Simultaneous Determination of Eight Ginsenosides in Rat Plasma by Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry: Application to Their Pharmacokinetics
Abstract
:1. Introduction
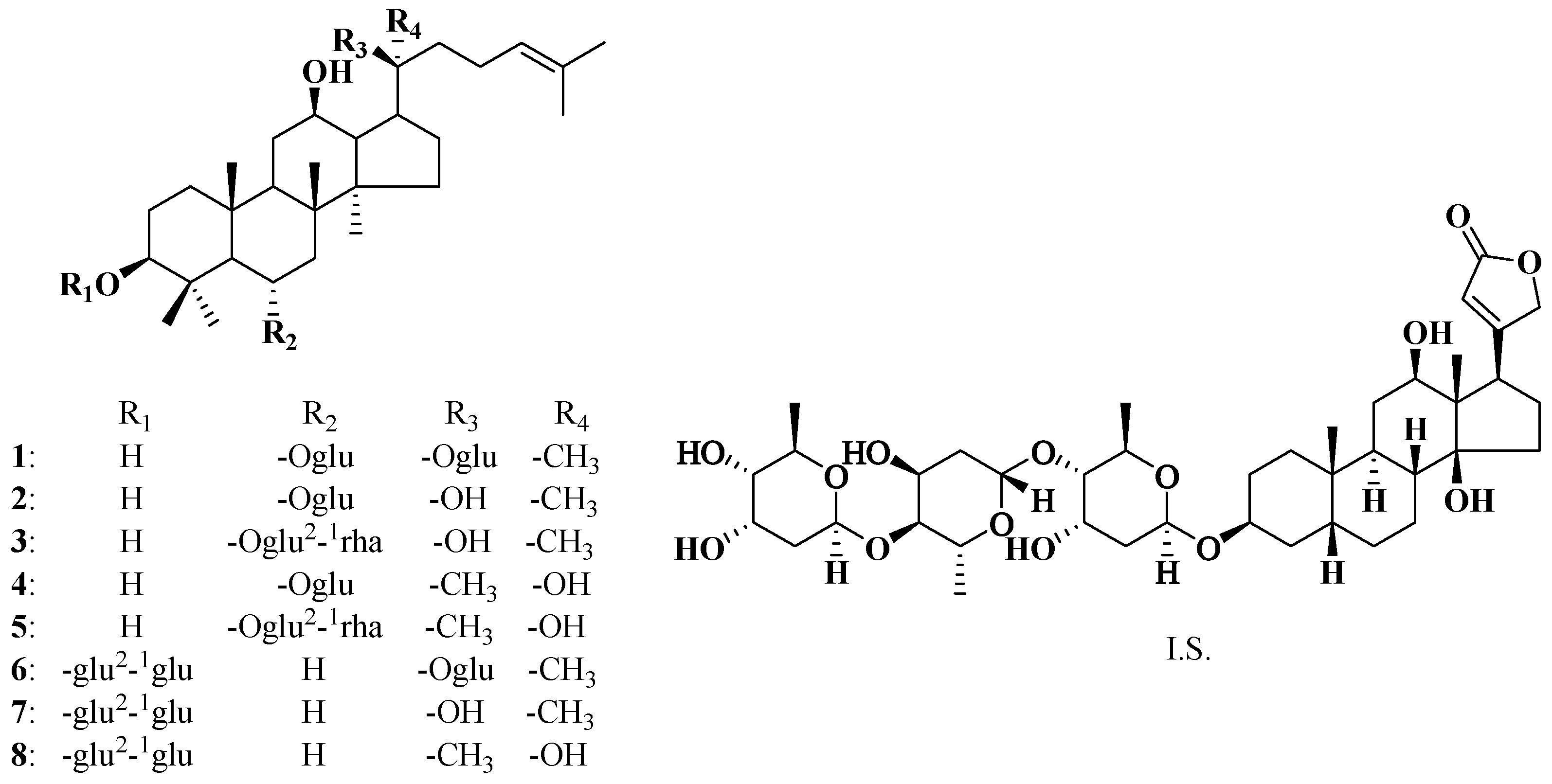
2. Results and Discussion
2.1. Identification and Determination of Ginsenosides in Rat Plasma
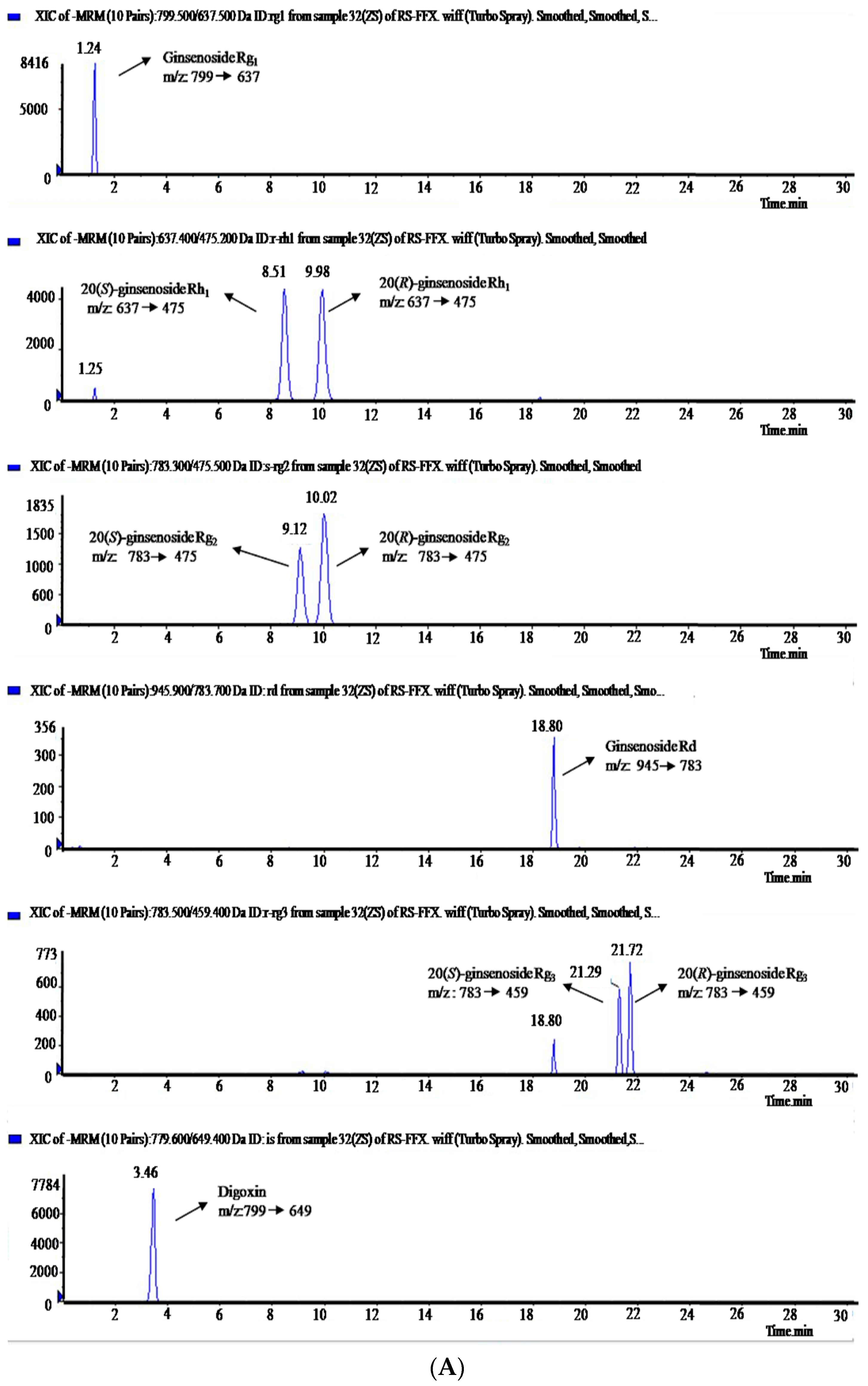
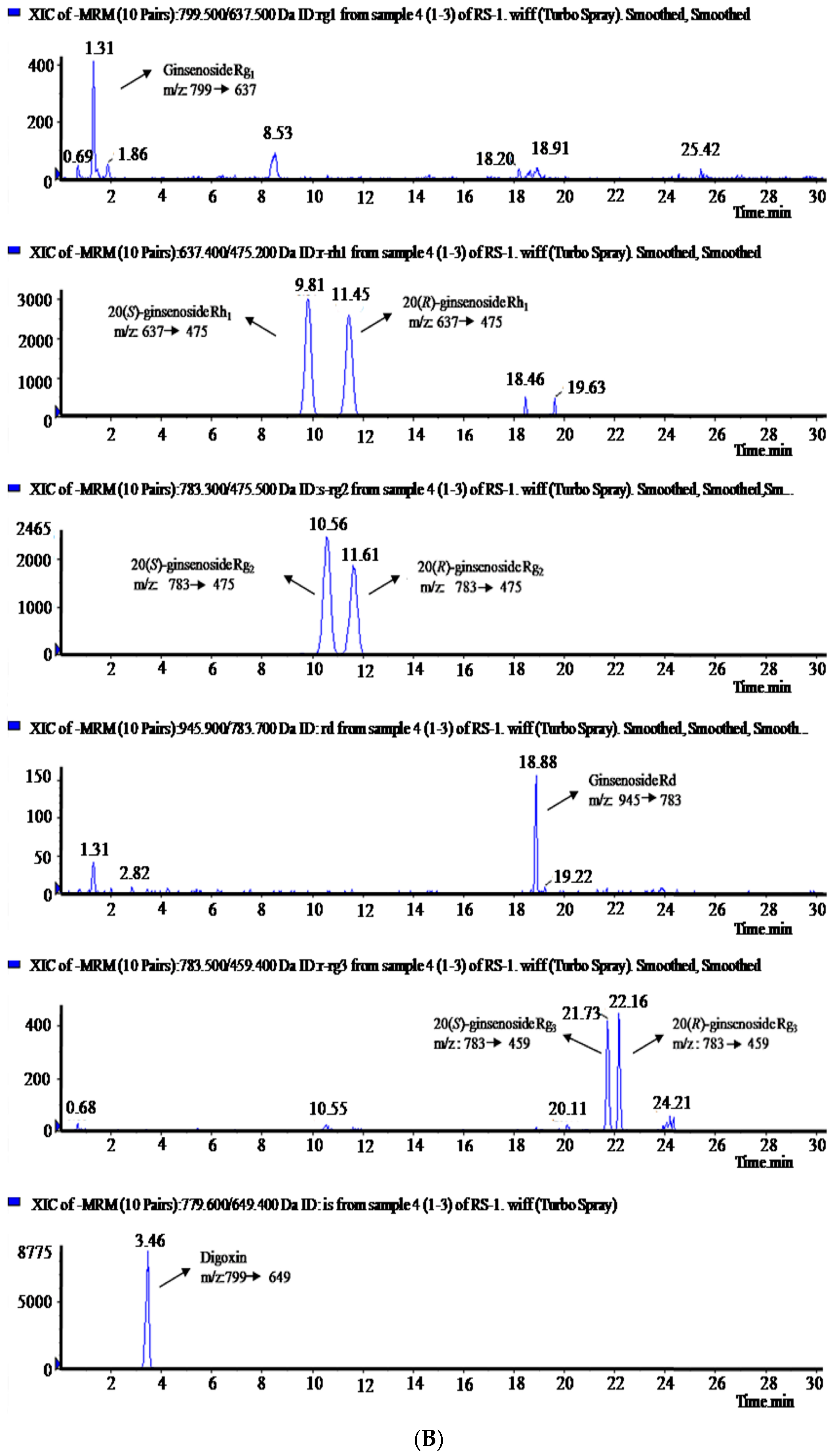
2.2. Method Validation
2.2.1. Selectivity and Linearity
| Analyte | RT (min) | Calibration Curve a | Correlation Coefficient (r2) | Linear Range (ng/mL) | LLOD (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|---|---|
| 1 | 1.24 | y = 0.0101x + 0.0032 | 0.9935 | 0.45–126.60 | 0.12 | 0.45 |
| 2 | 8.51 | y = 0.0122x + 0.0362 | 0.9909 | 0.30–85.54 | 0.07 | 0.30 |
| 3 | 9.12 | y = 0.0028x + 0.0127 | 0.9943 | 0.45–129.60 | 0.12 | 0.45 |
| 4 | 9.98 | y = 0.0105x + 0.0487 | 0.9959 | 0.21–59.62 | 0.05 | 0.21 |
| 5 | 10.02 | y = 0.006x + 0.0409 | 0.9903 | 0.34–98.50 | 0.08 | 0.34 |
| 6 | 18.80 | y = 0.0036x + 0.005 | 0.9921 | 0.95–182.40 | 0.27 | 0.95 |
| 7 | 21.29 | y = 0.0008x + 0.0022 | 0.9980 | 0.32–93.31 | 0.08 | 0.32 |
| 8 | 21.72 | y = 0.0009x + 0.0114 | 0.9910 | 0.38–108.86 | 0.09 | 0.38 |
2.2.2. Precision, Accuracy and Stability
| Ginsenoside | Spiked (ng/mL) | Recovery | Matrix Effects | ||
|---|---|---|---|---|---|
| Measured (%) | RSD (%) | Measured (%) | RSD (%) | ||
| 1 | 64.80 | 89.61 | 8.21 | 85.88 | 8.18 |
| 16.20 | 88.52 | 12.86 | 86.98 | 7.67 | |
| 0.45 | 77.98 | 16.21 | 85.31 | 12.70 | |
| 2 | 42.77 | 95.68 | 8.00 | 88.88 | 14.54 |
| 10.69 | 90.94 | 12.27 | 85.28 | 14.24 | |
| 0.30 | 84.77 | 11.65 | 86.33 | 12.63 | |
| 3 | 64.80 | 78.08 | 10.02 | 86.69 | 13.70 |
| 16.20 | 83.79 | 12.51 | 87.06 | 14.16 | |
| 0.45 | 75.69 | 15.77 | 86.50 | 10.95 | |
| 4 | 29.81 | 95.59 | 12.15 | 84.11 | 10.91 |
| 7.45 | 83.75 | 13.63 | 85.07 | 14.23 | |
| 0.21 | 79.82 | 14.22 | 85.77 | 9.59 | |
| 5 | 49.25 | 84.93 | 8.13 | 88.21 | 11.95 |
| 12.31 | 87.84 | 10.95 | 85.79 | 11.83 | |
| 0.34 | 77.62 | 15.07 | 87.42 | 9.88 | |
| 6 | 91.20 | 84.03 | 4.81 | 86.04 | 12.48 |
| 22.80 | 83.20 | 11.74 | 85.64 | 13.25 | |
| 0.95 | 79.05 | 8.68 | 85.90 | 11.45 | |
| 7 | 46.66 | 82.09 | 12.97 | 88.90 | 14.06 |
| 11.66 | 79.91 | 11.51 | 93.90 | 8.68 | |
| 0.32 | 78.19 | 12.14 | 85.40 | 12.40 | |
| 8 | 54.43 | 80.04 | 8.76 | 85.60 | 15.38 |
| 13.61 | 77.51 | 11.96 | 87.22 | 11.44 | |
| 0.38 | 84.78 | 12.15 | 87.34 | 9.93 | |
2.3. Pharmacokinetics of GTSSL
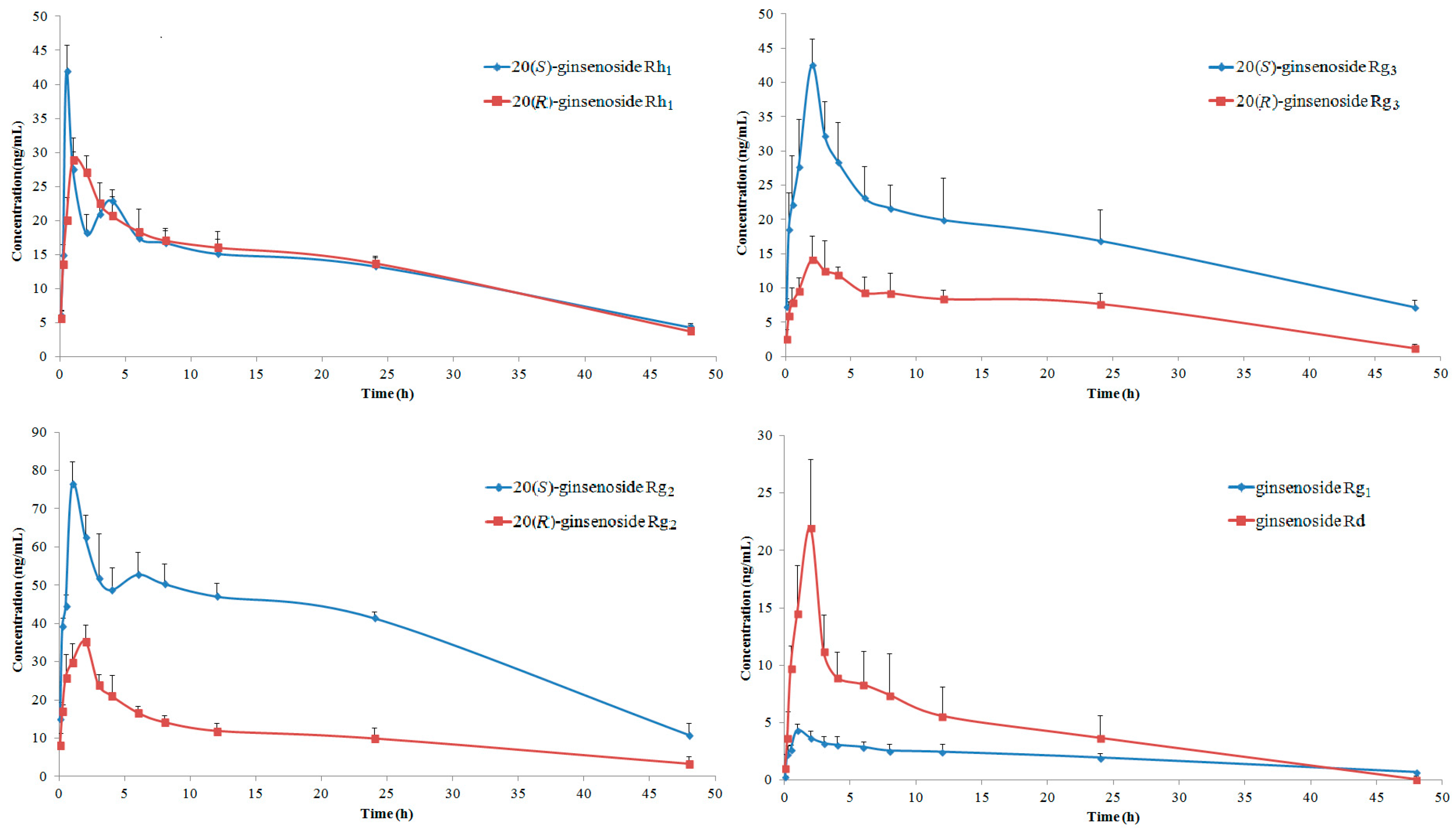
| Ginsenoside | AUC0→t (μg h/L) | AUC0→∞ (μg h/L) | MRT0→t (h) | MRT0→∞ (h) | t1/2 (h) | tmax (h) | Cmax (μg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 88.77 ± 22.08 | 105.64 ± 18.32 | 9.78 ± 0.48 | 12.70 ± 2.98 | 14.99 ± 3.77 | 2 | 4.32 ± 1.09 |
| 2 | 622.92 ± 46.37 | 753.19 ± 48.85 | 17.06 ± 0.14 | 27.61 ± 1.67 | 20.61 ± 1.18 | 0.5 | 41.99 ± 3.82 |
| 3 | 1778.47 ± 86.07 | 2302.22 ± 536.47 | 17.43 ± 0.55 | 25.65 ± 4.02 | 22.24 ± 9.88 | 1 | 76.42 ± 5.90 |
| 4 | 616.55 ± 49.28 | 716.65 ± 92.94 | 17.05 ± 0.67 | 24.78 ± 3.58 | 17.68 ± 2.99 | 1 | 29.74 ± 2.71 |
| 5 | 511.51 ± 63.84 | 617.80 ± 156.78 | 15.63 ± 1.92 | 25.55 ± 10.45 | 19.05 ± 8.05 | 2 | 35.68 ± 4.04 |
| 6 | 218.10 ± 18.69 | 221.75 ± 16.57 | 12.43 ± 1.46 | 13.46 ± 2.11 | 7.30 ± 3.32 | 2 | 22.05 ± 2.21 |
| 7 | 778.63 ± 56.72 | 922.70 ± 119.85 | 16.50 ± 1.02 | 23.83 ± 2.09 | 19.11 ± 3.34 | 2 | 42.91 ± 3.55 |
| 8 | 302.59 ± 32.45 | 311.84 ± 30.37 | 15.43 ± 0.74 | 16.91 ± 1.39 | 9.93 ± 2.34 | 2 | 15.42 ± 3.38 |
3. Experimental Section
3.1. Materials and Reagents
3.2. Animals
3.3. Instrumental and Chromatographic Conditions
3.4. Identification and Determination of Ginsenosides in Blood Samples
3.4.1. Preparation of Blood Samples
3.4.2. Preparation of Stock Solutions, Calibration Samples and Quality Control Samples
3.5. Method Validation
3.5.1. Linearity and Selectivity
3.5.2. Precision and Accuracy
3.5.3. Extraction Recovery and Matrix Effects
3.5.4. Stability
3.6. Application
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, J.Q.; Zhang, X.S.; Qu, F.Z.; Guo, Z.H.; Zhao, Y.Q. Dammarane triterpenoids for pharmaceutical use: A patent review (2005–2014). Expert Opin. Ther. Pat. 2015, 25, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Yang, X.W. A new triterpene natural product from stems and leaves of Panax ginseng. Chin. Tradit. Herb. Drugs 2015, 46, 169–173. [Google Scholar]
- Li, K.K.; Yang, X.B.; Yang, X.W.; Liu, J.X.; Gong, X.J. New triterpenoids from the stems and leaves of Panax ginseng. Fitoterapia 2012, 83, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Yao, C.M.; Yang, X.W. Four new dammarane-type triterpene saponins from the stems and leaves of Panax ginseng and their cytotoxicity on HL-60 cells. Planta Med. 2012, 78, 189–192. [Google Scholar]
- Yang, X.W. Complete assignment of 1H- and 13C-NMR chemical shifts of 20(R)-ginsenoside Rg2 and 20(S)-ginsenoside Rg2. Chin. J. Magn. Reson. 2000, 17, 9–15. [Google Scholar]
- Wang, H.P.; Yang, X.B.; Yang, X.W.; Liu, J.X.; Wang, Y.P.; Zhang, L.X. Chemical constituents from roots and rhizomes of Panax ginseng cultivated in Jilin province. China J. Chin. Mater. Med. 2013, 38, 2807–2817. [Google Scholar]
- Wang, H.P.; Yang, X.B.; Yang, X.W.; Liu, J.X.; Xu, W.; Zhang, Y.B.; Zhang, L.X.; Wang, Y.P. Ginsenjilinol, a new protopanaxatriol type saponin with inhibitory activity on LPS-activated NO production in macrophage RAW 264.7 cells from the roots and rhizomes of Panax ginseng. J. Asian Nat. Prod. Res. 2013, 15, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.J.; Yang, X.W.; Li, L.Y.; Tian, J.M. Simultaneous enantiomer determination of 20(R)- and 20(S)-ginsenoside-Rg2 in rat plasma after intravenous administration using HPLC method. J. Chromatogr. B 2007, 850, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, J.J.; Eun, S.H.; Kim, D.H. Anti-inflammatory effects of ginsenoside Rg1 and its metabolites ginsenoside Rh1 and 20(S)-protopanaxatriol in mice with TNBS-induced colitis. Eur. J. Pharmacol. 2015, 762, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Gao, Y.; Chu, S.F.; Zhang, Z.; Xia, C.Y.; Mou, Z.; Song, X.Y.; He, W.B.; Guo, X.F.; Chen, N.H. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol- and CCl4-induced hepatic fibrosis. Acta Pharmacol. Sin. 2014, 35, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.H.; Zhang, J.T. Ginsenoside Rg1 promotes proliferation of hippocampal progenitor cells. Neurol. Res. 2004, 26, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Lin, J.N.; Pei, Y.P.; Chen, Y.J.; Liu, C.M.; Xu, S.X.; Yao, X.S. Studies on the antiarrhythmic constituents from the rhizomes of Panax ginseng C.A. Meyer. J. Shenyang Coll. Pharm. 1992, 9, 103–105. [Google Scholar]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kim, Y.J.; Yang, D.C. Ginsenoside Rh1 induces mouse osteoblast growth and differentiation through the bone morphogenetic protein 2/runt-related gene 2 signalling pathway. J. Pharm. Pharmacol. 2014, 66, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Zhou, Q.L.; Yang, X.W. New SIRT1 activator from alkaline hydrolysate of total saponins in the stems-leaves of Panax ginseng. Bioorg. Med. Chem. Lett. 2015, 25, 5321–5325. [Google Scholar] [CrossRef] [PubMed]
- Siraj, F.M.; Natarajan, S.; Kim, Y.J.; Chun Yang, D. In silico screening of ginsenoside Rh1 with PPARγ and in vitro analysis on 3T3-L1 cell line. Mol. Simul. 2015, 41, 1219–1226. [Google Scholar] [CrossRef]
- Gai, Y.S.; Ma, Z.G.; Yu, X.F.; Qu, S.C.; Sui, D.Y. Effect of ginsenoside Rh1 on myocardial injury and heart function in isoproterenol-induced cardiotoxicity in rats. Toxicol. Mech. Methods 2012, 22, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Chen, J.; Chu, S.F.; Wang, Y.S.; Wang, X.Y.; Chen, N.H.; Zhang, J.T. Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1’s metabolites ginsenoside Rh1 and protopanaxatriol. J. Pharmacol. Sci. 2009, 109, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Lü, W.W.; Liu, J.; Tian, J.M.; Li, L.Y.; Ye, J.M.; Zhang, Z.W. Effect of 20-(S) and 20-(R)-ginsenoside Rg2 on hemodynamics and blood oxygen content of cardiogenic shock canine. Chin. Tradit. Herb. Drugs 2003, 34, 254–256. [Google Scholar]
- Zhang, Z.W.; Zhao, Y.J.; Ye, J.M.; Tian, J.M.; Guo, W.F.; Li, H.; Li, L.Y.; Jin, Y. Effects of ginsenoside Rg2 on myocardial damage induced by endotoxic diffuse intravascular coagulation and hemorrheology. Chin. Tradit. Herb. Drugs 2002, 33, 814–816. [Google Scholar]
- Liu, J.; Sun, W.J.; Lü, W.W.; Ye, J.M.; Li, L.Y. Comparison of cardiotonic and toxic effects between ginsenoside Rg2 and strophanthin K. Chin. Tradit. Herb. Drugs 2001, 32, 809–811. [Google Scholar]
- Liu, J.F.; Yan, X.D.; Li, L.; Li, Y.; Zhou, L.F.; Zhang, X.H.; Hu, X.H.; Zhao, G. Ginsenoside Rd improves learning and memory ability in APP transgenic mice. J. Mol. Neurosci. 2015, 57, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Yan, X.D.; Qi, L.S.; Li, L.; Hu, G.Y.; Li, P.; Zhao, G. Ginsenoside Rd attenuates Aβ25-35-induced oxidative stress and apoptosis in primary cultured hippocampal neurons. Chem. Biol. Interact. 2015, 239, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, M.; Bjoras, M.; Wang, W.; Zhang, G.Y.; Han, J.L.; Liu, Z.R.; Zhang, Y.X.; Wang, B.; Chen, J.; et al. Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front. Pharmacol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.J.; Chun, J.; Ha, Y.W.; Ko, H.J.; Xu, M.Y.; Kim, Y.S. Novel roles of ginsenoside Rg3 in apoptosis through downregulation of epidermal growth factor receptor. Chem. Biol. Interact. 2015, 233, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; Zhao, L.; Chen, W.; Hou, H.L.; Ye, Z.X.; Li, X. Ginsenoside 20(S)-Rg3 inhibits the Warburg effect through STAT3 pathways in ovarian cancer cells. Int. J. Oncol. 2015, 46, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Chen, L.L.; Wang, T.; Jiang, X.Q.; Zhang, H.; Li, P.; Lv, B.; Gao, X.M. Ginsenoside Rg3 antagonizes adriamycin-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine 2015, 22, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.E.; Liu, X.W.; Shi, L.; Zhang, Y.B.; Xu, T.T.; Gao, Y.; Hou, M.X. Effects of 20(R)-ginsenoside Rg3 on apoptosis of human glioma U87 cells. J. Chengdu Med. Coll. 2014, 9, 4–7. [Google Scholar]
- Kim, D.Y.; Yuan, H.D.; Huang, B.; Quan, H.Y.; Chung, S.H. Ginsenoside 20(R)-Rg3 stimulates glucose uptake in C2C12 myotubes via CaMKK-AMPK pathways. Food Sci. Biotechnol. 2010, 19, 1277–1282. [Google Scholar] [CrossRef]
- Guidance for Industry: Bioanalytical Method Validation. In Food and Drug Administration; Silver Spring, MD, USA, 2001; pp. 4–10. Available online: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070107.pdf (accessed on 10 October 2015).
- Saito, H.; Morita, M.; Takagi, K. Pharmacological studies of Panax ginseng leaves. Jpn. J. Pharmacol. 1973, 23, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.T.; Wang, G.J.; Sun, J.G.; Tucker, I.; Zhao, X.C.; Xie, Y.Y.; Li, H.; Jiang, X.L.; Wang, R.; Xu, M.J.; et al. High performance liquid chromatographic-mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J. Chromatogr. B 2005, 818, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.J.; Wang, G.J.; Xie, H.T.; Wang, R.; Wang, W.; Li, X.Y.; Sun, J.G. Determination of ginsenoside Rg2 in rat plasma by high-performance liquid chromatography-mass spectrometry after solid-phase extraction. Anal. Lett. 2006, 39, 113–126. [Google Scholar] [CrossRef]
- Wang, W.; Wang, G.J.; Xie, H.T.; Sun, J.G.; Zhao, S.; Jiang, X.L.; Li, H.; Lv, H.; Xu, M.J.; Wang, R. Determination of ginsenoside Rd in dog plasma by liquid chromatography-mass spectrometry after solid-phase extraction and its application in dog pharmacokinetics studies. J. Chromatogr. B 2007, 852, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Deng, Y.H.; Xu, S.J.; Zeng, X. In vivo pharmacokinetic and metabolism studies of ginsenoside Rd. J. Chromatogr. B 2007, 854, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, L.; Hu, C.J.; Jiang, X.H. Pharmacokinetics, tissue distribution, metabolism, and excretion of ginsenoside Rg1 in rats. Arch. Pharm. Res. 2010, 33, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.X.; Cai, Z.W.; Wong, R.N.S.; Mak, N.K.; Jiang, Z.H. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J. Chromatogr. B 2005, 816, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zou, H.F.; Kong, L.; Zhang, Y.K.; Pang, H.; Su, C.Y.; Liu, G.Y.; Hui, M.; Fu, L. Determination of ginsenoside Rg3 in plasma by solid-phase extraction and high-performance liquid chromatography for pharmacokinetic study. J. Chromatogr. B 1999, 731, 403–409. [Google Scholar] [CrossRef]
- Ma, L.Y.; Yang, X.W. Six new dammarane-type triterpenes from acidic hydrolysate of the stems-leaves of Panax ginseng and their inhibitory–activities against three human cancer cell lines. Phytochem. Lett. 2015, 13, 406–412. [Google Scholar] [CrossRef]
- Ma, L.Y.; Yang, X.W. Chemical constituents in acid hydrolysates of total saponins from stems and leaves of Panax ginseng. Chin. Tradit. Herb. Drugs 2015, 46, 2522–2533. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.-Y.; Zhang, Y.-B.; Zhou, Q.-L.; Yang, Y.-F.; Yang, X.-W. Simultaneous Determination of Eight Ginsenosides in Rat Plasma by Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry: Application to Their Pharmacokinetics. Molecules 2015, 20, 21597-21608. https://doi.org/10.3390/molecules201219790
Ma L-Y, Zhang Y-B, Zhou Q-L, Yang Y-F, Yang X-W. Simultaneous Determination of Eight Ginsenosides in Rat Plasma by Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry: Application to Their Pharmacokinetics. Molecules. 2015; 20(12):21597-21608. https://doi.org/10.3390/molecules201219790
Chicago/Turabian StyleMa, Li-Yuan, You-Bo Zhang, Qi-Le Zhou, Yan-Fang Yang, and Xiu-Wei Yang. 2015. "Simultaneous Determination of Eight Ginsenosides in Rat Plasma by Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry: Application to Their Pharmacokinetics" Molecules 20, no. 12: 21597-21608. https://doi.org/10.3390/molecules201219790
APA StyleMa, L.-Y., Zhang, Y.-B., Zhou, Q.-L., Yang, Y.-F., & Yang, X.-W. (2015). Simultaneous Determination of Eight Ginsenosides in Rat Plasma by Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry: Application to Their Pharmacokinetics. Molecules, 20(12), 21597-21608. https://doi.org/10.3390/molecules201219790






