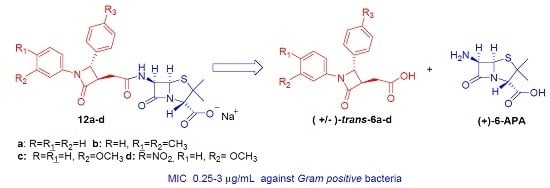
Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation
Abstract
:1. Introduction
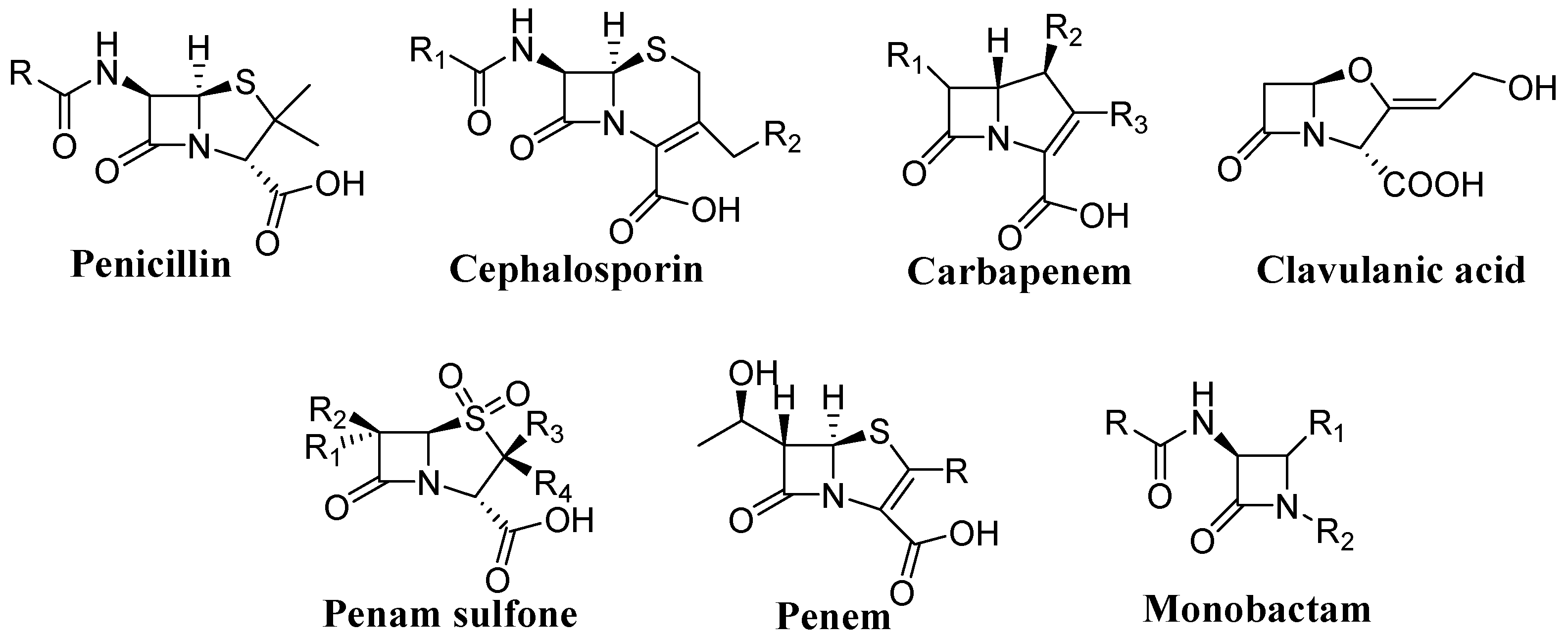
2. Results and Discussion
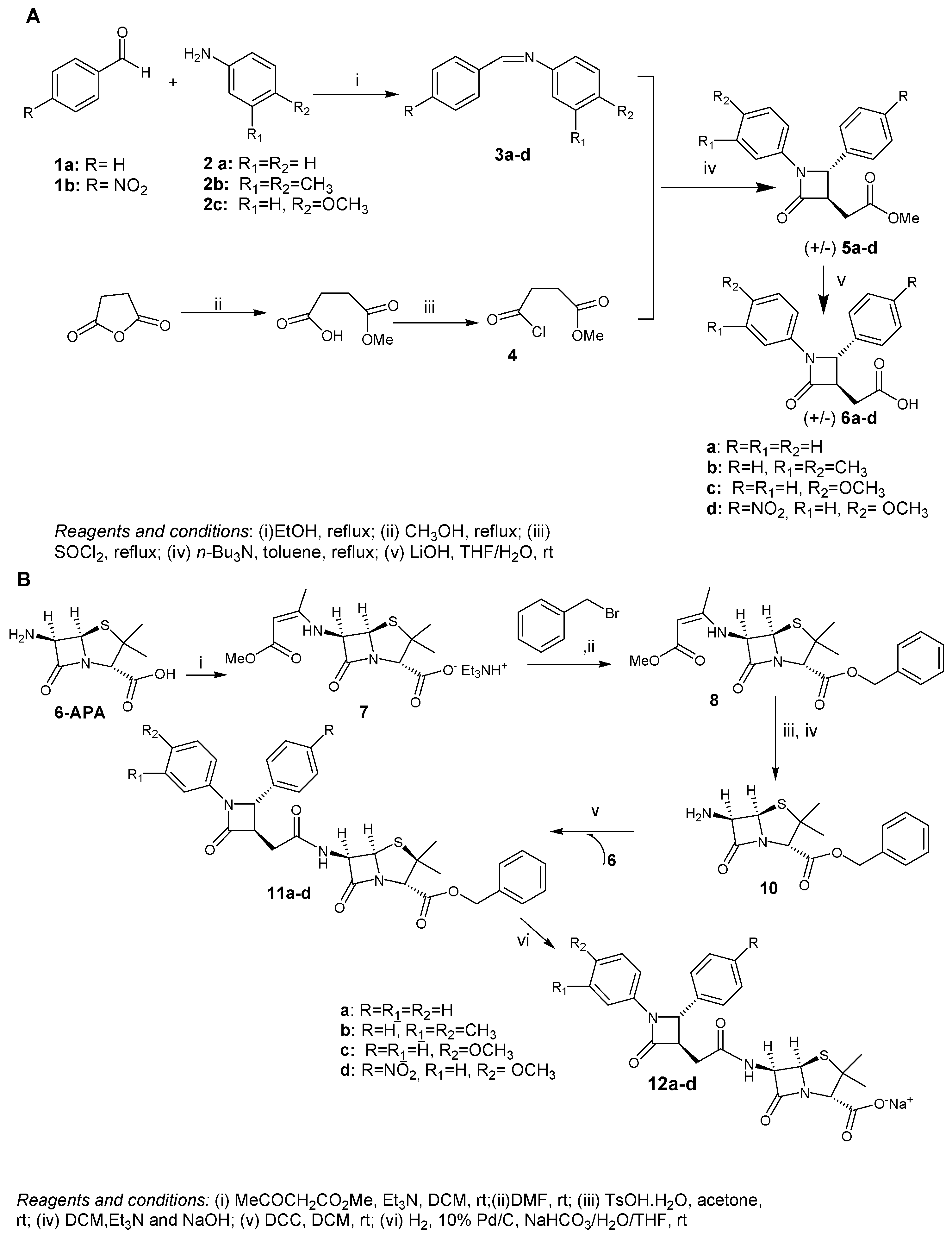
2.1. Chemistry
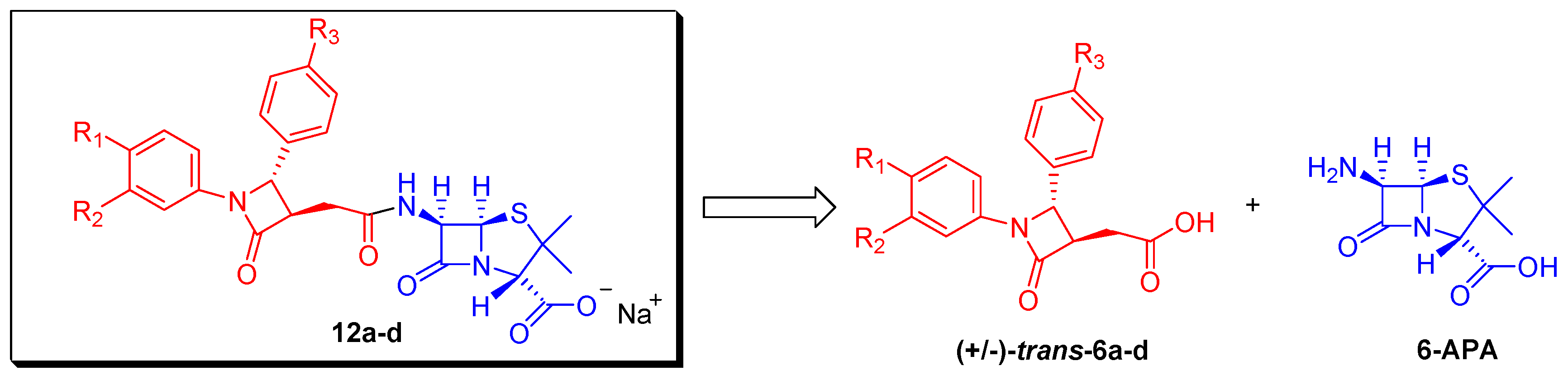
2.2. Biological Work
2.2.1. Antimicrobial Activity of Compounds 12a–d
| Compd. | Escherichia coli | Salmonella typhimurium | Pseudomonas spp. (fluorescens, aeruginosa) | Staphylococcus aureus | Staphylococcus epidermidis | Bacillus sp. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC100 | MLD | MIC100 | MLD | MIC100 | MLD | MIC100 | MLD | MIC100 | MLD | MIC100 | MLD | |
| Amp | 48 | ≤256 | 8.0 | ≤256 | >256 | >256 | 2.0 (5.8) | 8.0 (23.2) | 0.35 (1.0) | 16.0 (46.0) | 0.25 (0.72) | 8.0 (23.2) |
| 12a | >256 | >256 | >256 | >256 | >256 | >256 | 1.0 (2.1) | 4.0 (8,4) | 0.25 (0.52) | 16.0 (33.6) | 0.5 (1.05) | 32.0 (67.2) |
| 12b | >256 | >256 | >256 | >256 | >256 | >256 | <1.5(3.9) | 32.0 (63.4) | 0.35 (0.69) | ≥16.0 (≥31.7) | >1.0; <1.5 (>1.98; <2.97) | 48.0 (86.4) |
| 12c | >256 | >256 | >256 | >256 | >256 | >256 | >2.0; <3.0 (>3.9; <5.9) | <4.0 (<7.9) | <0.35 (<0.69) | 8.0(15.8) | 2.0(3.94) | 128.0 (252.2) |
| 12d | >256 | >256 | >256 | >256 | >256 | >256 | 1.75 (3.45) | 32.0 (57.6) | 0.75 (1.35) | ≥16.0 (≥28.8) | 2.0 (3.6) | 64.0 (115.2) |
| Compd. | S. aureus | S. epidermidis | Bacillus sp. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MICCmp/MICAmp | MLDCmp/MLDAmp | MICa–d/MICa | MICCmp/MICAmp | MLDCmp/MLDAmp | MICa–d/MICa | MICCmp/MICAmp | MLDCmp/MLDAmp | MICa–d/MICa | |
| 12a | 0.5 (0.36) | 0.5 (0.36) | 1 | 0.71 (0.52) | 1.0 (0.73) | 1 | 2.0 (1.46) | 4.0 (2.89) | 1 |
| 12b | <0.75 (<0.67) | 4.0 (2.73) | <1.5 (<1.86) | 1.0 (0.69) | ≥1.0 (≥0.69) | 1.4 (1.33) | >4.0, <6.0 (>2.75, <4.12) | 6.0 (3.72) | >2.0, <3.0 (>1.88, <2.83) |
| 12c | >1.0, <1.5 (>0,67, <1.01) | <0.5 (<0.34) | >2.0, <3.0 (>1.87, <2.81) | <1.0 (<0.69) | 0.5 (0.34) | <1.4 (<1.33) | 8.0 (5.47) | 16.0 (10.87) | 4.0 (3.75) |
| 12d | 0.87 (0.6) | 4.0 (2.48) | 1.75 (1.64) | 2.14 (1.35) | ≥1.0 (≥0.63) | 2.14 (2.6) | 8.0 (5.0) | 8.0 (4.96) | 4.0 (3.43) |
2.2.2. Cytotoxicity Assays
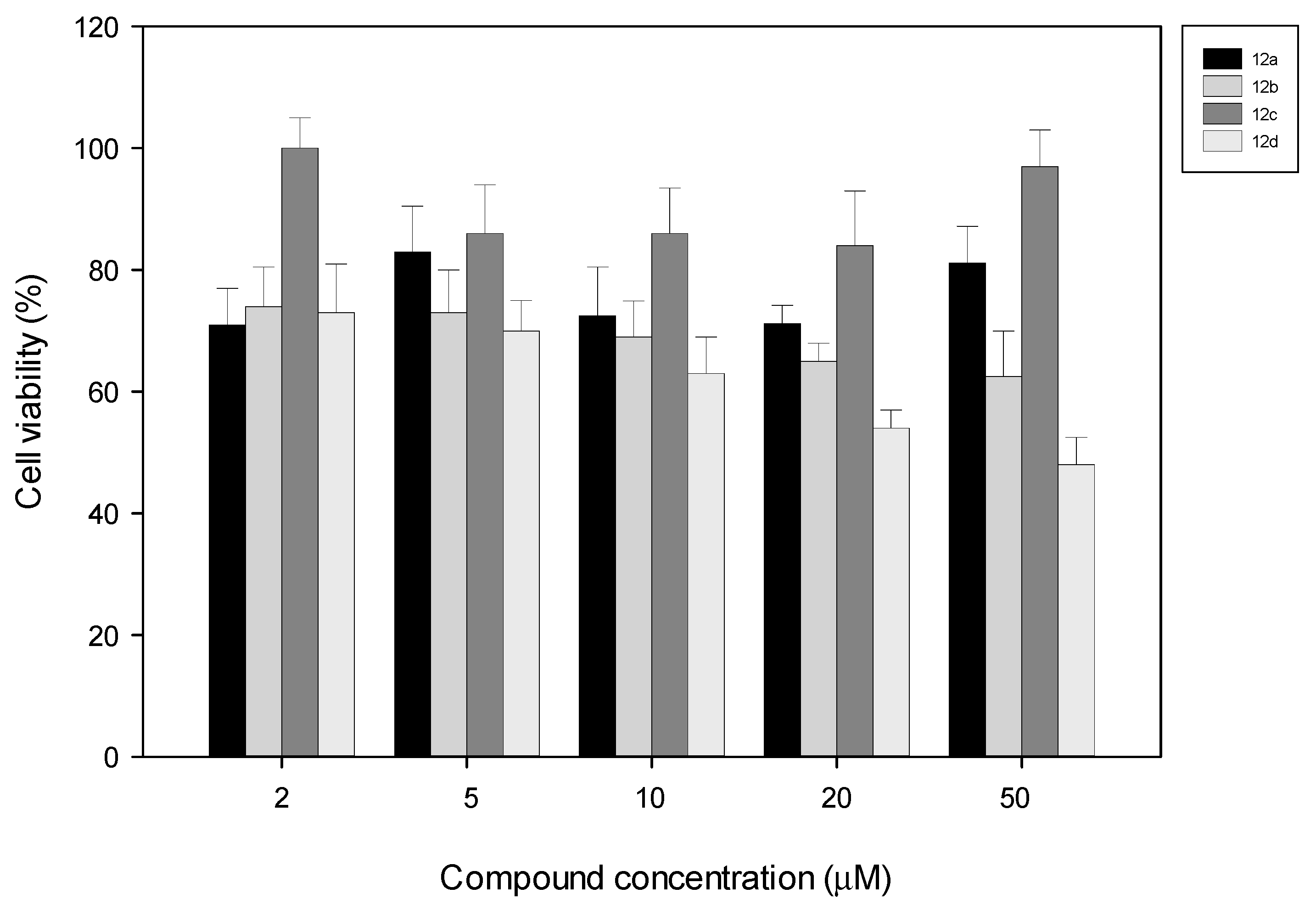
3. Experimental Section
3.1. Chemistry
3.1.1. Material and Methods
3.1.2. Synthesis of Compounds 3a–d. General Method
3.1.3. Methyl 4-chloro-4-oxobutanoate (4).
3.1.4. General procedure for the synthesis of azetidin-2-ones 5a–d
3.1.5. General Procedure for the Synthesis of 6a–d
3.1.6. Synthesis of (2S,5R,6R)-Benzyl 6-amino-3,3-dimethyl-7-oxo-4-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylate 10
3.1.7. General Procedure for the Synthesis of 11a–d
3.1.8. General Procedure for the Synthesis of 12a–d
3.2. Microbiological Assays and Bacterial Strains
3.3. In vitro NIH-3T3 Cells Line Cytotoxicity Testing
3.3.1. Cell Cultures
3.3.2. Cell Viability Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Troisi, L.; Granito, C.; Pindinelli, P. Novel and recent synthesis and applications of β-lactams. In Heterocyclic Scaffolds I-β-Lactams; Banik, B.K., Ed.; Springer-Verlag: Berlin, Germany, 2010; Volume 22, pp. 101–209. [Google Scholar]
- Fernandes, R.; Amador, P.; Prudêncio, C. β-lactams: Chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 2013, 24, 7–17. [Google Scholar] [CrossRef]
- Galletti, P.; Giacomini, D. Monocyclic β-lactams: New Structures for New Biological Activities. Curr. Med. Chem. 2011, 18, 4265–4283. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.D.; Sengar, N.P.S.; Pathak, A.K. 2-Azetidinone—A new profileof various pharmacological activities. Eur. J. Med. Chem. 2010, 45, 5541–5560. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S. β-Lactams in the New Millenium. Part-I: Monobactams and Carbapenems. Mini Rev. Med. Chem. 2004, 4, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S. β-Lactams in the New Millenium. Part-II: Cephems, Oxacephems, Penams and Sulbactam. Mini Rev. Med. Chem. 2004, 4, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Veinberg, G.; Vorona, M.; Shestakova, I.; Kanepe, I.; Lukevics, E. Design of β-Lactams with Mechanism Based Nonantibacterial Activities. Curr. Med. Chem. 2003, 10, 1741–1757. [Google Scholar] [CrossRef] [PubMed]
- Page, M.I. Structure-activity relationships: Chemical. In The Chemistry of β-Lactams; Page, M.I., Ed.; Springer-Science: Dordrecht, The Netherlands, 1992; pp. 79–99. [Google Scholar]
- Holden, K.G. Chemistry and Biology of β-Lactam Antibiotics; Morin, R.B., Gorman, M., Eds.; Academic Press: London, UK, 1982–1983; Volume 1–3, pp. 101–158. [Google Scholar]
- Fisher, J.F.; Meroueh, S.O.; Mobashery, S. Bacterial Resistance to β-Lactams Antibiotics: Compelling Opportunism, Compelling Opportunity. Chem. Rev. 2005, 105, 395–424. [Google Scholar] [CrossRef] [PubMed]
- Llarrull, L.I.; Testero, S.A.; Fisher, J.F.; Mobashery, S. The future of the β-lactams. Curr. Opin. Microbiol. 2010, 13, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.F.; Schneper, L.; Mathee, K. Beta-lactam antibiotics: From antibiosis to resistance and bacteriology. APMIS 2010, 118, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Wilke, M.S.; Lovering, A.L.; Strynadka, N.C.J. β-Lactam antibiotic resistance: A current structural perspective. Curr. Opin. Microbiol. 2005, 8, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Mechanisms of Resistance and Clinical Relevance of Resistance to β-Lactams, Glycopeptides, and Fluoroquinolones. Mayo Clin. Proc. 2012, 87, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.C.; Asgian, J.L.; Ekici, O.D.; James, K.E. Irreversible Inhibitors of Serine, Cysteine, and Threonine Proteases. Chem. Rev. 2002, 102, 4639–4750. [Google Scholar] [CrossRef] [PubMed]
- Hubschwerlen, C. β-Lactam Antibiotics. In Comprehensive Medicinal Chemistry, 2nd ed.; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Oxford, UK, 2007; pp. 479–517. [Google Scholar]
- Lakshmi, R.; Nusrin, K.S.; Ann, G.S.; Sreelakshmi, K.S. Role of Beta Lactamases in Antibiotic Resistance: A Review. Int. Res. J. Pharm. 2014, 5, 37–40. [Google Scholar] [CrossRef]
- Drawz, S.M.; Bonomo, R.A. Three Decades of β-Lactamase Inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–199. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Dis. 2013, 12, 371–386. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Zanfardino, A.; Notomista, E.; Wichelhaus, T.A.; Saturnino, C.; Varcamonti, M.; Soriente, A. Novel promising linezolid analogues: Rational design, synthesis and biological evaluation. Eur. J. Med. Chem. 2013, 69, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Y.; Du, D.-M.; Xu, J. Do Reaction Conditions Affect the Stereoselectivity in the Staudinger Reaction? J. Org. Chem. 2006, 71, 6983–6990. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, H. Ketenes. 1. Diphenylketene. Justus Liebigs Ann. Chem. 1907, 356, 51–123. [Google Scholar] [CrossRef]
- Jiao, L.; Liang, Y.; Xu, J. Origin of the Relative Stereoselectivity of the β-Lactam Formation in the Staudinger Reaction. J. Am. Chem. Soc. 2006, 128, 6060–6069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, H.; Zhou, J.; Zhang, H.; Ji, J.; Huang, W. Synthesis and Bioactivities of 2-Azetidinone Derivatives as Cholesterol Absorption Inhibitors. Med. Chem. 2011, 7, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Page, M.G.P. β-Lactam Antibiotics. In Antibiotic Discovery and Development; Dougherthy, T.J., Pucci, M.J., Eds.; Springer: US, 2012; Volume XVII, pp. 79–117. [Google Scholar]
- Arya, N.; Jagdale, A.Y.; Patil, T.A.; Yeramwar, S.S.; Holikatti, S.S.; Dwivedi, J.; Shishoo, C.J.; Jain, K.S. The chemistry and biological potential of azetidin-2-ones. Eur. J. Med. Chem. 2014, 74, 619–656. [Google Scholar] [CrossRef] [PubMed]
- Burchacka, E.; Walczak, M.; Sienczyk, M.; Dubin, G.; Zdzalik, M.; Potempa, J.; Oleksyszyn, J. The development of first Staphylococcus aureus SpIB protease inhibitors: Phosphonic analogues of glutamine. Bioorg. Med. Chem. 2012, 22, 5574–5578. [Google Scholar]
- Regourd, J.; Al-Sheikh Ali, A.; Thompson, A. Synthesis and Anti-Cancer Activity of C-Ring-Functionalized Prodigiosin Analogues. J. Med. Chem. 2007, 50, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.N.; Hamad, K.J.; Al-Joroshi, S.H. Synthesis and characterization of some Schiff bases. Asian J. Chem. 2006, 18, 2404–2406. [Google Scholar]
- Denmark, S.E.; Nakajima, N.; Stiff, C.M.; Nicaise, O.J.-C.; Kranz, M. Studies on the Bisoxazoline and (-)-Sparteine Mediated Enantioselective Addition of Organolithium Reagents to Imines. Adv. Synth. Catal. 2008, 350, 1023–1045. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Tseng, W.N.; Patel, H.V.; Wong, F.F.; Horng, D.-N.; Liaw, B.R.; Lin, L.C. Mono-deoxygenation of Nitroalkanes, Nitrones, and Heterocyclic N-Oxides by Hexamethyldisilane through 1,2-Elimination: Concept of “Counterattack Reagent”. J. Org. Chem. 1999, 64, 2211–2118. [Google Scholar] [CrossRef]
- Zarei, M.; Mohamadzadeh, M. 3-Thiolaterd 2-azetidinones: synthesis and in vitro antibacterial and antifungal activities. Tetrahedron 2011, 67, 5832–5840. [Google Scholar] [CrossRef]
- Favre, A.; Grugier, J.; Brans, A.; Joris, B.; Marchand-Brynaert, J. 6-Aminopenicillanic acid (6-APA) derivatives equipped with anchoring arms. Tetrahedron 2012, 68, 10818–10826. [Google Scholar] [CrossRef]
- Josephine, H.R.; Charlier, P.; Davies, C.; Nicholas, R.A.; Pratt, R.F. Reactivity of Penicillin-Binding Proteins with Peptidoglycan-Mimetic β-Lactams: What’s Wrong with These Enzymes? Biochemistry 2006, 45, 15873–15883. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 5th ed.; Approved Standard. NCCLS Document M7-A5; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2001. [Google Scholar]
- Promega. http://www.promega.com/products (acessed on 4 December 2015).
- DSMZ. Available online: http://www.dsmz.de/ (accessed on 4 December 2015).
- Sample Availability: Samples of the compounds are available upon request.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rosa, M.; Vigliotta, G.; Palma, G.; Saturnino, C.; Soriente, A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules 2015, 20, 22044-22057. https://doi.org/10.3390/molecules201219828
De Rosa M, Vigliotta G, Palma G, Saturnino C, Soriente A. Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules. 2015; 20(12):22044-22057. https://doi.org/10.3390/molecules201219828
Chicago/Turabian StyleDe Rosa, Margherita, Giovanni Vigliotta, Giuseppe Palma, Carmela Saturnino, and Annunziata Soriente. 2015. "Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation" Molecules 20, no. 12: 22044-22057. https://doi.org/10.3390/molecules201219828
APA StyleDe Rosa, M., Vigliotta, G., Palma, G., Saturnino, C., & Soriente, A. (2015). Novel Penicillin-Type Analogues Bearing a Variable Substituted 2-Azetidinone Ring at Position 6: Synthesis and Biological Evaluation. Molecules, 20(12), 22044-22057. https://doi.org/10.3390/molecules201219828