Synthesis and Biological Evaluation of Norcantharidin Derivatives Possessing an Aromatic Amine Moiety as Antifungal Agents
Abstract
:1. Introduction

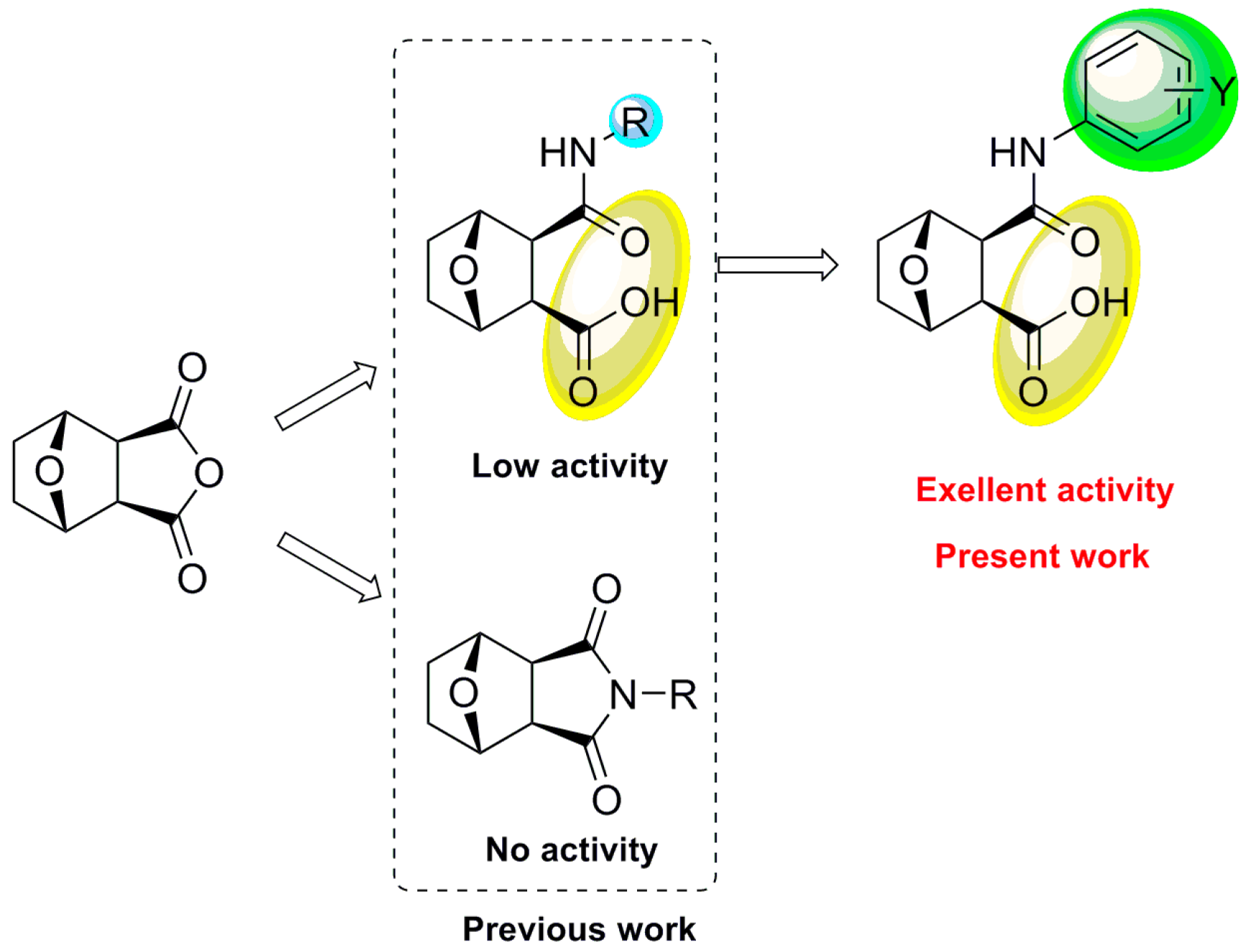
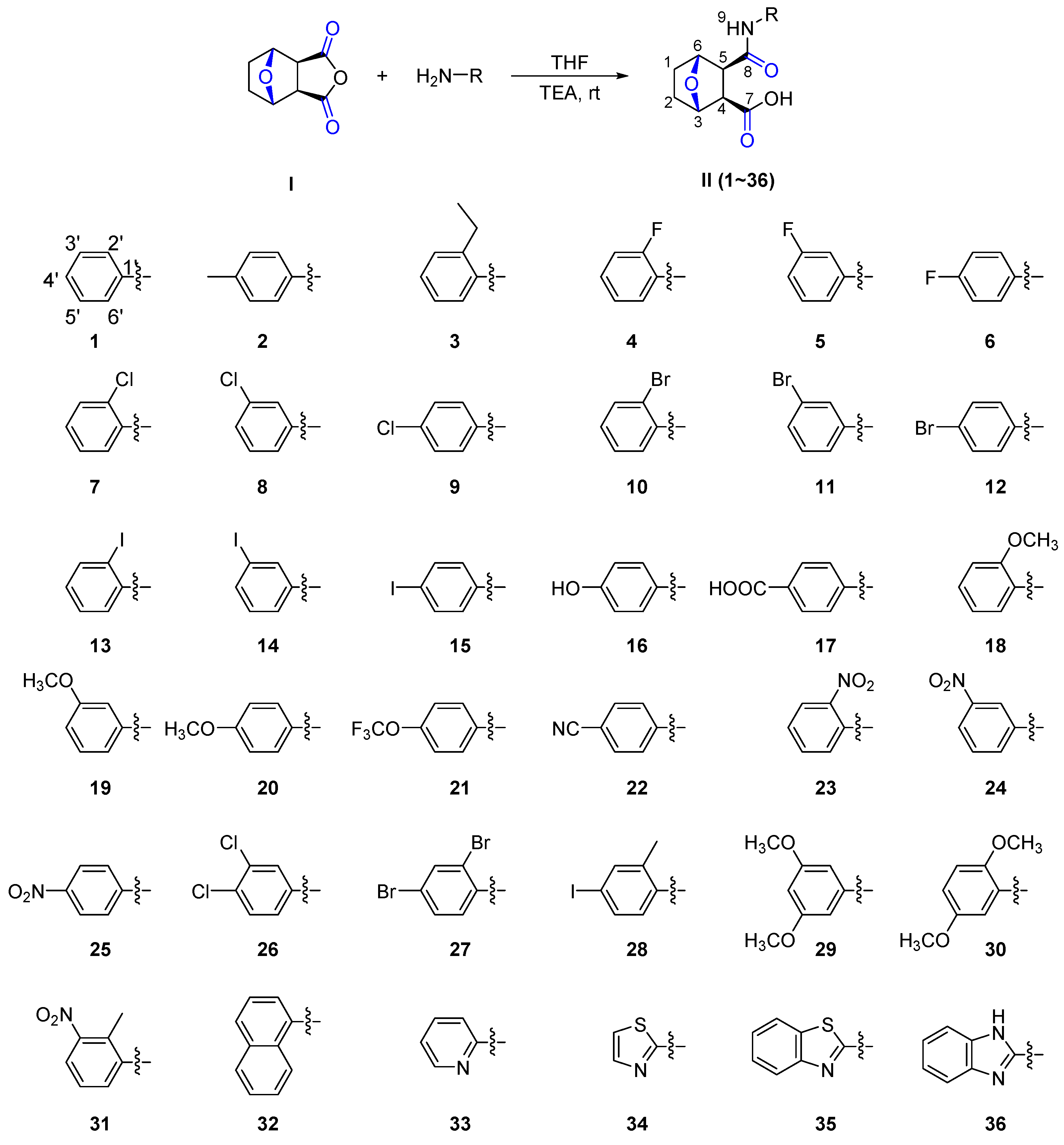
2. Results and Discussion
2.1. Chemistry
2.2. Antifungal Activity
| Compd. | Values of Inhibition Rate (%) to Eight Pathogens | |||||||
|---|---|---|---|---|---|---|---|---|
| Valsa mali | Botryosphaeria berengeriana | Sclerotinia fructigena | Glomerella cingulate | Alternaria alternate | Sclerotinia sclerotiorum | Alternaria solani | Cochliobolus sativum | |
| II-1 | 68.42 ± 1.99d * | 68.64 ± 0.65e | 72.20 ± 0.66e | 62.35 ± 0.99f | 65.52 ± 1.06de | 76.39 ± 0.92e | 61.10 ± 0.96e | 67.01 ± 0.59c |
| II-2 | 0.66 ± 0.06q | 1.27 ± 0.03qr | 0.53 ± 0.06qr | 5.38 ± 0.05tu | 9.66 ± 0.06no | 23.61 ± 0.06p | 1.52 ± 0.10u | 7.98 ± 0.06qr |
| II-3 | 18.42 ± 0.06mn | 11.02 ± 0.10l | 18.72 ± 0.06m | 6.99 ± 0.06st | 11.72 ± 0.06n | 20.17 ± 0.00q | 1.01 ± 0.06u | 10.11 ± 0.06pqr |
| II-4 | 74.99 ± 1.30c | 69.90 ± 1.17e | 76.48 ± 1.63d | 80.66 ± 1.18d | 70.34 ± 1.41c | 86.69 ± 0.85d | 76.77 ± 0.26c | 66.50 ± 0.30c |
| II-5 | 83.56 ± 1.05b | 87.70 ± 0.91c | 85.03 ± 1.79bc | 90.34 ± 2.64b | 84.82 ± 1.30a | 92.27 ± 0.06c | 83.35 ± 1.16b | 81.39 ± 0.38b |
| II-6 | 41.45 ± 0.47e | 39.39 ± 1.61f | 49.21 ± 1.37f | 55.40 ± 1.36g | 41.38 ± 2.14g | 46.34 ± 2.29f | 47.46 ± 1.21f | 51.59 ± 0.76e |
| II-7 | 34.21 ± 1.00f | 22.86 ± 1.85h | 45.47 ± 2.26g | 24.74 ± 0.83h | 38.62 ± 1.04g | 35.19 ± 1.78g | 30.29 ± 0.92g | 35.06 ± 1.80f |
| II-8 | 86.85 ± 1.00a | 91.54 ± 1.33b | 100.00 ± 0.00a | 92.96 ± 2.69a | 86.90 ± 2.32a | 100.00 ± 0.00a | 89.90 ± 0.79a | 91.50 ± 0.66a |
| II-9 | 32.88 ± 3.85f | 22.01 ± 2.89h | 28.36 ± 2.69h | 24.18 ± 1.08h | 30.34 ± 3.13h | 26.60 ± 2.79i | 28.27 ± 1.83hi | 17.53 ± 0.90j |
| II-10 | 25.01 ± 3.06g | 18.18 ± 3.79i | 23.00 ± 2.51i | 21.48 ± 1.28i | 23.44 ± 2.27i | 30.46 ± 3.04h | 18.17 ± 2.45j | 21.21 ± 2.53i |
| II-11 | 80.92 ± 1.26b | 81.77 ± 0.90d | 83.42 ± 2.49c | 84.97 ± 1.47c | 78.63 ± 2.28b | 96.13 ± 1.32b | 79.83 ± 1.81bc | 83.56 ± 1.80b |
| II-12 | 20.39 ± 1.04h | 15.24 ± 3.23i | 17.13 ± 2.59j | 18.25 ± 1.37j | 22.07 ± 3.14i | 20.59 ± 2.45j | 25.23 ± 1.87i | 13.78 ± 1.91k |
| II-13 | 15.14 ± 1.23i | 10.99 ± 2.47j | 22.46 ± 1.62i | 12.35 ± 0.59k | 17.22 ± 4.17j | 15.87 ± 1.37k | 16.16 ± 2.31j | 9.54 ± 1.22l |
| II-14 | 65.79 ± 1.00d | 66.51 ± 1.95e | 70.60 ± 2.19e | 60.77 ± 0.78f | 62.76 ± 2.13e | 73.80 ± 3.43e | 58.60 ± 1.33e | 63.86 ± 1.01d |
| II-15 | 3.29 ± 1.16j | −5.12 ± 3.86k | 1.07 ± 0.92k | 5.37 ± 0.86l | 3.44 ± 1.18k | 8.15 ± 1.43l | 11.10 ± 6.18k | −2.71 ± 1.98m |
| II-16 | 9.21 ± 0.10p | 0.85 ± 0.04pqr | 8.56 ± 0.06p | 9.14 ± 0.03rs | 10.34 ± 0.06no | 6.87 ± 0.06t | 2.02 ± 0.10u | 5.85 ± 0.10s |
| II-17 | 0.66 ± 0.06q | 0.42 ± 0.06qr | −0.53 ± 0.06r | 0.54 ± 0.06wx | 1.38 ± 0.06pq | 0.43 ± 0.08u | 7.58 ± 0.12s | 12.23 ± 0.10nopq |
| II-18 | 1.97 ± 0.06q | 6.36 ± 0.06mn | 0.00 ± 0.00qr | 0.00 ± 0.00x | 8.28 ± 0.06no | 0.00 ± 0.06u | 0.00 ± 0.00u | 0.00 ± 0.00t |
| II-19 | 0.66 ± 0.06q | 10.17 ± 0.06l | 2.67 ± 0.06qr | 11.83 ± 0.05pq | 15.86 ± 0.06m | 0.00 ± 0.06u | 2.53 ± 0.00u | 11.70 ± 0.06opq |
| II-20 | 0.66 ± 0.06q | 2.12 ± 0.10pqr | 0.53 ± 0.06qr | 0.00 ± 0.00x | 2.07 ± 0.06pq | 0.00 ± 0.06u | 0.00 ± 0.00u | 0.00 ± 0.00t |
| II-21 | 1.32 ± 0.10q | 10.59 ± 0.06l | 11.76 ± 0.10o | 15.59 ± 0.06o | 20.69 ± 0.06ijk | 13.30 ± 0.06s | 21.72 ± 0.06lm | 22.87 ± 0.06hi |
| II-22 | 24.34 ± 0.06k | 0.00 ± 0.066r | 8.02 ± 0.11p | 12.90 ± 0.05p | 17.93 ± 0.06jklm | 18.45 ± 0.06q | 22.73 ± 0.06kl | 12.23 ± 0.03nopq |
| II-23 | 19.74 ± 0.06m | 0.00 ± 0.00r | 9.09 ± 0.11p | 3.23 ± 0.03uv | 0.69 ± 0.10pq | 13.73 ± 0.10t | 2.53 ± 0.03u | 11.17 ± 0.06opq |
| II-24 | 25.00 ± 0.10q | 5.08 ± 0.06n | 18.18 ± 0.10m | 20.43 ± 0.06m | 28.28 ± 0.06h | 19.31 ± 0.06q | 7.07 ± 0.03st | 15.43 ± 0.10lm |
| II-25 | 23.03 ± 0.10kl | 2.54 ± 0.11pq | 12.30 ± 0.06o | 17.20 ± 0.05no | 21.38 ± 0.10ij | 7.73 ± 0.06u | 3.54 ± 0.03tu | 6.38 ± 0.11rs |
| II-26 | 32.89 ± 0.10j | 18.22 ± 0.06k | 48.13 ± 0.06g | 28.49 ± 0.06h | 35.86 ± 0.10g | 40.34 ± 0.06k | 33.33 ± 0.12op | 37.23 ± 0.06f |
| II-27 | 36.18 ± 0.06hi | 15.25 ± 0.06j | 39.57 ± 0.06i | 22.04 ± 0.06jkl | 20.69 ± 0.06ijk | 42.96 ± 0.06j | 29.80 ± 0.06ij | 14.89 ± 0.06lmn |
| II-28 | 8.55 ± 0.06p | 6.78 ± 0.06o | 39.04 ± 0.06i | 23.66 ± 0.06ijk | 38.62 ± 0.10fg | 42.06 ± 0.00jk | 16.67 ± 0.10nop | 12.77 ± 0.06mnop |
| II-29 | 30.26 ± 0.06j | 0.42 ± 0.06qr | 36.36 ± 0.06j | 21.51 ± 0.06kl | 40.00 ± 0.06f | 36.48 ± 0.06l | 20.20 ± 0.06lmn | 12.77 ± 0.10mnop |
| II-30 | 0.66 ± 0.06q | 0.00 ± 0.06r | 14.97 ± 0.10n | 4.30 ± 0.06tuv | 10.34 ± 0.16no | 26.18 ± 0.06no | 12.12 ± 0.10qr | 18.62 ± 0.10jk |
| II-31 | 15.13 ± 0.10o | 0.00 ± 0.06r | 17.11 ± 0.06m | 4.84 ± 0.00tuv | -1.38 ± 0.06q | 12.02 ± 0.06s | 7.07 ± 0.10st | 12.23 ± 0.11nopq |
| II-32 | 3.29 ± 0.10 q | 8.05 ± 0.06m | 6.42 ± 0.06p | 5.91 ± 0.06tu | 6.90 ± 0.06o | 7.30 ± 0.00t | 18.69 ± 0.06mno | 22.34 ± 0.06hi |
| II-33 | 0.66 ± 0.06q | 0.00 ± 0.11r | 28.88 ± 0.06k | 2.69 ± 0.06vw | -0.69 ± 0.05q | 23.61 ± 0.08p | 6.57 ± 0.06st | −1.60 ± 0.07t |
| II-34 | 36.84 ± 0.10h | 0.42 ± 0.06qr | 30.48 ± 0.06k | 0.54 ± 0.06x | 0.69 ± 0.06pq | 69.53 ± 0.06h | 13.13 ± 0.06pqr | 15.43 ± 0.10lm |
| II-35 | 30.26 ± 0.15g | 2.97 ± 0.06o | 21.39 ± 0.10l | 5.91 ± 0.06tu | 0.00 ± 0.03pq | 0.00 ± 0.00u | 0.51 ± 0.06u | 10.11 ± 0.03pqr |
| II-36 | 61.93 ± 0.10e | 72.88 ± 0.06e | 68.98 ± 0.15f | 62.37 ± 0.06e | 61.38 ± 0.03d | 77.25 ± 0.06f | 62.63 ± 0.06e | 65.43 ± 0.03cd |
| Norcantharidin | 88.17 ± 1.87a | 34.31 ± 1.69g | 86.64 ± 0.80b | 17.72 ± 1.21j | 20.00 ± 1.10ij | 24.46 ± 1.13i | 1.98 ± 2.24l | 29.75 ± 1.27g |
| Cantharidin | 88.16 ± 1.39a | 38.54 ± 2.68f | 100.00 ± 0.00a | 10.19 ± 1.61k | 67.59 ± 1.06cd | 84.54 ± 1.39d | 70.20 ± 0.84d | 68.05 ± 1.29c |
| Thiabendazole | 86.84 ± 1.22a | 100.00 ± 0.00a | 100.0 ± 0.00a | 66.67 ± 0.00e | 47.58 ± 1.60f | 100.00 ± 0.00a | 15.14 ± 1.18j | 24.99 ± 1.17h |
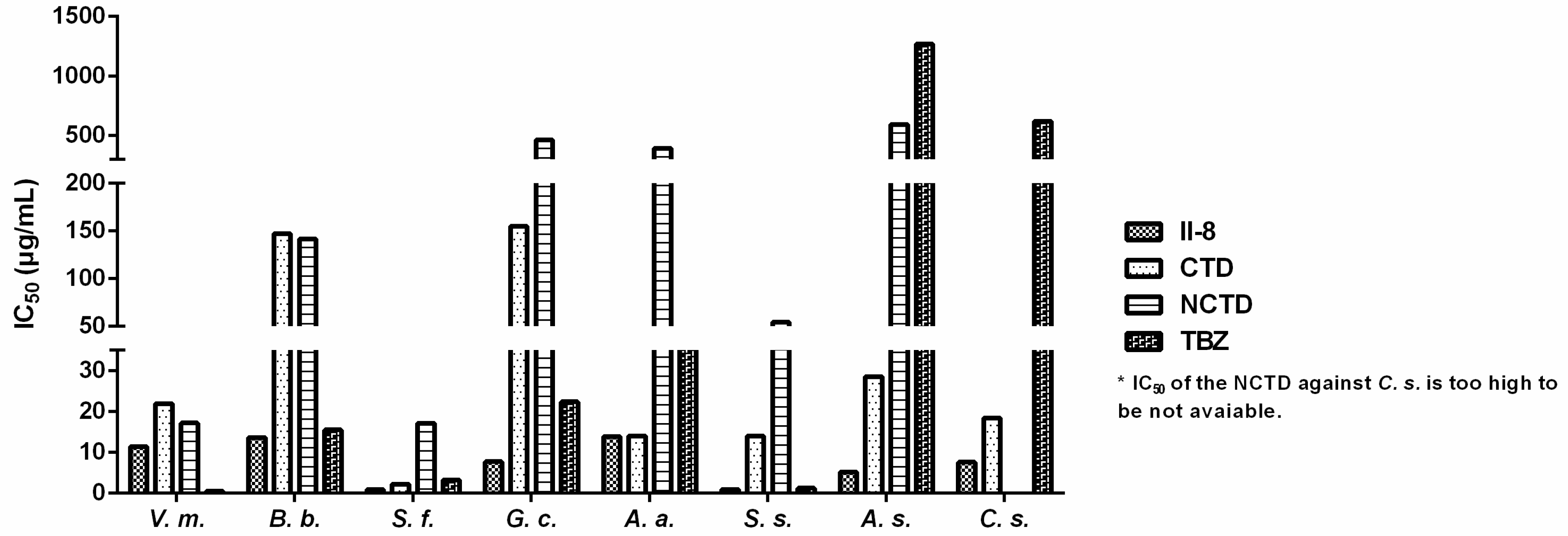
2.3. SAR
2.3.1. Effect of Introducing the Benzene Ring on Fungistatic Activity
2.3.2. Effect of Position Substituted on the Benzene Ring on Fungistatic Activity
| Compd. | IC50 (μg/mL) (CI 95%) ** | |||||||
|---|---|---|---|---|---|---|---|---|
| V. m. | B. b. | S. f. | G. c. | A. a. | S. s. | A. s. | C. s. | |
| II-1 | 32.0800 (26.1510–39.3533) | 31.2568 (23.7127–41.2012) | 14.6778 (10.7872–19.9716) | 36.3526 (29.1302–45.3658) | 42.2537 (33.7577–52.8879) | 7.2482 (2.9912–17.5637) | 30.1335 (23.3387–38.9065) | 22.7788 (16.0894–32.2493) |
| II-4 | 27.8016 (22.1312–34.9249) | 24.8446 (18.2585–33.8063) | 10.6665 (7.6148–14.9412) | 16.7764 (11.7587–23.9352) | 31.4258 (24.6239–40.1066) | 4.2858 (1.6648–11.0334) | 14.0938 (9.1043–21.8178) | 10.4051 (12.9130–24.7109) |
| II-5 | 20.1058 (14.8666–27.1914) | 19.8375 (14.7821–26.6217) | 2.0791 (1.3098–3.3003) | 11.2505 (6.9283–18.2691) | 18.1002 (13.1268–24.9579) | 3.4556 (1.6958–7.0418) | 10.4753 (6.7490–16.2590) | 10.4051 (6.4109–16.8880) |
| II-8 | 11.3756 (7.2790–17.7778) | 13.6528 (9.8721–18.8814) | 0.8805 (0.4243–1.8275) | 7.7364 (3.8335–15.6128) | 13.8916 (9.3377–20.6664) | 0.9698 (0.4790–1.9633) | 5.1863 (2.5133–10.7020) | 7.5908 (4.4564–12.9298) |
| II-11 | 17.0896 (11.9965–23.3450) | 17.0640 (12.6223–23.0689) | 2.3717 (1.4367–3.9152) | 9.1071 (5.1797–16.0125) | 22.1465 (16.7792–29.2308) | 2.1783 (0.9142–5.1904) | 12.1081 (7.9591–18.4200) | 12.9298 (8.9552–18.6685) |
| II-14 | 47.7810 (39.0992–58.39.4) | 37.3895 (28.8394–48.4746) | 21.9666 (16.3757–29.4664) | 57.0963 (45.2452–72.0517) | 49.1546 (39.1730–61.6795) | 9.6192 (4.2886–21.5754) | 34.8290 (27.5093–44.0964) | 29.8409 (16.0894–32.2493) |
| Norcantharidin | 17.1862 (9.2272–32.0104) | 141.8133 (61.1287–328.9946) | 17.1673 (10.0952–29.1937) | 465.1719 (86.5242–2500.8598) | 394.9566 (73.9654–2108.9689) | 54.5899 (32.0147–93.0839) | 594.2606 (211.1064–1672.8327) | NA *** |
| Cantharidin | 21.9320 (13.3453–36.0436) | 147.4225 (46.8714–463.6810) | 2.2175 (0.6116–12.0751) | 155.0286 (76.7546–313.1261) | 14.0428 (5.6729–34.7622) | 14.0622 (8.0013–24.7142) | 28.5223 (18.6015–43.7340) | 18.3900 (8.3854–40.3309) |
| TBZ | 0.5191 (0.0218–12.3413) | 15.5378 (8.8501–27.2793) | 3.1998 (1.2133–8.4391) | 22.3806 (10.4428–47.9653) | 47.8696 21.2217–107.9790 | 1.2947 (0.6661–2.5165) | 1268.5889 (110.6555–1453.4930) | 621.7874 60.1464–6427.9804 |
2.3.3. Effect of Various Substituents on the Benzene Ring on Fungistatic Activity
3. Experiment Section
3.1. General Information
3.2. Synthesis of the Title Compounds II(1–36)
3.3. Screening of Antifungal Activity in Vitro
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bai, Y.B.; Zhang, A.L.; Tang, J.J.; Gao, J.M. Synthesis and antifungal activity of 2-chloromethyl-1H-benzimidazole derivatives against phytopathogenic fungi in vitro. J. Agric. Food Chem. 2013, 61, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Schirra, M.; Aquino, S.D.; Cabras, P.; Angioni, A. Control of postharvest diseases of fruit by heat and fungicides: Efficacy, residue levels, and residue persistence. A review. J. Agric. Food Chem. 2011, 59, 8531–8542. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Teng, P.S.; Willocquet, L.; Nutter, F.W., Jr. Quantification and modeling of crop losses: A review of purposes. Annu. Rev. Phytopathol. 2006, 44, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Yu, J.J.; Zhang, Y.N.; Zhang, X.; Cheng, C.J.; Wang, J.X.; Hollomon, D.W.; Fan, P.S.; Zhou, M.G. Effect of carbendazim resistance on trichothecene production and aggressiveness of Fusarium graminearum. Mol. Plant Microbe Interact. 2009, 22, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Seifi, A.; Visser, R.G.F.; Bai, Y.L. How to effectively deploy plant resistances to pests and pathogens in crop breeding. Euphytica 2013, 190, 321–334. [Google Scholar] [CrossRef]
- Dossey, A.T. Insects and their chemical weaponry: New potential for drug discovery. Nat. Prod. Rep. 2010, 27, 1737–1757. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.J.; Li, A.Y.; Pritchard, S.; Tangtrakulwanich, K.; Baxendale, F.P.; Brewer, G. Contact and Fumigant Toxicity of a Botanical-Based Feeding Deterrent of the Stable Fly, Stomoxys calcitrans (Diptera: Muscidae). J. Agric. Food Chem. 2011, 59, 10394–10400. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Cantrell, C.L.; Duke, S.O. Natural products in crop protection. Bioorg. Med. Chem. 2009, 17, 4022–4034. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, L.C.; Teare, D. Poisoning by cantharides. Br. J. Med. 1954, 2, 1384–1386. [Google Scholar] [CrossRef]
- Wang, G.S. Medical uses of Mylabris in ancient China and recent studies. J. Ethnopharmacol. 1989, 26, 147–162. [Google Scholar] [CrossRef]
- Sakoff, J.A.; Ackland, S.P.; Baldwin, M.L.; Keane, M.A.; McCluskey, A. Anticancer activity and protein phosphatase 1 and 2A inhibition of a new generation of cantharidin analogues. Investig. New Drugs 2002, 20, 1–11. [Google Scholar] [CrossRef]
- Li, W.; Xie, L.; Chen, Z.; Zhu, Y.; Sun, Y.J.; Miao, Y.; Xu, Z.K.; Han, X. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci. 2010, 101, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, A.; Ackland, S.P.; Bowyer, M.C.; Baldwin, M.L.; Garner, J.; Walkom, C.C.; Sakoff, J.A. Cantharidin analogues: Synthesis and evaluation of growth inhibition in a panel of selected tumour cell lines. Bioorg. Chem. 2003, 31, 68–79. [Google Scholar] [CrossRef]
- Sagawa, M.; Nakazato, T.; Uchida, H.; Ikeda, Y.; Kizaki, M. Cantharidin induces apoptosis of human multiple myeloma cells via inhibition of the JAK/STAT pathway. Cancer Sci. 2008, 99, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Carrel, J.E.; Eisner, T. Cantharidin potent feeding deterrent to insects. Science 1974, 183, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Carrel, J.E.; Doom, J.P.; McCormick, J.P. Identification of cantharidin in false blister beetles (Coleoptera. Meloidae) from Florida. J. Chem. Ecol. 1986, 12, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Chen, X.S.; Hou, X.H. The toxicity effect of cantharidin on six pests. Guizhou Agric. Sci. 2008, 36, 65–66. [Google Scholar]
- Liu, R.R.; Ma, Y.; Ma, Z.Q.; Zhang, Y.L. Bioactivity of cantharidin against eleven pests. J. Northwest A F Univ. 2010, 38, 181–185. [Google Scholar]
- Bajsa, J.; Duke, S.O.; Tekwani, B.L. Plasmodium falciparum serine/threonine phosphoprotein phosphatases (PPP): From housekeeper to the “Holy Grail”. Curr. Drug Targets 2008, 9, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.H.; Walter, J.; Scheidmann, K.; Ohst, K.; Newport, J.; Walter, G. Protein phosphatase 2A is required for the initiation of chromosomal DNA replication. Proc. Natl. Acad. Sci. USA 1998, 95, 14693–14698. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, G.B.; de Wever, V.; Templeton, G.; Kerk, D. Evolution of protein phosphatases in plants and Animals. Biochem. J. 2009, 417, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Calderone, V.; Fragai, M.; Luchinat, C.; Talluri, E. Structural basis of serine/threonine phosphatase inhibition by the archetypal small molecules cantharidin and norcantharidin. J. Med. Chem. 2009, 52, 4838–4843. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Mackintosh, C.; Casida, J.E. Protein phosphatase 2A and its [3H] cantharidin/[3H] endothall thioanhydride binding site. Inhibitor specificity of cantharidin and ATP analogues. Biochem. Pharmacol. 1993, 46, 1435–1443. [Google Scholar] [CrossRef]
- Chen, X.E.; Liu, J.Y.; Zhang, Y.L. Cantharidin impedes the activity of protein serine/threonine phosphatase in Plutella xylostella. Mol. BioSyst. 2014, 10, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Bajsa, J.; Pan, Z.Q.; Dayan, F.E.; Owens, D.K.; Duke, S.O. Validation of serine/threonine protein phosphatase as the herbicide target site of endothall. Pestic. Biochem. Physiol. 2012, 102, 38–44. [Google Scholar] [CrossRef]
- Sun, W.B.; Liu, Z.Y.; Zhang, Y.L. Cantharidin and its anhydride-modified derivatives: Relation of structure to insecticidal activity. Int. J. Mol. Sci. 2013, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhang, Q.; Zhang, A.L.; Gao, J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 2012, 60, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.A.; Stewart, S.G.; Gordon, C.P.; Ackland, S.P.; Gilbert, J.; Sauer, B.; Sakoff, J.A.; McCluskey, A. Norcantharidin Analogues: Synthesis, Anticancer Activity and Protein Phosphatase 1 and 2A Inhibition. ChemMedChem 2008, 3, 1878–1892. [Google Scholar] [CrossRef] [PubMed]
- Saremi, H.; Okhovvat, S.M. Mycotoxin producing Fusarium species associated with plant disease on potato, wheat, corn and animal diseases in northwest Iran. Commun. Agric. Appl. Biol. Sci. 2006, 71, 1175–1185. [Google Scholar] [PubMed]
- Popov, I.I.; Narezhnaya, V.N.; Zubenko, A.A. Research on unsaturated azole derivatives VII. New syntheses in the 2-vinylbenzimidazole series. Khim. Geterotsikl. Soedin. 1978, 8, 1104–1107. [Google Scholar]
- Sample Availability: Samples of the compounds II (1–36) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sun, W.; Zha, S.; Wang, H.; Zhang, Y. Synthesis and Biological Evaluation of Norcantharidin Derivatives Possessing an Aromatic Amine Moiety as Antifungal Agents. Molecules 2015, 20, 21464-21480. https://doi.org/10.3390/molecules201219782
Wang Y, Sun W, Zha S, Wang H, Zhang Y. Synthesis and Biological Evaluation of Norcantharidin Derivatives Possessing an Aromatic Amine Moiety as Antifungal Agents. Molecules. 2015; 20(12):21464-21480. https://doi.org/10.3390/molecules201219782
Chicago/Turabian StyleWang, Yang, Wenbo Sun, Shunqing Zha, Huan Wang, and Yalin Zhang. 2015. "Synthesis and Biological Evaluation of Norcantharidin Derivatives Possessing an Aromatic Amine Moiety as Antifungal Agents" Molecules 20, no. 12: 21464-21480. https://doi.org/10.3390/molecules201219782
APA StyleWang, Y., Sun, W., Zha, S., Wang, H., & Zhang, Y. (2015). Synthesis and Biological Evaluation of Norcantharidin Derivatives Possessing an Aromatic Amine Moiety as Antifungal Agents. Molecules, 20(12), 21464-21480. https://doi.org/10.3390/molecules201219782







