Quaternary Alkylammonium Conjugates of Steroids: Synthesis, Molecular Structure, and Biological Studies
Abstract
:1. Introduction
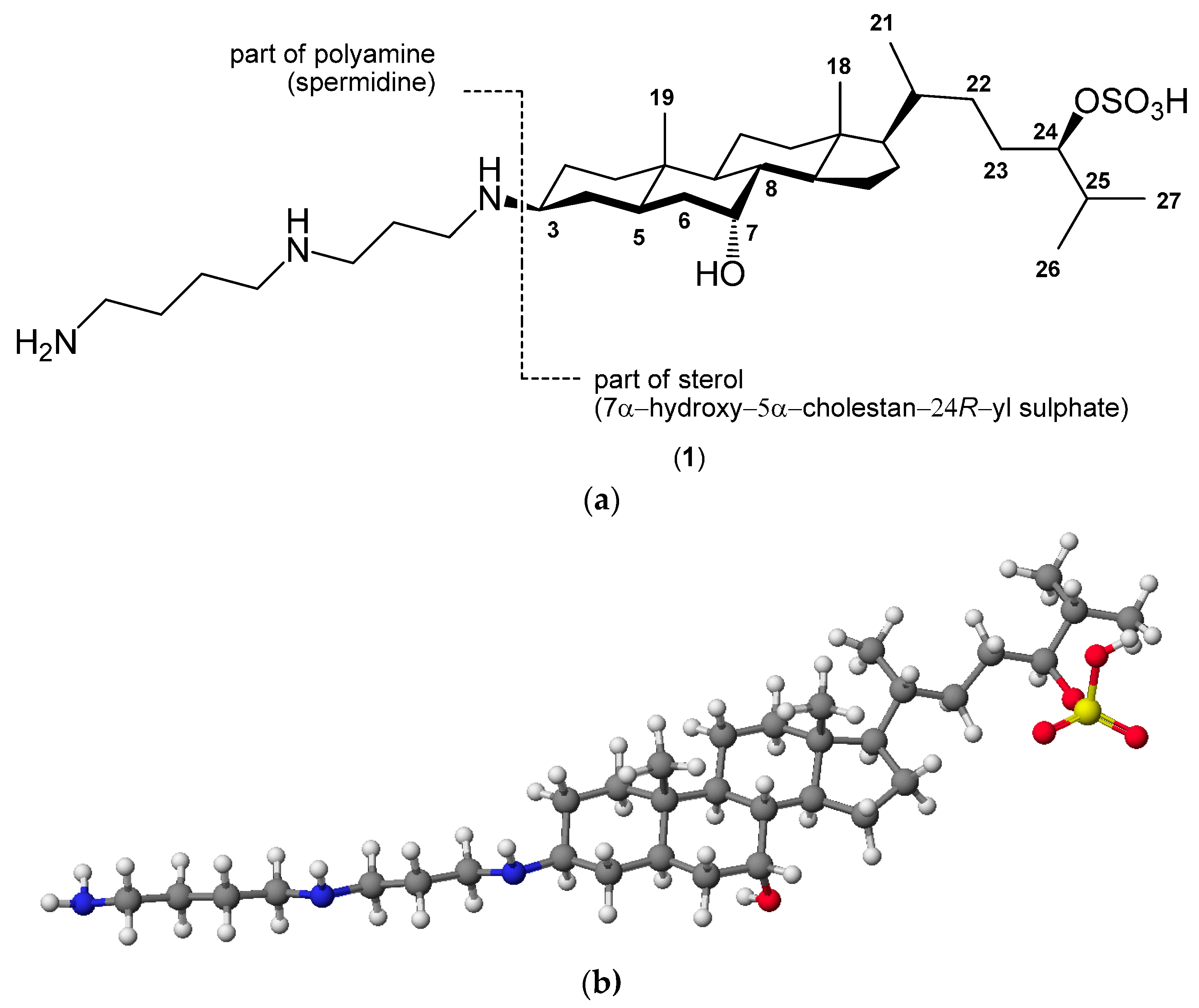
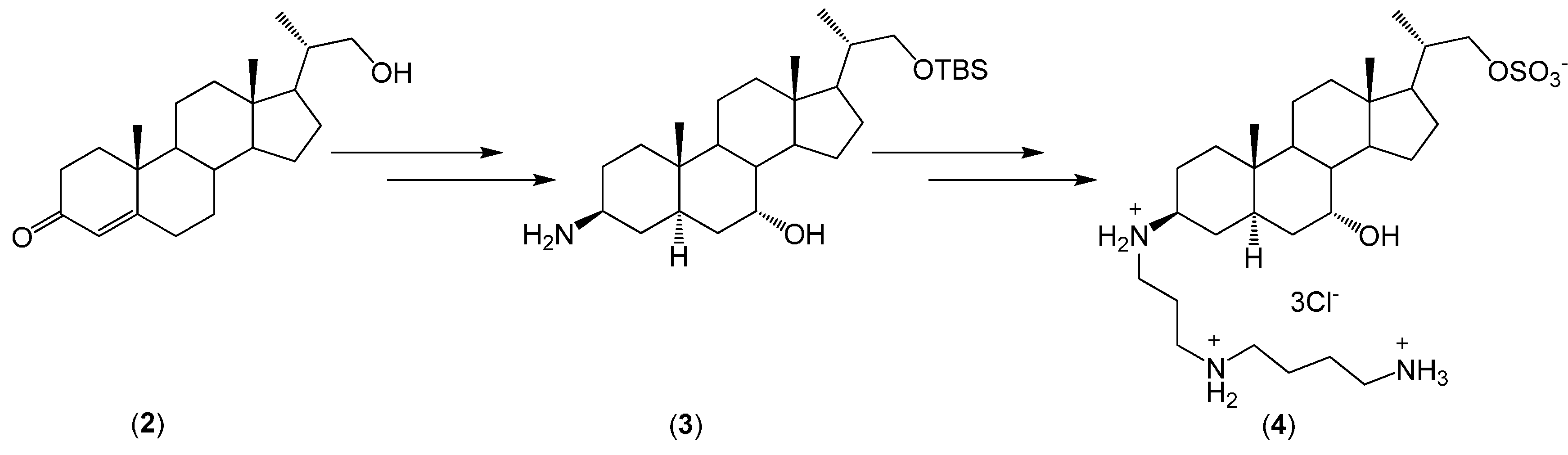
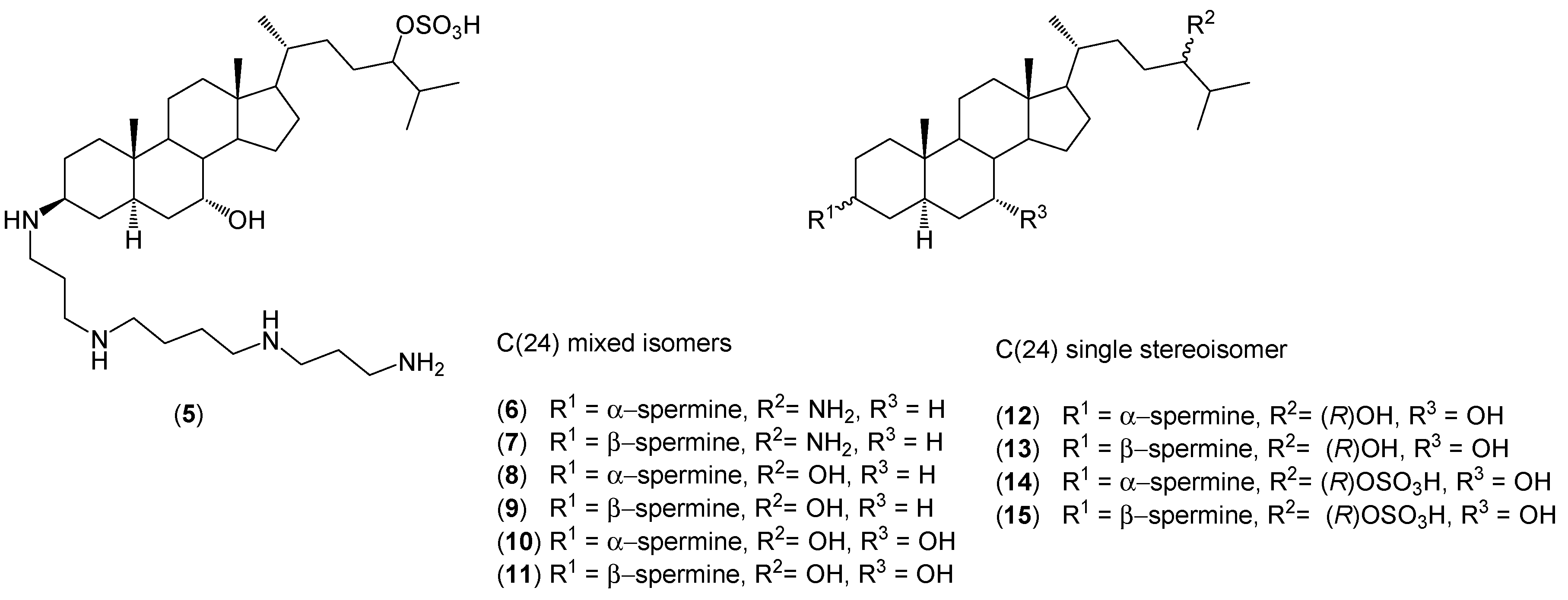
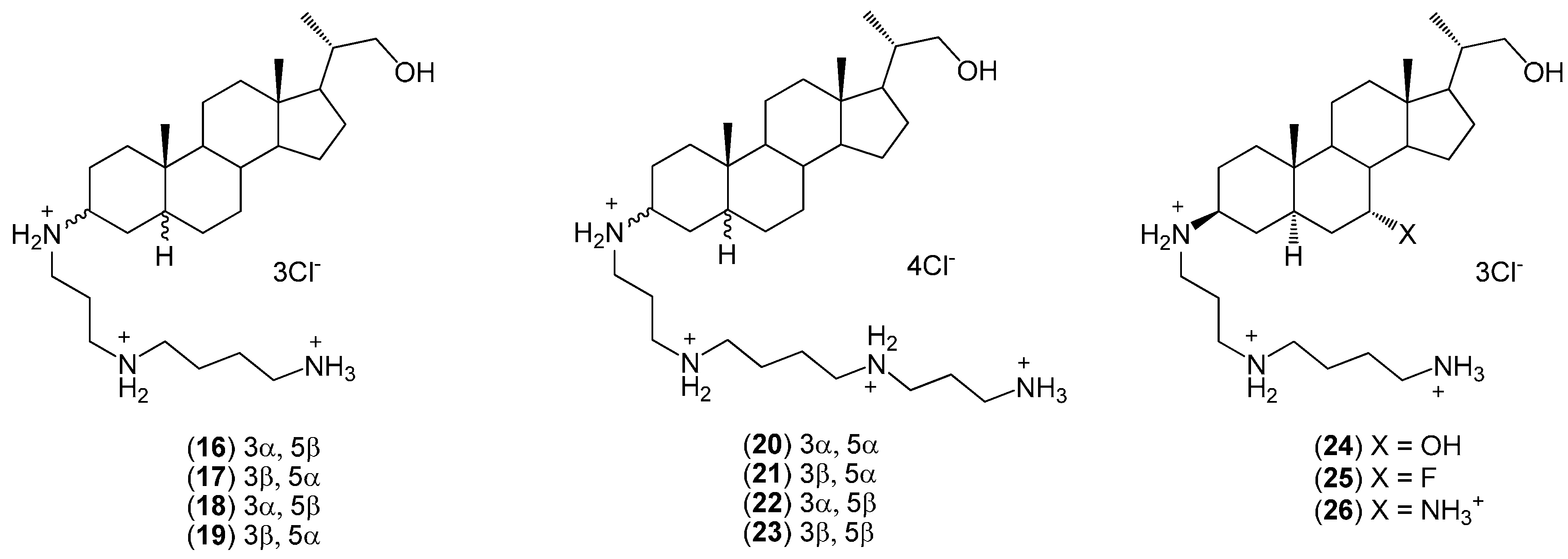
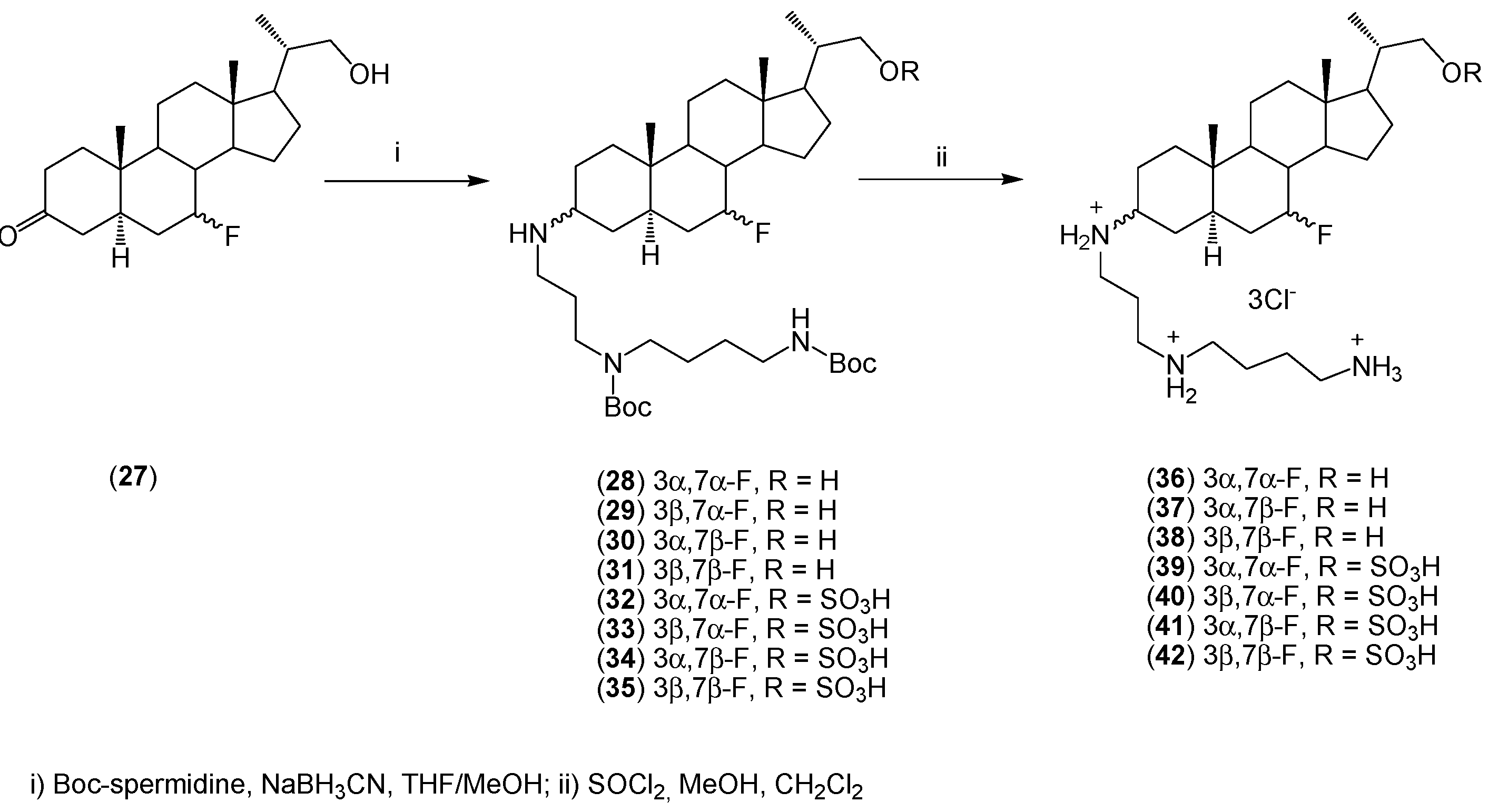
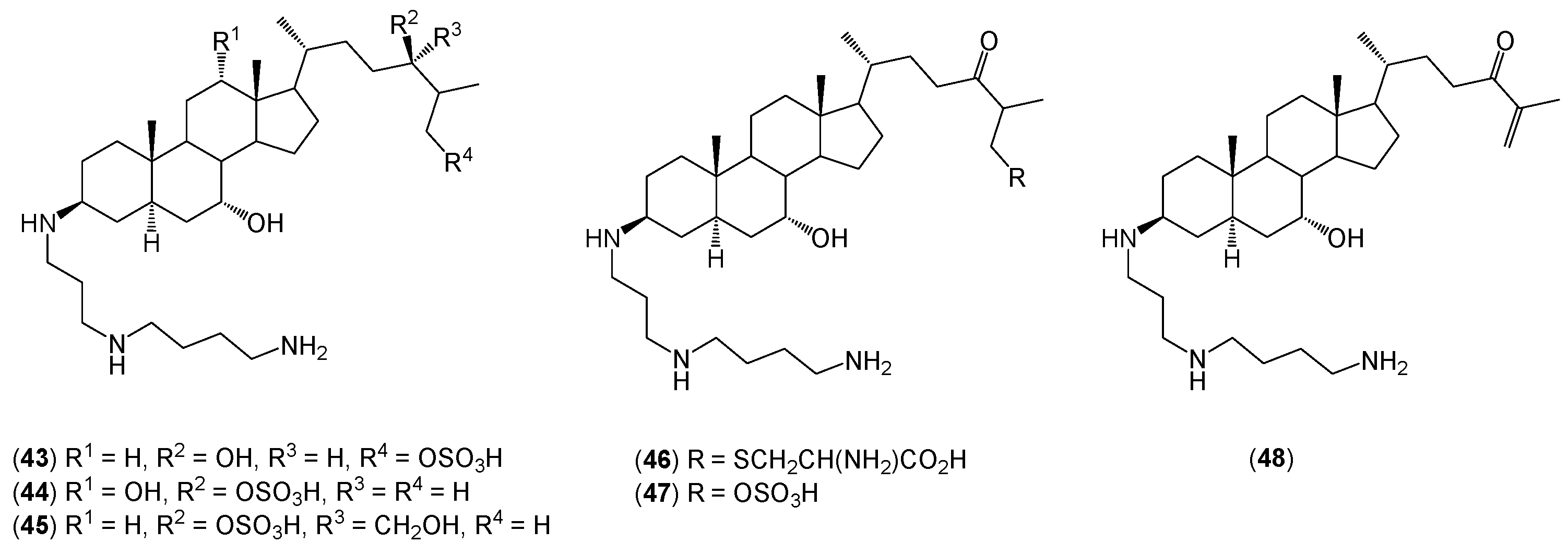
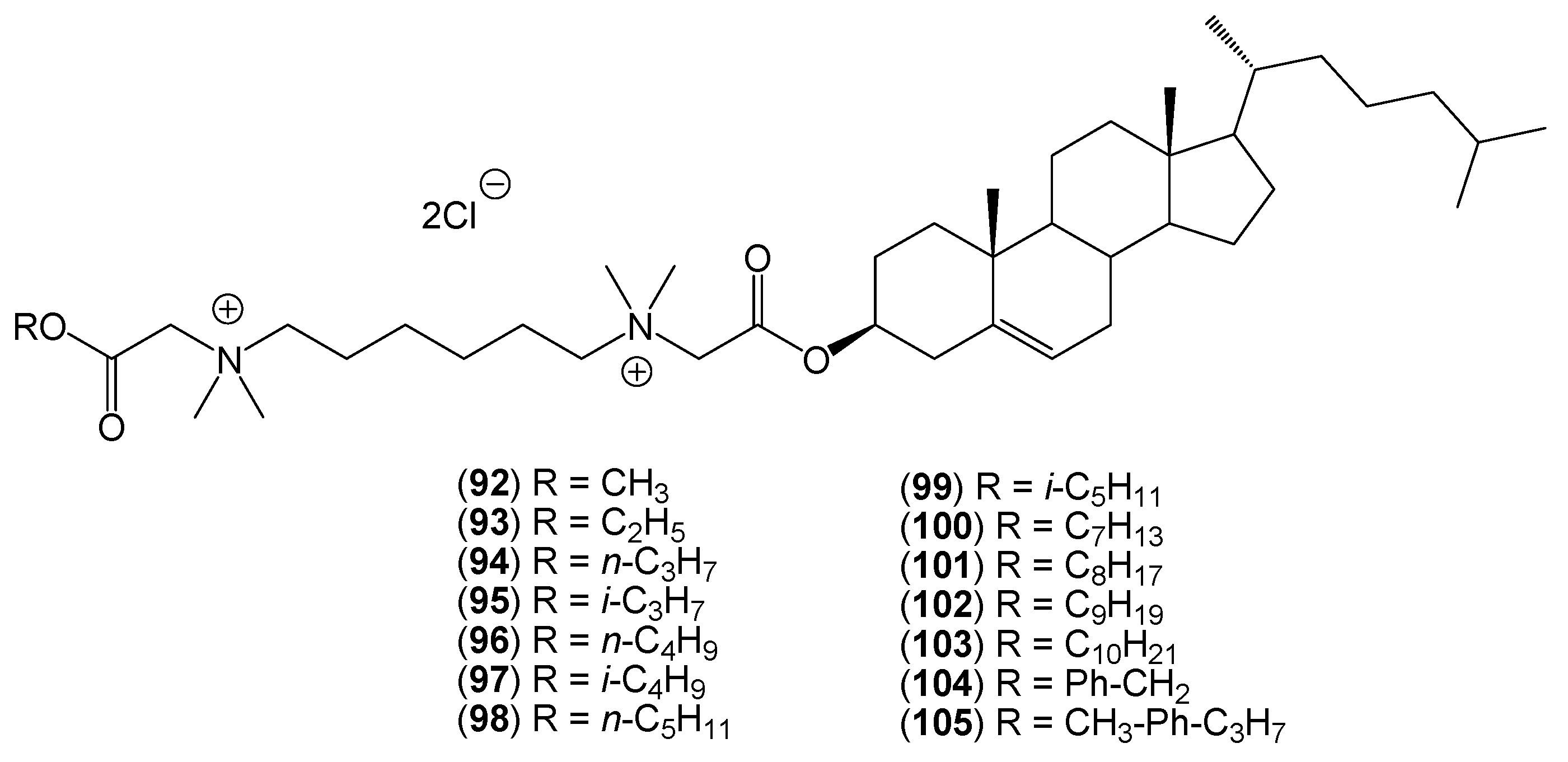
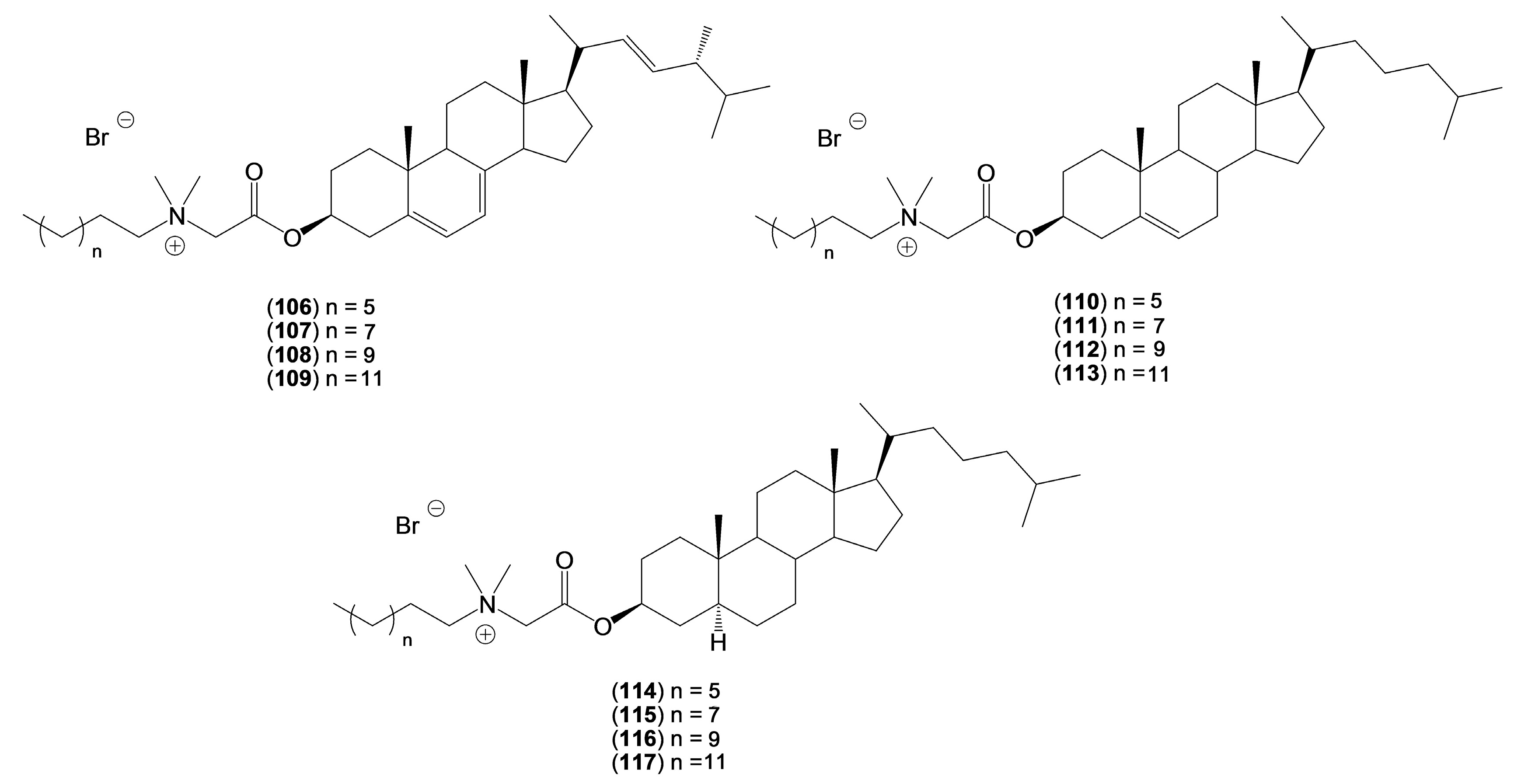
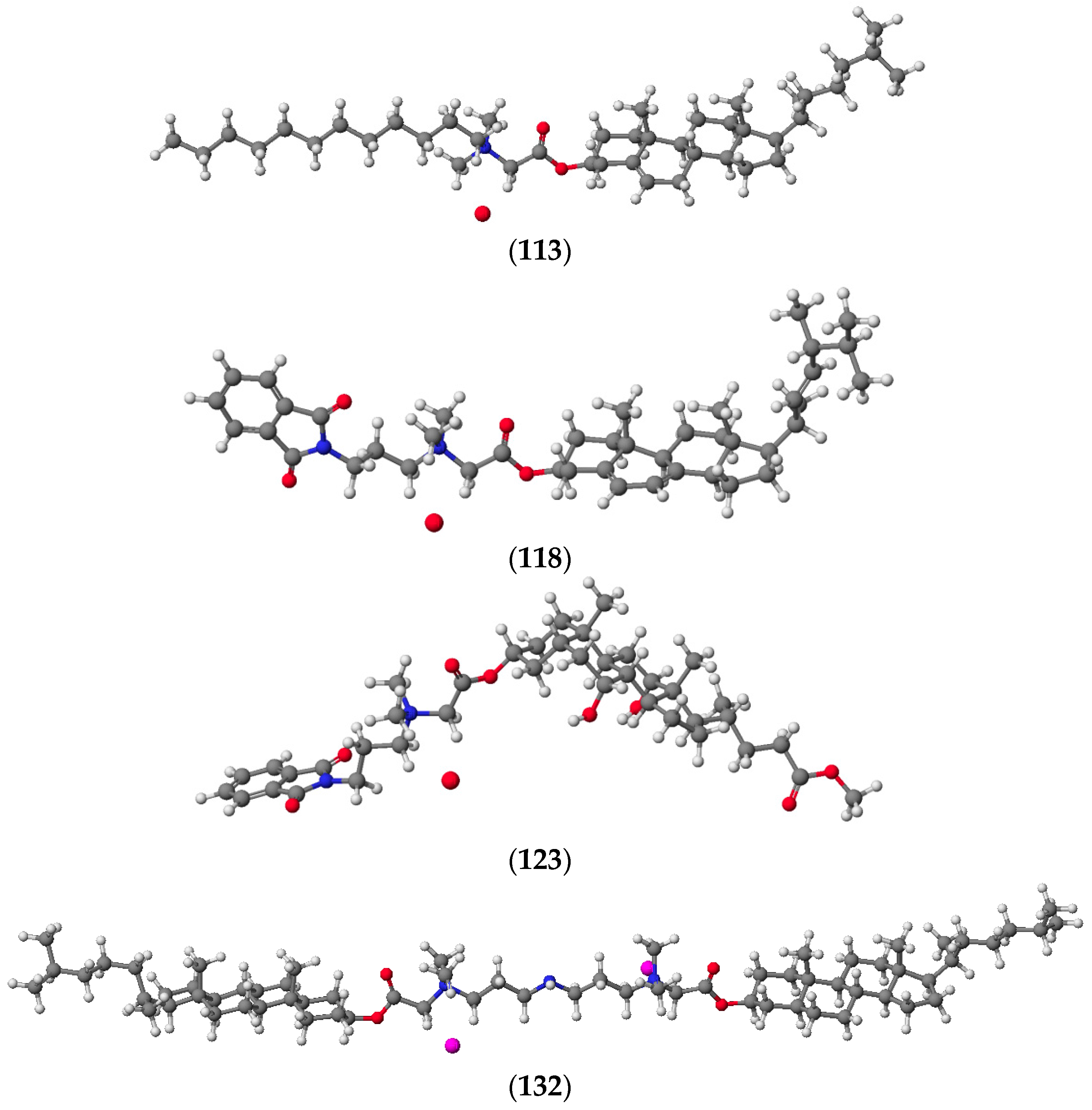
2. Quaternary Alkylammonium Conjugates of Steroids
| Microorganisms | Conjugate/MIC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 25 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | |
| S. pyogenes 308A | 6.3 | 25.0 | 12.5 | 12.5 | 12.5 | 50.0 | 25.0 | 50.0 |
| S. pyogenes 77A | 6.3 | 12.5 | 6.3 | 12.5 | 12.5 | 50.0 | 12.5 | 50.0 |
| S. ureus 503 | 6.3 | 12.5 | 6.3 | 6.3 | 12.5 | 25.0 | 6.3 | 50.0 |
| E. coli DC2 | 6.3 | 50.0 | 12.5 | 12.5 | 25.0 | 50.0 | 25.0 | 25.0 |
| P. aeruginosa 9027 | 6.3 | 50.0 | 12.5 | 12.5 | 25.0 | 12.5 | 50.0 | 50.0 |
| P. aeruginosa 1771M | 3.1 | 100.0 | 25.0 | 6.3 | 50.0 | 25.0 | 50.0 | 50.0 |
| S. typhimurium | 100.0 | 100.0 | 50.0 | 100.0 | 100.0 | 100.0 | 50.0 | 50.0 |
| E. cloacae 1321E | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 50.0 | 50.0 |
| Microorganisms | Conjugates/MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 43 | 44 | 45 | 46 | 47 | 48 | |
| S. aureus (29213) | 1 | 4–8 | 8–16 | 2 | 8–16 | 8 | 2 |
| E. coli (25922) | 4 | 128 | 16 | 8 | 256 | 128 | 16 |
| P. aeruginosa (27853) | 16 | 32 | 16 | 16 | 256 | 128 | 16 |
| C. albicans (90028) | 16 | 16 | 32 | 32 | 128 | 32 | 2 |

| Microorganisms | Conjugates/MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 53 | 54 | 55 | 56 | 57 | 58 | |
| S. aureus | 0.5–1 | 16 | 1 | 2–4 | 2 | >256 | 16 |
| E. coli | 2–4 | 32–64 | 8–16 | 32 | 32 | >256 | 16 |
| P. aeruginosa | 16 | 128 | 64 | 128 | 32 | 128 | 8 |
| C. albicans | 8 | 8 | 2–4 | 4 | 2 | >256 | 4 |




| Focal Predicted Activity (PA > 80) | Conjugates | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 106–109 | 110–113 | 114–117 | 118 | 119 | 120 | 121 | 122 | 123 | 124 | 125 | 126 | 127 | 128 | 129 | 130 | 131 | 132 | |
| Cholesterol antagonist | 88 | 90 | 87 | – | – | – | – | – | – | 81 | 85 | – | 87 | 89 | 82 | 82 | 86 | – |
| Antihypercholesterolemic | 91 | 87 | – | – | – | – | – | – | – | 88 | 83 | 86 | 85 | 80 | 83 | – | 94 | – |
| Glyceryl-ether monooxygenase inhibitor | 89 | 92 | 95 | 87 | 91 | 93 | 93 | 94 | 95 | 89 | 89 | 88 | 92 | 92 | 91 | 95 | 95 | 94 |
| Acylcarnitine hydrolase inhibitor | – | 87 | 97 | – | – | 81 | 83 | 91 | 94 | – | – | – | 85 | 80 | – | 96 | – | 93 |
| Alcohol O-acetyltransferase inhibitor | 91 | – | – | – | – | – | – | – | – | 91 | 90 | 90 | – | – | – | – | – | – |
| Oxidoreductase inhibitor | 81 | – | – | – | – | – | – | – | – | 87 | 86 | 85 | – | – | – | – | – | – |
| Prostaglandin-E2 9-reductase inhibitor | – | 86 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Alkylacetylglycerophosphatase inhibitor | – | – | 92 | – | – | 84 | 82 | 90 | 86 | – | – | – | – | – | – | 90 | 87 | 83 |
| Alkenylglycerophosphocholine hydrolase inhibitor | – | – | 90 | – | – | – | – | 80 | – | – | – | – | – | – | – | 88 | 82 | 80 |
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dewick, P.M. Medicinal Natural Products A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 275–277. [Google Scholar]
- Nicolaou, K.C.; Montagnon, T. Molecules that Changed the World; John Wiley & Sons, Ltd.: Weinheim, UK, 2008; pp. 79–90. [Google Scholar]
- Fieser, L.F.; Fieser, M. Steroids; Reinhold Publishing Corporation: New York, NY, USA, 1959; pp. 341–364. [Google Scholar]
- Templeton, W. An Introduction to the Chemistry of Terpenoids and Steroids; Butterworths: London, UK, 1969; pp. 158–190. [Google Scholar]
- Lednicer, D. Steroid Chemistry at a Glance; John Wiley & Sons, Ltd.: Chichester, UK, 2011. [Google Scholar]
- Hayat, S.; Ahmad, A. Brassinosteroids: A Class of Plant Hormone; Springer: New York, NY, USA, 2011. [Google Scholar]
- Lawrence, S.A. Amines: Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Rato, C.; Amirova, S.R.; Bates, D.G.; Stansfield, I.; Wallace, H.M. Translational recoding as a feedback controller: Systems approaches reveal polyamine-specific effects on the antizyme ribosomal frameshift. Nucleic Acid Res. 2011, 39, 4587–4597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lee, H.K.; Pruess, T.H.; White, H.S.; Bulaj, G.J. Synthesis and applications of polyamine amino acid residues: Improving the bioactivity of an analgesic neuropeptide, neurotensin. Med. Chem. 2009, 52, 1514–1517. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Ranade, S.A.; Nagar, P.K.; Kumar, N. Role of polyamines and ethylene as modulators of plant senescence. J. Biosci. 2000, 25, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Salunke, D.B.; Hazra, B.G.; Pore, V.S. Steroidal conjugates and their pharmacological applications. Curr. Med. Chem. 2006, 13, 813–847. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.S.; Wehrli, S.; Roder, H.; Rogers, M., Jr.; Forrest, J.N., Jr.; McCrimmon, D.; Zasloff, M. Squalamine: An aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. USA 1993, 90, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, S.; Moore, K.S.; Roder, H.S.; Durell, S.; Zasloff, M. Structure of the novel steroidal antibiotic squalamine determined by two-dimensional NMR spectroscopy. Steroids 1993, 58, 370–378. [Google Scholar] [CrossRef]
- Sadownik, A.; Deng, G.; Janout, V.; Regen, S.L.; Bernard, E.M.; Kikuchi, K.; Armstrong, D. Rapid Construction of a Squalamine Mimic. J. Am. Chem. Soc. 1995, 117, 6138–6139. [Google Scholar] [CrossRef]
- Rao, M.N.; Shinnar, A.E.; Noecker, L.A.; Chao, T.L.; Feibush, B.; Snyder, B.; Sharkansky, I.; Sarkahian, A.; Zhang, X.; Jones, S.R.; et al. Aminosterols from the Dogfish Shark Squalus acanthias. J. Nat. Prod. 2000, 63, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choi, B.S.; Kwon, K.C.; Lee, S.O.; Kwak, H.J.; Lee, C.H. Synthesis and Antimicrobial Activity of Squalamine Analogue. Bioorg. Med. Chem. 2000, 8, 2059–2065. [Google Scholar] [CrossRef]
- Bhargava, P.; Marshall, J.L.; Dahut, W.; Rizvi, N.; Trocky, N.; Williams, J.I.; Hait, H.; Song, S.; Holroyd, K.J.; Hawkins, M.J. A phase I and pharmacokinetic study of squalamine, a novel antiangiogenic agent, in patients with advanced cancers. Clin. Cancer Res. 2001, 7, 3912–3919. [Google Scholar] [PubMed]
- Teicher, B.A.; Williams, J.I.; Takeuchi, H.; Ara, G.; Herbst, R.S.; Buxton, D. Potential of the aminosterol, squalamine in combination therapy in the rat 13,762 mammary carcinoma and the murine Lewis lung carcinoma. Anticancer Res. 1998, 18, 2567–2573. [Google Scholar] [PubMed]
- Schiller, J.H.; Bittner, G. Potentiation of platinum antitumor effects in human lung tumor xenografts by the angiogenesis inhibitor squalamine: effects on tumor neovascularisation. Clin. Cancer Res. 1999, 5, 4287–4294. [Google Scholar] [PubMed]
- Williams, J.I.; Weitman, S.; Gonzalez, C.M.; Jundt, C.H.; Marty, J.; Stringer, S.D.; Holroyd, K.J.; McLane, M.P.; Chen, Q.; Zasloff, M.; et al. Squalamine treatment of human tumors in nu/nu mice enhances platinum-based chemotherapies. Clin. Cancer Res. 2001, 7, 724–733. [Google Scholar] [PubMed]
- Li, D.; Williams, J.I.; Pietras, R.J. Squalamine and cisplatin block angiogenesis and growth of human ovarian cancer cells with or without HER-2 gene overexpression. Oncogene 2002, 21, 2805–2814. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.T.; Houston, T.A. Squalamine and its derivatives as potential antitubercular compounds. Tuberculosis 2013, 93, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Sills, A.K., Jr.; Williams, J.I.; Tyler, B.M.; Epstein, D.S.; Sipos, E.P.; Davis, J.D.; McLane, M.P.; Pitchford, S.; Cheshire, K.; Gannon, F.H.; et al. Squalamine inhibits angiogenesis and solid tumor growth in vivo and perturbs embryonic vasculature. Cancer Res. 1998, 58, 2784–2792. [Google Scholar] [PubMed]
- Novotná, E.; Waisser, K.; Kuneš, J.; Palát, K.; Buchta, V.; Stolaříková, J.; Beckert, R.; Wsól, V. Synthesis and Biological Activity of Quaternary Ammonium Salt-Type Agents Containing Cholesterol and Terpenes. Arch. Pharm. Chem. Life Sci. 2014, 347, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M.; Adams, A.P.; Beckerman, B.; Campbell, A.; Han, Z.; Luijten, E.; Meza, I.; Julander, J.; Mishra, A.; Qu, W.; et al. Squalamine as a broad-spectrum systemic antiviral agent with therapeutic potential. Proc. Natl. Acad. Sci. USA 2011, 108, 15978–15983. [Google Scholar] [CrossRef] [PubMed]
- Kinney, W.A.; Zhang, X.; Williams, J.I.; Johnston, S.; Michalak, R.S.; Deshpande, M.; Dostal, L.; Rosazza, J.P.N. A short formal synthesis of squalamine from a microbial metabolite. Org. Lett. 2000, 2, 2921–2922. [Google Scholar] [CrossRef]
- Zhou, X.D.; Cai, F.; Zhou, W.S. A new highly stereoselective construction of the side chain of squalamine through improved Sharpless catalytic asymmetric dihydroxylation. Tetrahedron Lett. 2001, 42, 2537–2539. [Google Scholar] [CrossRef]
- Zhou, X.-D.; Cai, F.; Zhou, W.-S. A stereoselective synthesis of squalamine. Tetrahedron 2002, 58, 10293–10299. [Google Scholar] [CrossRef]
- Weis, A.L.; Bakos, T.; Alferiev, I.; Zhang, X.; Shao, B.; Kinney, W.A. Synthesis of an azido spermidine equivalent. Tetrahedron Lett. 1999, 40, 4863–4864. [Google Scholar] [CrossRef]
- Khabnadideh, S.; Tan, C.L.; Croft, S.L.; Kendrick, H.; Yardley, V.; Gilbert, I.H. Squalamine analogues as potential anti-trypanosomal and anti-leishmanial compounds. Bioorg. Med. Chem. Lett. 2000, 10, 1237–1239. [Google Scholar] [CrossRef]
- Choucair, B.; Dherbomez, M.; Roussakis, C.; Khiel, L.E. Synthesis of spermidinylcholestanol and spermidinylcholesterol, squalamine analogues. Tetrahedron 2004, 60, 11477–11486. [Google Scholar] [CrossRef]
- Hussey, S.L.; He, E.; Peterson, B.R. Synthesis of chimeric 7 alpha-substituted estradiol derivatives linked to cholesterol and cholesterylamine. Org. Lett. 2002, 4, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Jones, R.S.; Kinney, W.A.; Selinsky, B.S. The synthesis of spermine analogs of the shark aminosterol squalamine. Steroids 2002, 67, 291–304. [Google Scholar] [CrossRef]
- Kim, H.S.; Khan, S.N.; Jadhav, J.R.; Jeong, J.W.; Jung, K.; Kwak, J.H. A concise synthesis and antimicrobial activities of 3-polyamino-23,24-bisnorcholanes as steroid–polyamine conjugates. Bioorg. Med. Chem. Lett. 2011, 21, 3861–3865. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kwon, K.C.; Kim, K.S.; Lee, C.H. Synthesis and antimicrobial activity of new 3α-hydroxy-23,24-bisnorcholane polyamine carbamates. Bioorg. Med. Chem. Lett. 2001, 11, 3065–3068. [Google Scholar] [CrossRef]
- Kim, B.-K.; Doh, K.-O.; Bae, Y.-U.; Seu, Y.-B. Synthesis and Optimization of Cholesterol-Based Diquaternary Ammonium Gemini Surfactant (Chol-GS) as a New Gene Delivery Vector. J. Microbiol. Biotechnol. 2011, 21, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Kim, B.J.; Kim, H.-S. Synthesis and antimicrobial activity of 7-fluoro-3-aminosteroids. Bioorg. Med. Chem. Lett. 2007, 17, 5139–5142. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Nakamura, Y.; Takeuchi, S.; Kato, I.; Fujimoto, Y.; Ikekawa, N. Formal Synthesis of Squalamine from Desmosterol. Chem. Pharm. Bull. 2003, 51, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, R.M.; Tuladhar, S.M.; Guo, L.; Wehril, S. Synthesis of squalamine. A steroidal antibiotic from the shark. Tetrahedron Lett. 1994, 44, 8103–8106. [Google Scholar] [CrossRef]
- Moriarty, R.M.; Enache, L.A.; Kinney, W.A.; Allenc, C.S.; Canary, J.W.; Tuladhar, S.M.; Guo, L. Stereoselective synthesis of squalamine dessulfate. Tetrahedron Lett. 1995, 36, 5139–5142. [Google Scholar] [CrossRef]
- Jones, S.R.; Selinsky, B.S.; Rao, M.N.; Zhang, X.H.; Kinney, W.A.; Tham, F.S. Efficient Route to 7α-(Benzoyloxy)-3-dioxolane Cholestan-24(R)-ol, a Key Intermediate in the Synthesis of Squalamine. J. Org. Chem. 1998, 63, 3786–3789. [Google Scholar] [CrossRef]
- Zhang, X.H.; Rao, M.N.; Jones, S.R.; Shao, B.; Feibush, P.; McGuigan, M.; Tzodikov, N.; Feibubush, B.; Sharkansky, I.; Snyder, B.; et al. Synthesis of Squalamine Utilizing a Readily Accessible Spermidine Equivalent. J. Org. Chem. 1998, 63, 8599–8603. [Google Scholar] [CrossRef]
- Brunel, J.M.; Letourneux, Y. Recent Advances in the Synthesis of Spermine and Spermidine Analogs of the Shark Aminosterol Squalamine. Eur. J. Org. Chem. 2003, 20, 3897–3907. [Google Scholar] [CrossRef]
- Jones, S.R.; Kinney, W.A.; Zhang, W.; Jones, L.M.; Selinsky, B.S. The synthesis and characterization of analogs of the antimicrobial compound squalamine: 6β-hydroxy-3-aminosterols synthesized from hyodeoxycholic acid. Steroids 1996, 61, 565–571. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Balasubramanian, R.; Maitra, U.; Ghosh, S.; Raju, A.R. Novel Cationic and Neutral Analogues of Bile Acids: Synthesis and Preliminary Study of Their Aggregation Properties. Langmuir 2002, 18, 7154–7157. [Google Scholar] [CrossRef]
- Bhat, S.; Maitra, U. Low molecular mass cationic gelators derived from deoxycholic acid: Remarkable gelation of aqueous solvents. Tetrahedron 2007, 63, 7309–7320. [Google Scholar] [CrossRef]
- Lopushanskii, A.I.; Udovitskaya, V.V. Quaternary ammonium derivatives of cholesterol. Pharm. Chem. J. 1970, 4, 425–429. [Google Scholar] [CrossRef]
- Brycki, B.; Koenig, H.; Kowalczyk, I.; Pospieszny, T. Synthesis, Spectroscopic and Semiempirical Studies of New Quaternary Alkylammonium Conjugates of Sterols. Molecules 2013, 18, 14961–14976. [Google Scholar] [CrossRef] [PubMed]
- Aher, N.G.; Pore, V.S.; Patil, S.P. Design, synthesis, and micellar properties of bile acid dimers and oligomers linked with a 1,2,3-triazole ring. Tetrahedron 2007, 63, 12927–12934. [Google Scholar] [CrossRef]
- Brycki, B.; Koenig, H.; Kowalczyk, I.; Pospieszny, T. Synthesis, Spectroscopic and Theoretical Studies of New Quaternary N,N-Dimethyl-3-phthalimidopropylammonium Conjugates of Sterols and Bile Acids. Molecules 2014, 19, 4212–4233. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.; Koenig, H.; Kowalczyk, I.; Pospieszny, T. Synthesis, Spectroscopic and Theoretical Studies of New Dimeric Quaternary Alkylammonium Conjugates of Sterols. Molecules 2014, 19, 9419–9434. [Google Scholar] [CrossRef] [PubMed]
- Fujitsu. CAChe 5.04 User Guide; Fujitsu: Chiba, Japan, 2003. [Google Scholar]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods. III Extension of PM3 to Be, Mg, Zn, Ga, Ge, As, Se, Cd, In, Sn, Sb, Te, Hg, Tl, Pb, and Bi. J. Comput. Chem. 1991, 12, 320–341. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Pharma Expert Predictive Services © 2011–2013, Version 2.0. Available online: http://www.pharmaexpert.ru/PASSOnline/ (accessed on 18 November 2013).
- Poroikov, V.V.; Filimonov, D.A.; Borodina, Y.V.; Lagunin, A.A.; Kos, A. Robustness of biological activity spectra predicting by computer program PASS for noncongeneric sets of chemical compounds. J. Chem. Inf. Comput. Sci. 2000, 40, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Poroikov, V.V.; Filimonov, D.A. How to acquire new biological activities in old compounds by computer prediction. J. Comput. Aided. Mol. Des. 2002, 16, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Poroikov, V.V.; Filimonov, D.A. Predictive Toxicology; Helma, C., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 459–478. [Google Scholar]
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. Prediction of biological activity spectra for substances: Evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 106–132 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brycki, B.; Koenig, H.; Pospieszny, T. Quaternary Alkylammonium Conjugates of Steroids: Synthesis, Molecular Structure, and Biological Studies. Molecules 2015, 20, 20887-20900. https://doi.org/10.3390/molecules201119735
Brycki B, Koenig H, Pospieszny T. Quaternary Alkylammonium Conjugates of Steroids: Synthesis, Molecular Structure, and Biological Studies. Molecules. 2015; 20(11):20887-20900. https://doi.org/10.3390/molecules201119735
Chicago/Turabian StyleBrycki, Bogumił, Hanna Koenig, and Tomasz Pospieszny. 2015. "Quaternary Alkylammonium Conjugates of Steroids: Synthesis, Molecular Structure, and Biological Studies" Molecules 20, no. 11: 20887-20900. https://doi.org/10.3390/molecules201119735
APA StyleBrycki, B., Koenig, H., & Pospieszny, T. (2015). Quaternary Alkylammonium Conjugates of Steroids: Synthesis, Molecular Structure, and Biological Studies. Molecules, 20(11), 20887-20900. https://doi.org/10.3390/molecules201119735






