rodriguez-Saôna, L.E.; Giusti, M.M.; Wrolstad, R.E. Color and pigment stability of red radish and red-fleshed potato anthocyanins in juice model systems. J. Food Sci. 1999, 64, 451–456. [Google Scholar] [CrossRef]
- Worthington, K.; Worthington, V. Worthington Enzyme Manual. (2011) Worthington Biochemical Corporation. Available online: http://www.worthington-biochem.com/TY/default.html (accessed on 1 June 2015).
- Rocha, A.; Morais, A.M. Polyphenoloxidase activity and total phenolic content as related to browning of minimally processed “Jonagored” apple. J. Sci. Food Agric. 2002, 82, 120–126. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, O.; Hernández-Brenes, C. Use of Modified Phenolic Thyme Extracts (Thymus vulgaris) with Reduced Polyphenol Oxidase Substrates as Anthocyanin Color and Stability Enhancing Agents. Molecules 2015, 20, 22422-22434. https://doi.org/10.3390/molecules201219854
Aguilar O, Hernández-Brenes C. Use of Modified Phenolic Thyme Extracts (Thymus vulgaris) with Reduced Polyphenol Oxidase Substrates as Anthocyanin Color and Stability Enhancing Agents. Molecules. 2015; 20(12):22422-22434. https://doi.org/10.3390/molecules201219854
Chicago/Turabian StyleAguilar, Oscar, and Carmen Hernández-Brenes. 2015. "Use of Modified Phenolic Thyme Extracts (Thymus vulgaris) with Reduced Polyphenol Oxidase Substrates as Anthocyanin Color and Stability Enhancing Agents" Molecules 20, no. 12: 22422-22434. https://doi.org/10.3390/molecules201219854
APA StyleAguilar, O., & Hernández-Brenes, C. (2015). Use of Modified Phenolic Thyme Extracts (Thymus vulgaris) with Reduced Polyphenol Oxidase Substrates as Anthocyanin Color and Stability Enhancing Agents. Molecules, 20(12), 22422-22434. https://doi.org/10.3390/molecules201219854






