Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology
Abstract
:1. Introduction
| Iron Overload |
| Haemoglobinopathies: β-thalassaemia major, β-thalassaemia intermedia, HbE β-thalassaemia, HbS β-thalassaemia, sickle cell anaemia |
| Anaemias: Aplastic anaemia, sideroblastic anaemia, Blackfan-Diamond anaemia, Fanconis anaemia, pernicious anaemias, congenital dyserythropoietic anaemia, hereditary hypochromic anaemia |
| Hereditary conditions: Idiopathic haemochromatosis, hereditary spherocytosis, pyruvate-kinase deficiency, congenital atransferrinaemia, porphyria cutanea tarda |
| Iatrogenic: Intramuscular iron dextran, dietary or iatrogenic iron intake, iron poisoning |
| Other conditions: Haemolytic disease of the newborn, iron overload in liver disease, iron overload in haemodialysis |
| Iron Imbalance and Oxidative Stress |
| Friedreich’s ataxia, Hallevorden-Spatz syndrome, Parkinson’s disease, Alzheimer’s disease |
| Cyclooxygenase and lipoxygenase inhibitors |
| Congestive cardiac failure, liver disease, acute kidney disease, rheumatoid arthritis |
| Ischaemia reperfusion injury |
| Drug toxicity, e.g., doxorubicin induced cardiac damage |
| Iron Imbalance |
| Anaemia of chronic disease in inflammatory, infectious and neoplasmic diseases |
| Iron deficiency anaemia. |
| Free Radical Pathology |
| All diseases affected by free radical damage and oxidative stress |
| Ageing |
| Metal Toxicity, Diagnostics and Therapeutics |
| Aluminium overload |
| Actinide contamination, e.g., plutonium and uranium |
| Diagnostic metal complexes, e.g., gallium, indium and gadolinium |
| Therapeutic metal complexes, e.g., gold and platinum |
| Other Metal Imbalance and Toxicity Conditions |
| All cancer types with increased iron requirements, neoplasmic disease, neuroblastoma, hepatocellular carcinoma (Adjuvant therapies with anticancer drugs) |
| Infectious Diseases |
| All microbial infections, e.g., meningitis, malaria and other parasitic infections, mucormycosis. (Adjuvant therapies with antimicrobial drugs) |

2. Molecular Aspects of Iron Chelation Therapy
| Chelator | log β | MWt | Kpar | Kpar Iron Complex | Charge |
|---|---|---|---|---|---|
| Deferoxamine | 31 | 561 | 0.02 | 0.02 | positive |
| Deferiprone | 35 | 139 | 0.18 | 0.05 | neutral |
| Deferasirox | 27 | 373 | 6.30 | - | negative |
| Maltol | 30 | 126 | 1.23 | 0.32 | neutral |
| Tropolone | 32 | 122 | 3.04 | 4.50 | neutral |
| Mimosine | 36 | 198 | 0.01 | 0.01 | zwitterionic |
3. The Role and Effect of Low Molecular Weight Iron Chelators, Including Phytochelators, on Iron Metabolism and Toxicity
| Protein | Function |
|---|---|
| Haemoglobin | Oxygen transport |
| Myoglobin | Oxygen transport |
| Cytochromes | Electron transport. Respiration |
| Adrenodoxin | Electron transport. Oxidation/reduction |
| Ferredoxin | Electron transport. Oxidation/reduction. |
| Cyt P450 and b5 | Drug detoxification |
| Ribonucleotide reductase | DNA synthesis |
| Proline hydroxylase | Collagen synthesis |
| Peroxidases | Decomposition of hydroperoxides |
| Catalase | Decomposition of hydronen peroxide |
| Lipoxygenase | HPETE and leukotriene synthesis |
| Cyclooxygenase | Prostaglandin and thromboxane synthesis |
| Aconitase | Tricarboxylic acid cycle |
| Succinate dehydrogenase | Tricarboxylic acid cycle |
| NADH dehydrogenase | Electron transport. Respiration |
| Xanthine oxidase | Conversion of xanthine to uric acid |
| Aldehyde oxidase | Metabolism of aldehydes |
| Transferrin | Iron transport in plasma |
| Lactoferrin | Iron binding in milk and other secretions |
| Ferritin | Iron storage |
| Haemosiderin | Iron storage |
| Hephaestin | Protein affecting iron metabolism |
| Ferroportin | Protein affecting iron metabolism |
| Hepcidin | Protein affecting iron metabolism |
4. The Role of Free Radicals and Antioxidants in Biological Systems
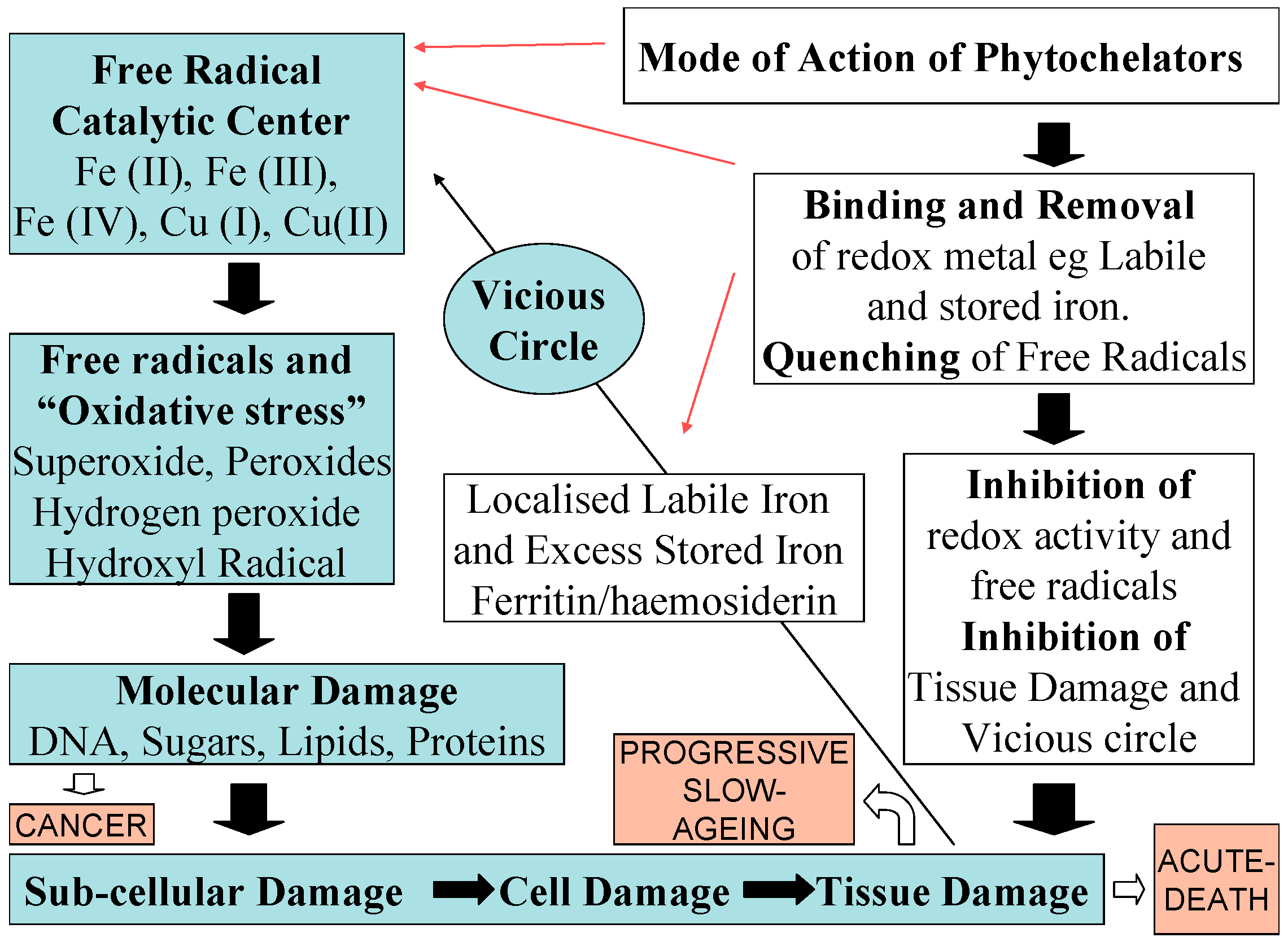
5. Antioxidant Targeting Aspects of Iron Phytochelators and Chelating Drugs
6. Phytochelators with Iron Binding Properties
Chemistry of Phytochelators
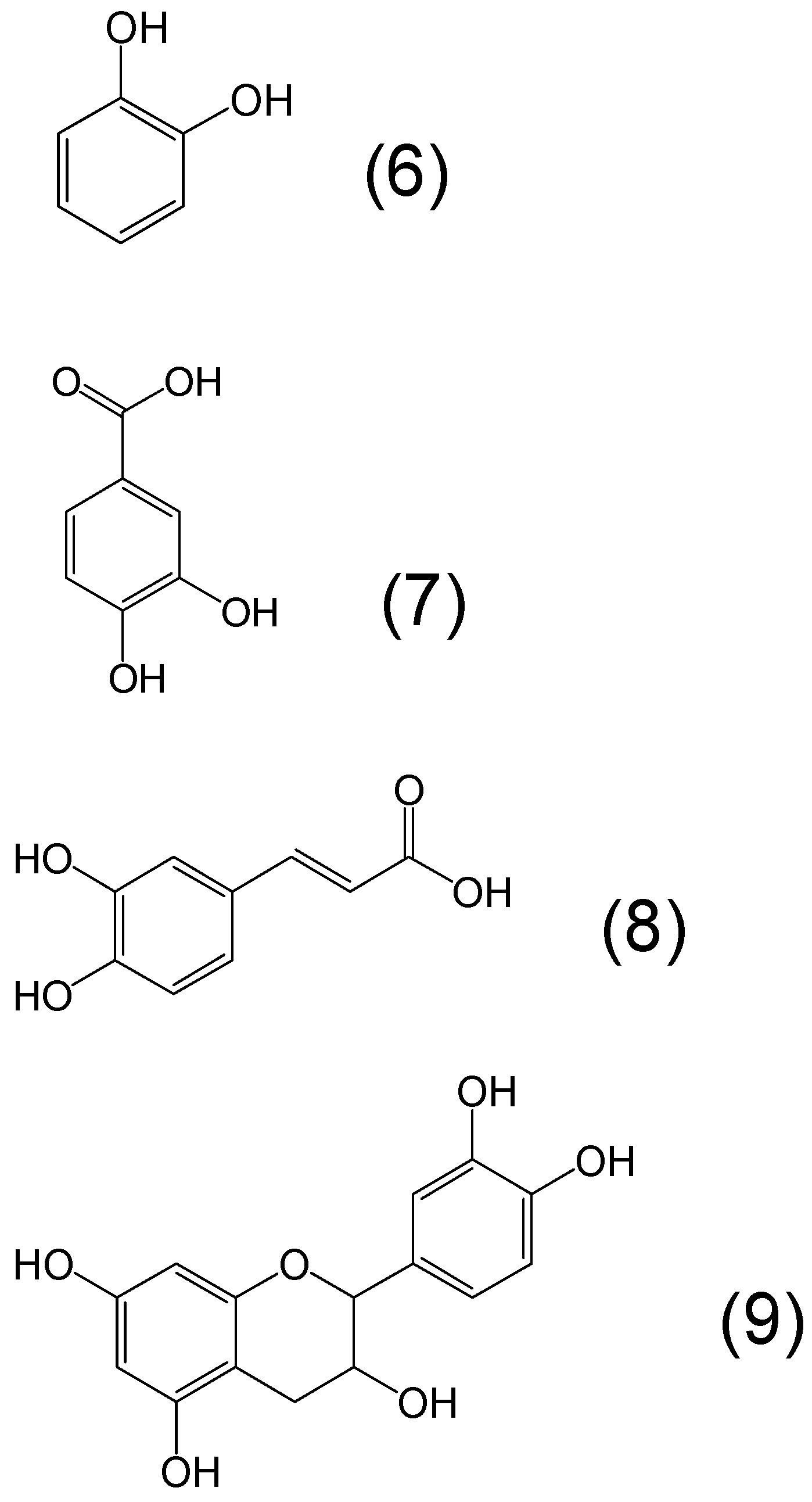
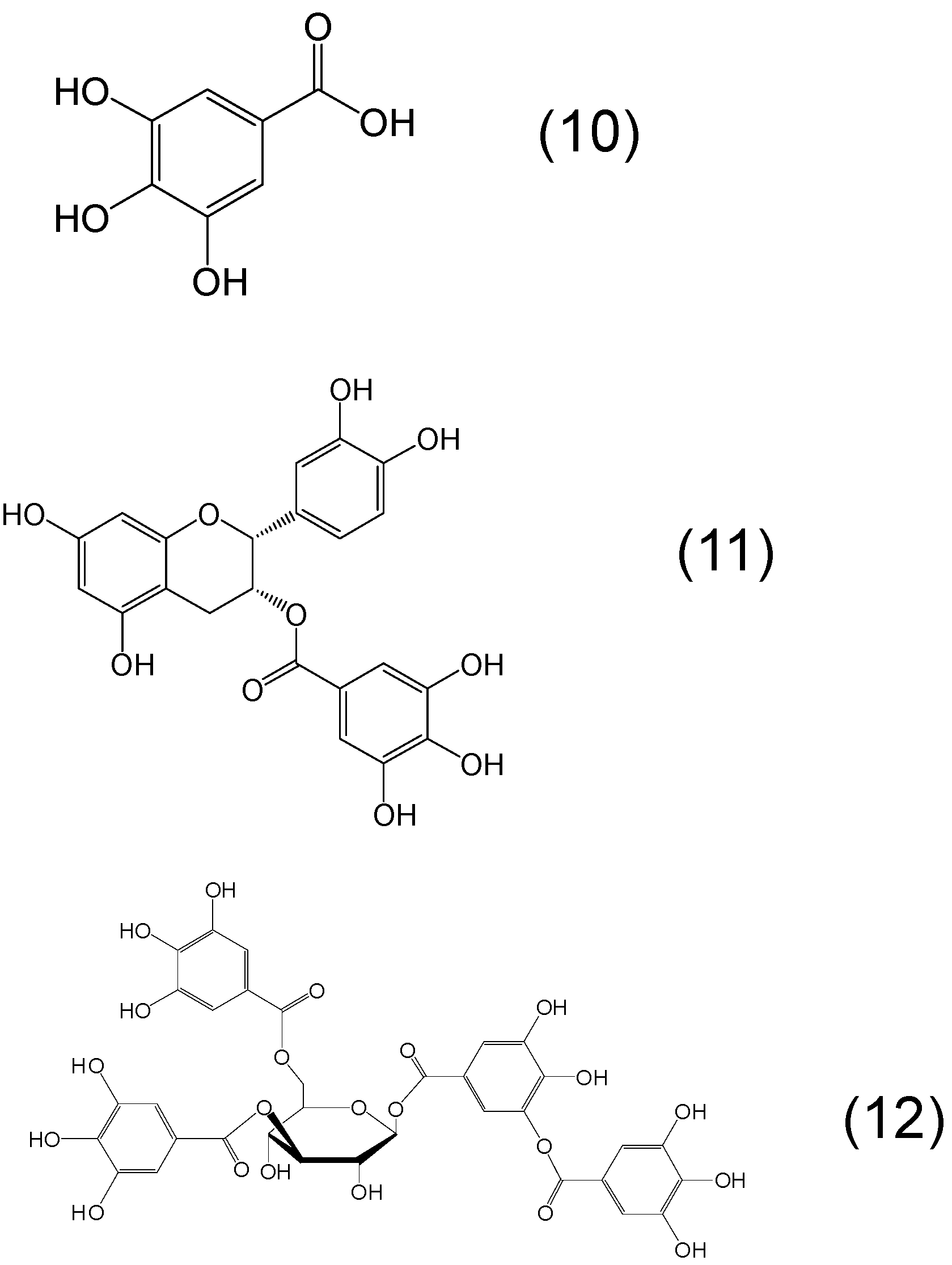
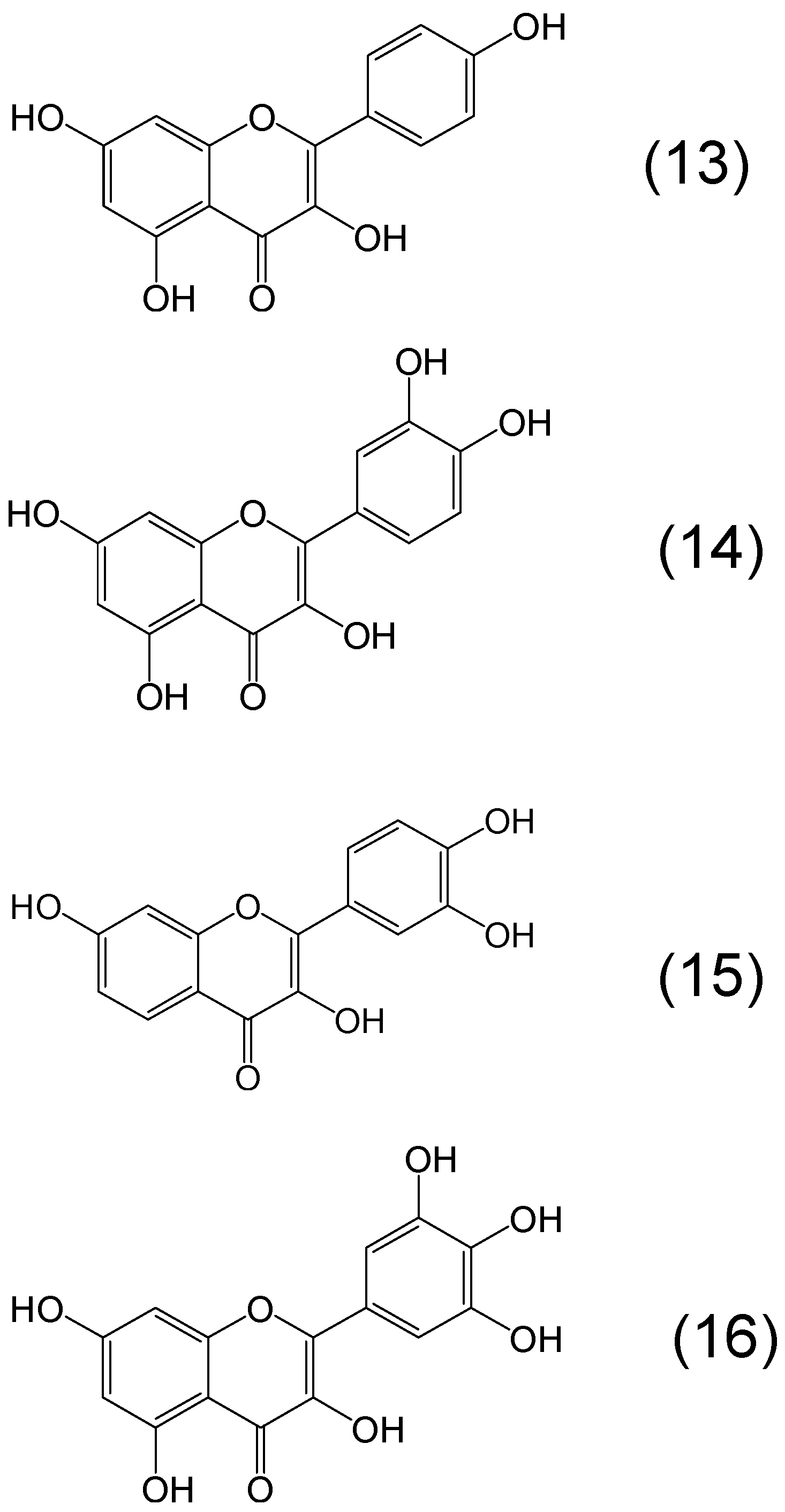
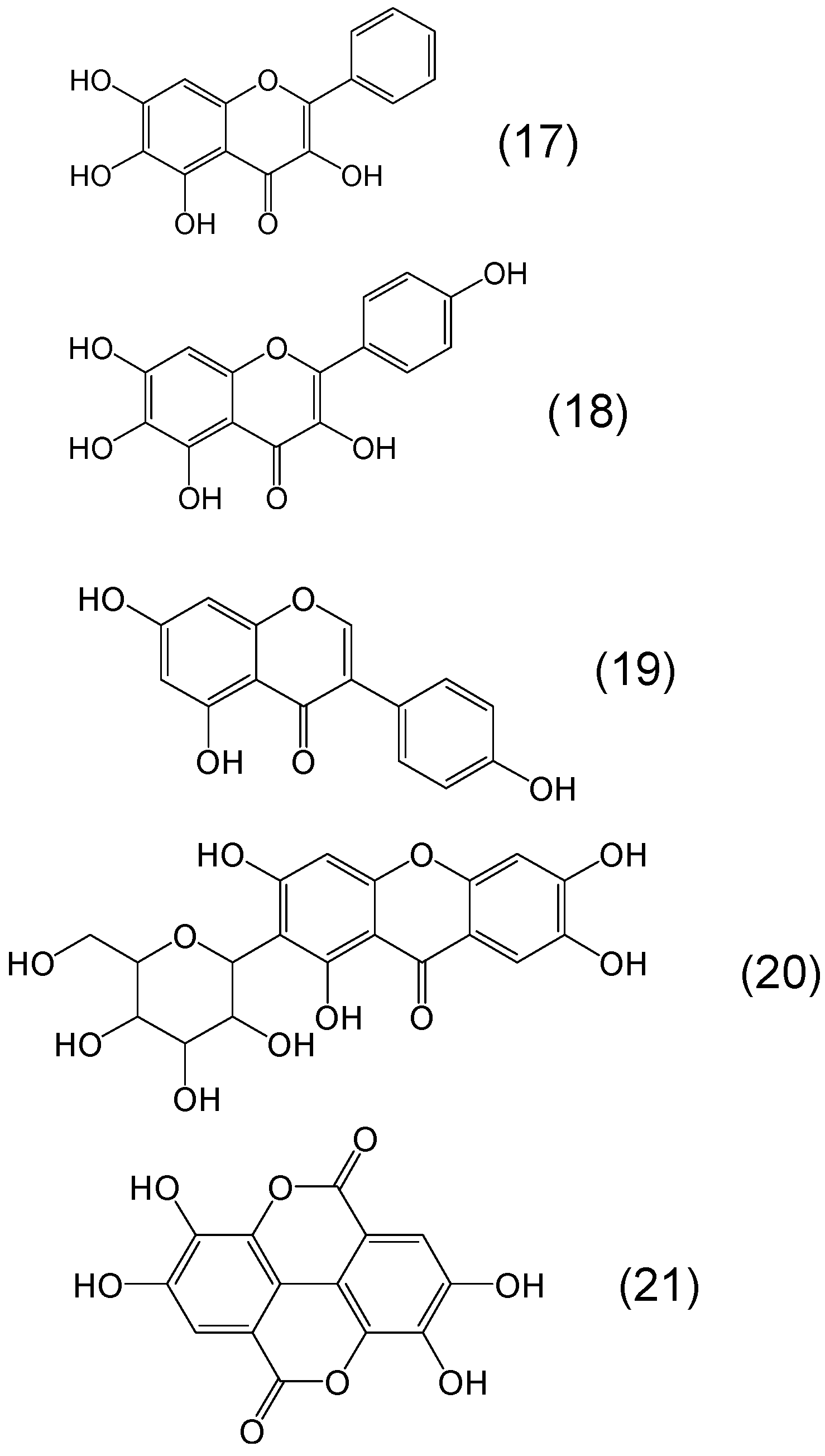
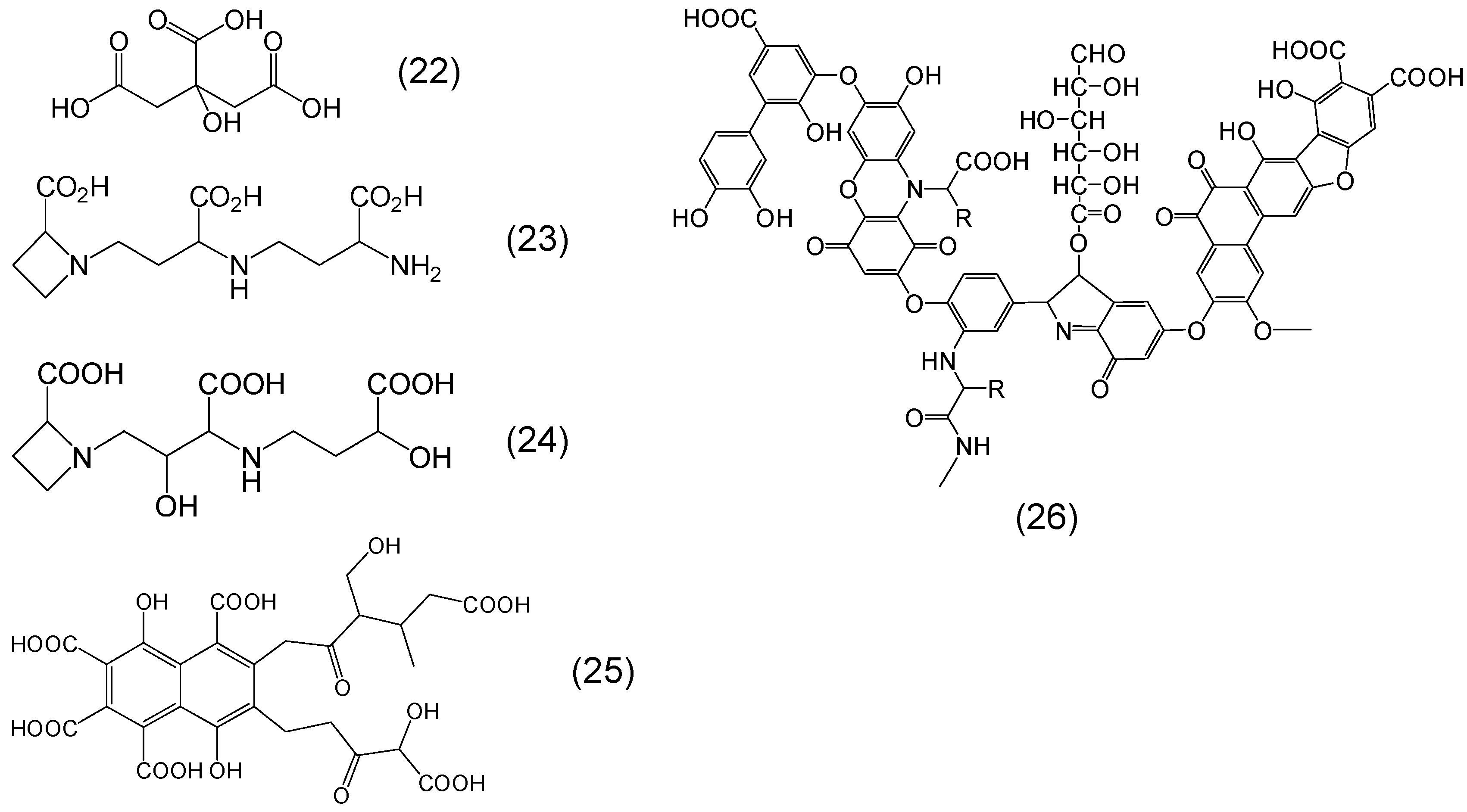
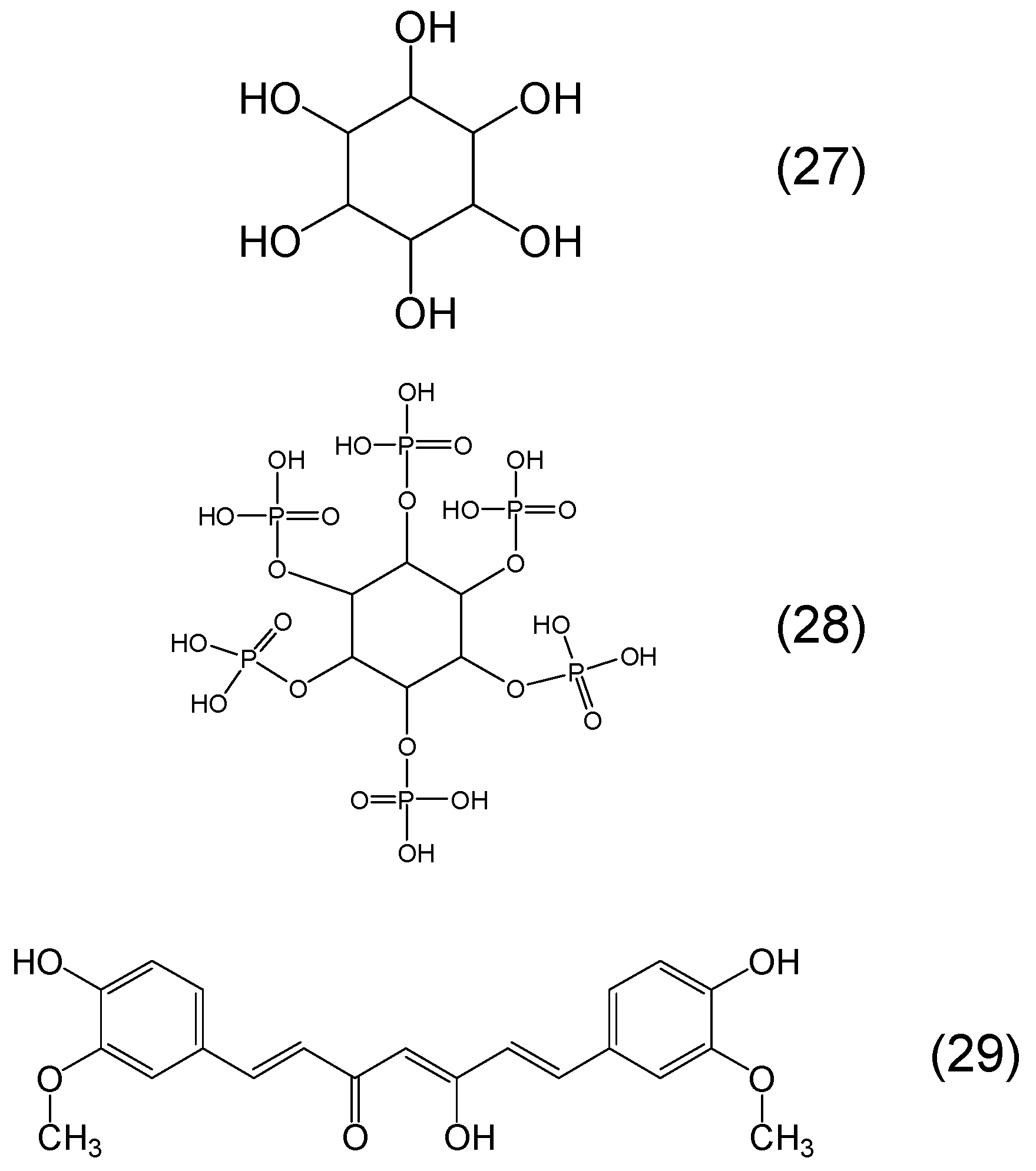
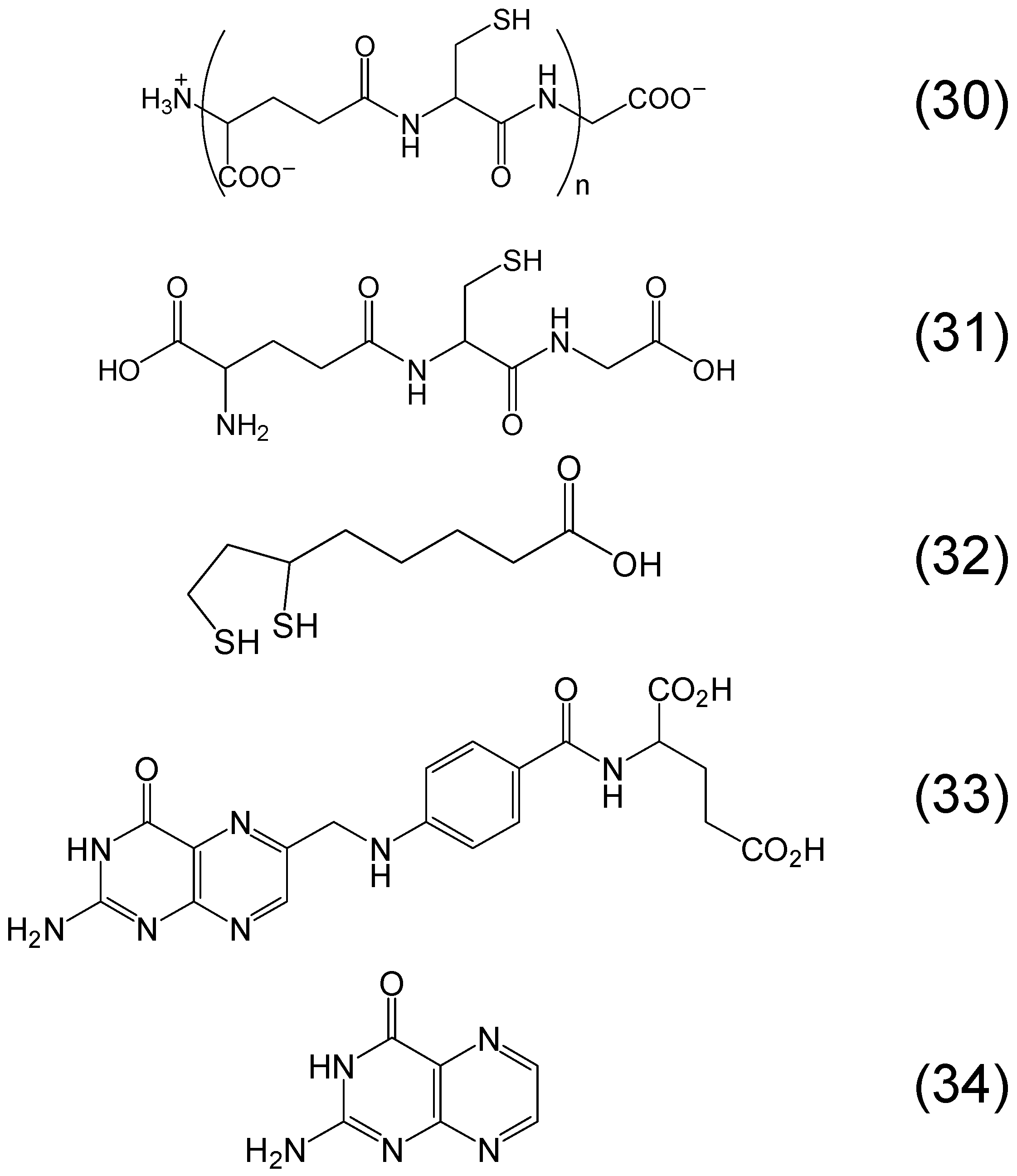

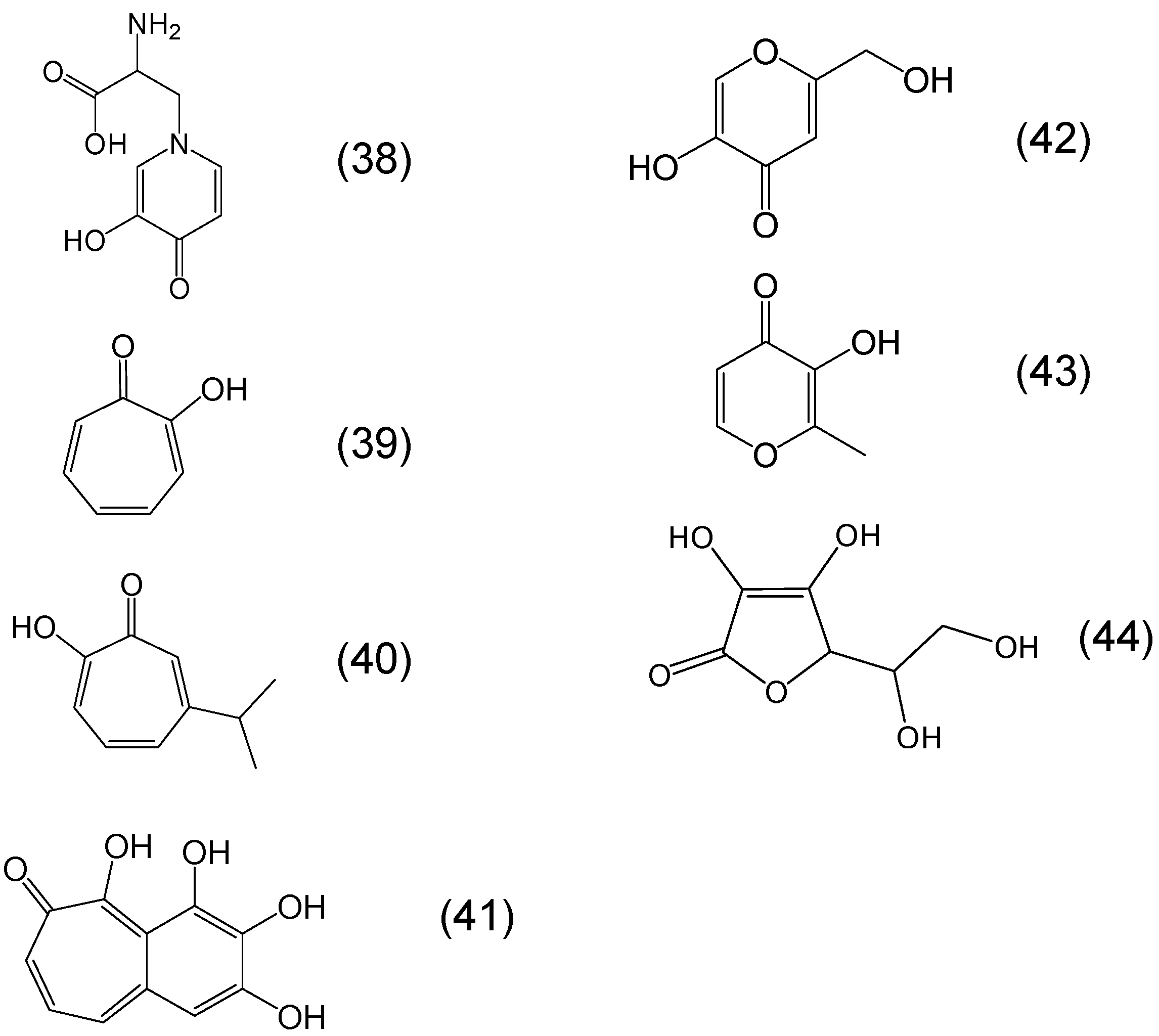
7. Screening of Phytochelators Intended for Clinical Use in the Treatment of Iron Overload
7.1. Initial Screening of Phytotchelators Intended for Clinical Use in Conditions of Iron Metabolism and Free Radical Pathology
7.2. The Screening of Iron Phytochelators and Other Chelators in Cell Models of Iron Metabolism
7.3. The Selection of the Iron Phytochelators Mimosine, Tropolone and Maltol for the Treatment of Iron Overload and Other Diseases of Iron Imbalance and Toxicity
7.4. Clinical Investigations and Human Use of Mimosine, Tropolone, Maltol and Other Iron Phytochelators
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Prospects for introducing deferiprone as potent pharmaceutical antioxidant. Front. Biosci. 2009, 1, 161–178. [Google Scholar]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C.; Cross, C.E. Free radicals, antioxidants and human disease: Where are we now? J. Lab. Clin. Med. 1992, 119, 598–620. [Google Scholar] [PubMed]
- Kontoghiorghes, G.J. Iron chelation in biochemistry and medicine. In Free Radicals, Oxidant Stress and Drug Action; Rice-Evans, C., Ed.; Rechelieu Press: London, UK, 1987; pp. 277–303. [Google Scholar]
- Karogodina, T.Y.; Sergeeva, S.V.; Stass, D.V. Stability and reactivity of free radicals: A physicochemical perspective with biological implications. Hemoglobin 2011, 35, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Andreou, N.; Constantinou, K.; Kontoghiorghes, G.J. World health dilemmas: Orphan and rare diseases, orphan drugs and orphan patients. World J. Methodol. 2014, 4, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lodi, G.; Iriti, M. Efficacy behind activity-phytotherapeutics are not different from pharmaceuticals. Pharm. Biol. 2015, 53, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Neocleous, K.; Kolnagou, A. Benefits and risks of deferiprone in iron overload in thalassaemia and other conditions. Comparison of epidemiological and therapeutic aspects with deferoxamine. Drug Saf. 2003, 26, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Eracleous, E.; Economides, C.; Kolnagou, A. Advances in iron overload therapies. Prospects for effective use of deferiprone (L1), deferoxamine, the new experimental chelators ICL670, GT56–252, L1NAll and their combination. Curr. Med. Chem. 2005, 12, 2663–2681. [Google Scholar] [CrossRef] [PubMed]
- Wruss, J.; Lanzerstorfer, P.; Huemer, S.; Himmelsbach, M.; Mangge, H.; Höglinger, O.; Weghuber, D.; Weghuber, J. Differences in pharmacokinetics of apple polyphenols after standardized oral consumption of unprocessed apple juice. Nutr. J. 2015, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- De Biase, S.; Merlino, G.; Lorenzut, S.; Valente, M.; Gigli, G.L. ADMET considerations when prescribing novel therapeutics to treat restless legs syndrome. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1365–1380. [Google Scholar] [CrossRef] [PubMed]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Community control of hereditary anaemias: Memorandum from a WHO meeting. Bull. World Health Org. 1983, 61, 63–80. [Google Scholar] [PubMed]
- Zurlo, M.G.; de Stefano, P.; Borgna-Pignatti, C.; di Palma, A.; Piga, A.; Melevendi, C.; di Gregorio, F.; Burattini, M.G.; Terzoli, S. Survival and causes of death in thalassaemia major. Lancet 1989, 2, 27–30. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The proceedings of the 20th International Conference on Chelation held in the USA: Advances on new and old chelation therapies. Toxicol. Mech. Methods 2013, 23, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.S.; Williams, G.M.; Frangel, H.H.; Weisburger, J.H. 8-hydroxyquinoline: Chronic toxicity and inhibitory effect on the carcinogenicity of N-2-fluorenylacetamide. Toxicol. Appl. Pharmacol. 1971, 19, 687–698. [Google Scholar] [CrossRef]
- Vreugdenhil, G.; Kontoghiorghes, G.J.; van Eijk, H.G.; Swaak, A.J.G. Impaired erythropoietin responsiveness to the anemia in rheumatoid arthritis. A possible inverse relationship with iron stores and effects of the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one. Clin. Exp. Rheumatol. 1991, 9, 35–40. [Google Scholar] [PubMed]
- Vreughtenhil, G.; Kontoghiorghes, G.J.; van Eijk, H.G.; Swaak, A.J.G. Efficacy and safety of the oral chelator L1 in anaemic rheumadoit arthritis patients. Lancet 1989, 2, 1398–1399. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Antioxidant targeting by deferiprone in diseases related to oxidative damage. Front. Biosci. 2014, 19, 862–885. [Google Scholar] [CrossRef]
- Neilands, J.B. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, Á.; Briones, L.; Andrews, M.; Arredondo, M.; Olivares, M.; Brito, A.; Pizarro, F. Effect of phytic acid, tannic acid and pectin on fasting iron bioavailability both in the presence and absence of calcium. J. Trace Elem. Med. Biol. 2015, 30, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, L.N.; Kontoghiorghes, G.J. Competition between deferiprone, desferrioxamine and other chelators for iron and the effect of other metals. Arzneimittelforschung 1993, 43, 659–663. [Google Scholar] [PubMed]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Potential clinical applications of chelating drugs in diseases targeting transferrin-bound iron and other metals. Expert Opin. Investig. Drugs 2013, 22, 591–618. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Iron mobilization from transferrin and non-transferrin bound iron by deferiprone. Implications in the treatment of thalassaemia, anaemia of chronic disease, cancer and other conditions. Hemoglobin 2006, 30, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Pippard, M.J.; Jackson, M.J.; Hoffman, K.; Petrou, M.; Modell, C.B. Iron chelation using subcutaneous infusion of diethylene triamine penta-acetic acid (DTPA). Scand. J. Haematol. 1986, 36, 466–472. [Google Scholar] [CrossRef] [PubMed]
- De Virgilis, S.; Cognia, M.; Turco, M.P.; Frau, F.; Dessi, C.; Argiolou, F.; Sorcinelli, R.; Sitzia, A.; Cao, A. Depletion of trace elements and acute ocular toxicity induced by desferrioxamine in patients with thalassaemia. Arch. Dis. Child. 1988, 63, 250–255. [Google Scholar] [CrossRef]
- AlRefai, F.N.; Wonke, B.; Wickens, D.G.; Aydinok, Y.; Fielding, A.; Hoffbrand, A.V. Zinc concentration in patients with iron overload receiving oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one or desferrioxamine. J. Clin. Pathol. 1994, 47, 657–660. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Barr, J.; Baillod, R.A. Studies of aluminium mobilisation in renal dialysis patients using the oral chelator 1, 2-dimethyl-3-hydroxypyrid-4-one. Arzneimittelforschung 1994, 44, 522–526. [Google Scholar] [PubMed]
- Jacobs, A. An intracellular transit iron pool. Ciba Found. Symp. 1976, 51, 91–106. [Google Scholar] [PubMed]
- Kolnagou, A.; Kontoghiorghe, C.N.; Kontoghiorghes, G.J. Transition of Thalassaemia and Friedreich ataxia from fatal to chronic diseases. World J. Methodol. 2014, 4, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kolnagou, A. Molecular factors and mechanisms affecting iron and other metal excretion or absorption in health and disease. The role of natural and synthetic chelators. Curr. Med. Chem. 2005, 12, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C.; Graham, G.; Bates, G.W.; Rachmilewitz, E.A. Non-specific serum iron in thalassaemia: An abnormal serum iron fraction of potential toxicity. Br. J. Haematol. 1978, 40, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Chelators affecting iron absorption in mice. Arzneimittelforschung 1990, 40, 1332–1335. [Google Scholar] [PubMed]
- Kontoghiorghes, G.J.; Barr, J.; Nortey, P.; Sheppard, L. Selection of a new generation of orally active alpha-ketohydroxypyridine iron chelators intended for use in the treatment of iron overload. Am. J. Hematol. 1993, 42, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Dresow, B.; Fischer, R.; Nielsen, P.; Gabbe, E.E.; Piga, A. Effect of oral iron chelator L1 on iron absorption in man. Ann. N. Y. Acad. Sci. 1998, 850, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Berkovitch, M.; Livne, A.; Lushkov, G.; Segal, M.; Talmor, C.; Bentur, Y.; Klein, J.; Koren, G. The efficacy of oral deferiprone in acute iron poisoning. Am. J. Emerg. Med. 2000, 18, 36–40. [Google Scholar] [CrossRef]
- Wu, M.L.; Tsai, W.J.; Ger, J.; Deng, J.F. Clinical experience of acute ferric chloride poisoning. Vet. Hum. Toxicol. 2003, 45, 243–246. [Google Scholar] [PubMed]
- Djaldetti, M.; Fishman, P.; Notti, I.; Bessler, H. The effect of tetracycline administration on iron absorption in mice. Biomedicine 1981, 35, 150–152. [Google Scholar] [PubMed]
- Kontoghiorghes, G.J.; Jackson, M.I.; Lunec, J. In vitro screening of iron chelators using models of free radical damage. Free Radic. Res. Commun. 1986, 2, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Srichairatanakool, S.; Ounjaijean, S.; Thephinlap, C.; Khansuwan, U.; Phisalpong, C.; Fuchareon, S. Iron-chelating and free-radical scavenging activities in microwave-processed green tea in iron overload. Hemoglobin 2006, 30, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.C.; Mathur, R. Correction of anemia and iron deficiency in vegetarians by administration of ascorbic acid. Indian J. Physiol. Pharmacol. 1995, 39, 403–406. [Google Scholar] [PubMed]
- Hussain, M.A.; Green, N.; Flynn, D.M.; Hoffbrand, A.V. Effect of dose, time, and ascorbate on iron excretion after subcutaneous desferrioxamine. Lancet 1977, 1, 977–979. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Iwasaki, K.; Sakamoto, K. Iron chelators may help prevent photoaging. J. Cosmet. Dermatol. 2006, 5, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Efstathiou, A.; Kleanthous, M.; Michaelides, Y.; Kolnagou, A. Risk/benefit assessment, advantages over other drugs and targeting methods in the use of deferiprone as a pharmaceutical antioxidant in iron loading and non iron loading conditions. Hemoglobin 2009, 33, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Iyer, S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Inhibition of Fe(2+)- and Fe(3+)-induced hydroxyl radical production by the iron-chelating drug deferiprone. Free Radic. Biol. Med. 2015, 78, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Reeder, B.J.; Wilson, M.T. Hemoglobin and myoglobin associated oxidative stress: From molecular mechanisms to disease states. Curr. Med. Chem. 2005, 12, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Orally active α-ketohydroxypyridine iron chelators. Effects on iron and other metal mobilisations. Acta Haematol. 1987, 78, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.M.; Wilson, M.T. Kinetic studies on the reduction of cytochrome c. Reaction with dihydroxyconjugated compounds (catechols and quinols). Biochem. J. 1982, 20, 433–444. [Google Scholar] [CrossRef]
- Moridani, M.Y.; O’Brien, P.J. Iron complexes of deferiprone and dietary plant catechols as cytoprotective superoxide radical scavengers. Biochem. Pharmacol. 2001, 62, 1579–1585. [Google Scholar] [CrossRef]
- Chakraborty, D.; Bhattacharrya, M. Deferiprone (L1) induced conformation change of hemoglobin: A fluorescence and CD spectroscopic study. Mol. Cell. Biochem. 2000, 204, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.W.; Hiller, M.C. Development of Iron Chelators for Clinical Use; Bethesda: Rockville, MD, USA, 1975; pp. 1–275, DHEW Publication No. NIH 77–994. [Google Scholar]
- Kontoghiorghes, G.J. The Design of Orally Active Iron Chelators for the Treatment of Thalassaemia. Ph.D. Thesis, University of Essex, Colchester, UK, 1982; pp. 1–243. [Google Scholar]
- Kontoghiorghes, G.J. Design, properties and effective use of the oral chelator L1 and other α-ketohydroxypyridines in the treatment of transfusional iron overload in thalassaemia. Ann. N. Y. Acad. Sci. 1990, 612, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Pattichis, K.; Neocleous, K.; Kolnagou, A. The design and development of deferiprone (L1) and other iron chelators for clinical use: Targeting methods and application prospects. Curr. Med. Chem. 2004, 11, 2161–2183. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.R.; Ford, S.J.; Horniblow, R.D.; Iqbal, T.H.; Tselepis, C. Iron chelation in the treatment of cancer: A new role for deferasirox? J. Clin. Pharmacol. 2013, 53, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kolnagou, A.; Skiada, A.; Petrikkos, G. The role of iron and chelators on infections in iron overload and non iron loaded conditions: Prospects for the design of new antimicrobial therapies. Hemoglobin 2010, 34, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Blanusa, M.; Varnai, V.M.; Piasek, M. Kostial, K. Chelators as antidotes of metal toxicity: Therapeutic and experimental aspects. Curr. Med. Chem. 2005, 12, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Queen, B.L.; Tollefsbol, T.O. Polyphenols and aging. Curr. Aging Sci. 2010, 3, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Covas, M.I.; Fitó, M.; Kušar, A.; Pravst, I. Health effects of olive oil polyphenols: Recent advances and possibilities for the use of health claims. Mol. Nutr. Food Res. 2013, 57, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; de la Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Varoni, E.M. Chemopreventive potential of flavonoids in oral squamous cell carcinoma in human studies. Nutrients 2013, 5, 2564–2576. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch. Biochem. Biophys. 2008, 476, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nkhili, E.; Loonis, M.; Mihai, S.; El Hajji, H.; Dangles, O. Reactivity of food phenols with iron and copper ions: Binding, dioxygen activation and oxidation mechanisms. Food Funct. 2014, 5, 1186–1120. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G.; Afanas’ev, I.B. Antioxidant and chelating properties of flavonoids. Adv. Pharmacol. 1997, 38, 151–163. [Google Scholar] [PubMed]
- Namba, K.; Murata, Y. Toward mechanistic elucidation of iron acquisition in barley: Efficient synthesis of mugineic acids and their transport activities. Chem. Rec. 2010, 10, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.; von Wirén, N.; Hayen, H. Investigation of ascorbate-mediated iron release from ferric phytosiderophores in the presence of nicotianamine. Biometals 2008, 21, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Yuan, D.; Zhang, L.; Feng, L.; Chen, Y.; Wang, Y. Effect of environmental factors on the complexation of iron and humic acid. J. Environ. Sci. 2015, 27, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Van Schaik, J.W.; Persson, I.; Kleja, D.B.; Gustafsson, J.P. EXAFS study on the reactions between iron and fulvic acid in acid aqueous solutions. Environ. Sci. Technol. 2008, 42, 2367–2373. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; van den Berg, C.M. Metal complexation by humic substances in seawater. Environ. Sci. Technol. 2009, 43, 7192–7193. [Google Scholar] [CrossRef] [PubMed]
- Christides, T.; Sharp, P. Sugars increase non-heme iron bioavailability in human epithelial intestinal and liver cells. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Huebbe, P.; Frank, J.; Rimbach, G.; Pallauf, K. Curcumin may impair iron status when fed to mice for six months. Redox Biol. 2014, 2, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, V.; Pullakhandam, R.; Nair, K.M. Dietary ligands as determinants of iron-zinc interactions at the absorptive enterocyte. J. Food Sci. 2010, 75, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Egli, I.; Zeder, C.; Walczyk, T.; Hurrell, R. Polyphenols and phytic acid contribute to the low iron bioavailability from common beans in young women. J. Nutr. 2010, 140, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Ogawa, S.; Shamsuddin, A.K.M. Proceedings of the first international symposium on disease prevention by IP6, and other rice components. Anticancer Res. 1999, 19, 3633–3808. [Google Scholar] [PubMed]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Pivato, M.; Fabrega-Prats, M.; Masi, A. Low-molecular-weight thiols in plants: Functional and analytical implications. Arch. Biochem. Biophys. 2014, 560, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Gorąca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid—Biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Campbell, N.R.; Hasinoff, B.B. Iron supplements: A common cause of drug interactions. Br. J. Clin. Pharmacol. 1991, 31, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, C.; Tolrà, R.P.; Barceló, J. A role for cyclic hydroxamates in aluminium resistance in maize? J. Inorg. Biochem. 2005, 99, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, A.; Ishihara, A.; Hasegawa, M.; Kodama, O.; Iwamura, H. Induced accumulation of 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one glucoside (HDMBOA-Glc) in maize leaves. Phytochemistry 2001, 56, 669–675. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C. Chelation of intracellular iron enhances endothelial barrier function: A role for vitamin C? Arch. Biochem. Biophys. 2010, 500, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Fuchinoue, S.; Teraoka, S.; Ota, K. The in vivo cytoprotection of ascorbic acid against ischemia/reoxygenation injury of rat liver. Arch. Biochem. Biophys. 1995, 318, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Mostert, L.J.; van Dorst, J.A.L.M.; Koster, J.F.; van Eijk, H.G.; Kontoghiorghes, G.J. Free radical and cytotoxic effects of chelators and their iron complexes in the hepatocyte. Free Radic. Res. Commun. 1987, 3, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Structure/red blood cell permeability activity of iron(III) chelator complexes. Inorg. Chim. Acta 1988, 151, 101–106. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; May, A. Uptake and intracellular distribution of iron from transferrin and chelators in erythroid cells. Biol. Met. 1990, 3, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Piga, A.; Hoffbrand, A.V. Cytotoxic and DNA-inhibitory effects of iron chelators on human leukaemic cell lines. Hematol. Oncol. 1986, 4, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Forsbeck, K.; Nilsson, K.; Kontoghiorghes, G.J. Variation in iron accumulation, transferrin membrane binding and DNA synthesis in the K-562 and U-937 cell lines induced by chelators and their iron complexes. Eur. J. Haematol. 1987, 39, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.H.; Liceaga, J.; Arthur, H.M.; Kontoghiorghes, G.J. Effect of novel 1-alkyl-3-hydroxy-2-methylpyrid-4-one chelators on uptake and release of iron from macrophages. Am. J. Hematol. 1990, 34, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.H.; Liceaga, J.; Kontoghiorghes, G.J. The effect of synthetic iron chelators on bacterial growth in human serum. FEMS Microbiol. Immunol. 1988, 1, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. The study of iron mobilisation from transferrin using α-ketohydroxy heteroaromatic chelators. Biochim. Biophys. Acta 1986, 869, 141–146. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Iron mobilisation from lactoferrin by chelators at physiological pH. Biochim. Biophys. Acta 1986, 882, 267–270. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Chambers, S.; Hoffbrand, A.V. Comparative study of iron mobilisation from haemosiderin, ferritin and iron(III) precipitates by chelators. Biochem. J. 1987, 241, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Decreased solubilisation of ferritin iron and fresh iron(III) precipitate following repeated chelator treatments. Inorg. Chim. Acta 1987, 138, 36–40. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Evans, R.W. Site specificity of iron removal from transferrin by α-ketohydroxypyridine chelators. FEBS Lett. 1985, 189, 141–144. [Google Scholar] [CrossRef]
- Hegarty, M.P.; Lee, C.P.; Christie, G.S.; Court, R.D.; Haydock, K.P. The goitrogen 3-hydroxy-4(1H)-pyridone, a ruminal metabolite from Leucaena leucocephala: Effects in mice and rats. Aust. J. Biol. Sci. 1979, 32, 27–40. [Google Scholar] [PubMed]
- Hammond, A.C. Leucaena toxicosis and its control in ruminants. J. Anim. Sci. 1995, 73, 1487–1492. [Google Scholar] [PubMed]
- Tsai, W.C.; Ling, K.H. Study of the stability constant of some metal ion chelates of mimosine and 3,4-dihydroxypyridine. J. Chin. Biochem. Soc. 1973, 2, 72–86. [Google Scholar]
- Tsai, W.C.; Ling, K.H. Effect of metals on the absorption and excretion of mimosine and 3,4-dihydroxypyridine in rat in vivo. J. Formosan Med. Assoc. 1971, 73, 543–549. [Google Scholar]
- Harvey, R.S.; Reffitt, D.M.; Doig, L.A.; Meenan, J.; Ellis, R.D.; Thompson, R.P.; Powell, J.J. Ferric trimaltol corrects iron deficiency anaemia in patients intolerant of iron. Aliment. Pharmacol. Ther. 1998, 12, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Reffitt, D.M.; Burden, T.J.; Seed, P.T.; Wood, J.; Thompson, R.P.; Powell, J.J. Assessment of iron absorption from ferric trimaltol. Ann. Clin. Biochem. 2000, 37, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Gasche, C.; Ahmad, T.; Tulassay, Z.; Baumgart, D.C.; Bokemeyer, B.; Büning, C.; Howaldt, S.; Stallmach, A. AEGIS Study Group. Ferric maltol is effective in correcting iron deficiency anemia in patients with inflammatory bowel disease: Results from a phase-3 clinical trial program. Inflamm. Bowel Dis. 2015, 21, 579–588. [Google Scholar] [PubMed]
- Dai, Y.; Gold, B.; Vishwanatha, J.K.; Rhode, S.L. Mimosine inhibits viral DNA synthesis through ribonucleotide reductase. Virology 1994, 205, 210–206. [Google Scholar] [CrossRef] [PubMed]
- Zalatnai, A. P-glycoprotein expression is induced in human pancreatic cancer xenografts during treatment with a cell cycle regulator, mimosine. Pathol. Oncol. Res. 2005, 11, 164–169. [Google Scholar] [CrossRef] [PubMed]
- White, G.P.; Jacobs, A.; Grady, R.W.; Cerami, A. The effect of chelating agents on iron mobilization in Chang cell cultures. Blood 1976, 48, 923–929. [Google Scholar] [PubMed]
- Inamori, Y.; Muro, C.; Sajima, E.; Katagiri, M.; Okamoto, Y.; Tanaka, H.; Sakagami, Y.; Tsujibo, H. Biological activity of purpurogallin. Biosci. Biotechnol. Biochem. 1997, 61, 890–892. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.P.; Wu, T.W.; Lui, C.P. Purpurogallin inhibits DNA synthesis of murine fibrosarcoma L-929 and human U-87 MG glioblastoma cells in vitro. Chemotherapy 1996, 42, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Hsu, W.H.; Yen, T.L.; Luo, J.Y.; Kuo, Y.C.; Fong, T.H.; Sheu, J.R. Hinokitiol, a natural tropolone derivative, offers neuroprotection from thromboembolic stroke in vivo. Evid. Based Complement. Altern. Med. 2013, 2013, 840487. [Google Scholar] [CrossRef] [PubMed]
- Iha, K.; Suzuki, N.; Yoneda, M.; Takeshita, T.; Hirofuji, T. Effect of mouth cleaning with hinokitiol-containing gel on oral malodor: A randomized, open-label pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Borsari, M.; Gabbi, C.; Ghelfi, F.; Grandi, R.; Saladini, M.; Severi, S.; Borella, F. Silybin, a new iron-chelating agent. J. Inorg. Biochem. 2001, 85, 123–129. [Google Scholar] [CrossRef]
- Hutchinson, C.; Bomford, A.; Geissler, C.A. The iron-chelating potential of silybin in patients with hereditary haemochromatosis. Eur. J. Clin. Nutr. 2010, 64, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Hagag, A.A.; Elfrargy, M.S.; Gazar, R.A.; El-Lateef, A.E. Therapeutic value of combined therapy with deferasirox and silymarin on iron overload in children with Beta thalassemia. Mediterr. J. Hematol. Infect. Dis. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, A.; González-Osnaya, L.; Sánchez-Chinchillas, A.; Trejo, A. Role of oxate, phytate, tannins and cooking on iron bioavailability from foods commonly consumed in Mexico. Int. J. Food Sci. Nutr. 2010, 61, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Tuntawiroon, M.; Sritongkul, N.; Brune, M.; Rossander-Hultén, L.; Pleehachinda, R.; Suwanik, R.; Hallberg, L. Dose-dependent inhibitory effect of phenolic compounds in foods on nonheme-iron absorption in men. Am. J. Clin. Nutr. 1991, 53, 554–557. [Google Scholar] [PubMed]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Inderjit, Bajpai, D.; Rajeswari, M.S. Interaction of 8-hydroxyquinoline with soil environment mediates its ecological function. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Quintana, N.; El Kassis, E.G.; Stermitz, F.R.; Vivanco, J.M. Phytotoxic compounds from roots of Centaurea diffusa Lam. Plant Signal. Behav. 2009, 4, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Adão, P.; Roy, S.; Wahba, M.; Matos, C.; Maurya, M.R.; Marques, F.; Pavan, F.R.; Leite, C.Q.; Avecilla, F.; Costa Pessoa, J. Hydroxyquinoline derived vanadium(IV and V) and copper(II) complexes as potential anti-tuberculosis and anti-tumor agents. J. Inorg. Biochem. 2014, 141, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; de Vries, E.F.; Jamar, F.; Israel, O.; Signore, A. Guidelines for the labelling of leucocytes with (111)In-oxine. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Chobot, V.; Drage, S.; Hadacek, F. Redox properties of 8-quinolinol and implications for its mode of action. Nat. Prod. Commun. 2011, 6, 597–602. [Google Scholar] [PubMed]
- Kontoghiorghes, G.J. Comparative efficacy and toxicity of desferrioxamine, deferiprone and other iron and aluminium chelating drugs. Toxicol. Lett. 1995, 80, 1–18. [Google Scholar] [CrossRef]
- Born, T.; Kontoghiorghe, C.N.; Spyrou, A.; Kolnagou, A.; Kontoghiorghes, G.J. EDTA chelation reappraisal following new clinical trials and regular use in millions of patients: Review of preliminary findings and risk/benefit assessment. Toxicol. Mech. Methods 2013, 23, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Leszczyszyn, O.I.; Imam, H.T.; Blindauer, C.A. Diversity and distribution of plant metallothioneins: A review of structure, properties and functions. Metallomics 2013, 5, 1146–1169. [Google Scholar] [CrossRef] [PubMed]
- Grennan, A.K. Metallothioneins, a diverse protein family. Plant Physiol. 2011, 155, 1750–1751. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. A new era in iron chelation therapy: The design of optimal, individually adjusted iron chelation therapies for the complete removal of iron overload in thalassemia and other chronically transfused patients. Hemoglobin 2009, 33, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C. Unravelling of the health effects of polyphenols is a complex puzzle complicated by metabolism. Arch. Biochem. Biophys. 2014, 559, 100–105. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Riddell, L.J.; Nowson, C.A.; Booth, A.O.; Szymlek-Gay, E.A. Iron and zinc nutrition in the economically-developed world: A review. Nutrients 2013, 5, 3184–3211. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.Y.; Kovacevic, Z.; Richardson, V.; Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Targeting cancer by binding iron: Dissecting cellular signaling pathways. Oncotarget 2015, 6, 18748–18779. [Google Scholar] [CrossRef] [PubMed]
- Rajapurkar, M.M.; Hegde, U.; Bhattacharya, A.; Alam, M.G.; Shah, S.V. Effect of deferiprone, an oral iron chelator, in diabetic and non-diabetic glomerular disease. Toxicol. Mech. Methods 2013, 23, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Arkadopoulos, N.; Nastos, C.; Kalimeris, K.; Economou, E.; Theodoraki, K.; Kouskouni, E.; Pafiti, A.; Kostopanagiotou, G.; Smyrniotis, V. Iron chelation for amelioration of liver ischemia-reperfusion injury. Hemoglobin 2010, 34, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Sánchez, D.; Aracil, A.; Montero, R.; Mas, A.; Jiménez, L.; O’Callaghan, M.; Tondo, M.; Capdevila, A.; Blanch, J.; Artuch, R.; et al. Combined therapy with idebenone and deferiprone in patients with Friedreich’s ataxia. Cerebellum 2011, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Ghosh, K.; Pathare, A.V.; Karnad, D. Deferiprone (L1) as an adjuvant therapy for Plasmodium falciparum malaria. Indian J. Med. Res. 2002, 115, 17–21. [Google Scholar] [PubMed]
- Galaris, D.; Pantopoulos, K. Oxidative stress and iron homeostasis: Mechanistic and health aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.C.; Richardson, D.R.; Kalinowski, D.S.; Bernhardt, P.V. Synthetic and natural products as iron chelators. Curr. Top. Med. Chem. 2011, 11, 591–607. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules 2015, 20, 20841-20872. https://doi.org/10.3390/molecules201119725
Kontoghiorghe CN, Kolnagou A, Kontoghiorghes GJ. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules. 2015; 20(11):20841-20872. https://doi.org/10.3390/molecules201119725
Chicago/Turabian StyleKontoghiorghe, Christina N., Annita Kolnagou, and George J. Kontoghiorghes. 2015. "Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology" Molecules 20, no. 11: 20841-20872. https://doi.org/10.3390/molecules201119725
APA StyleKontoghiorghe, C. N., Kolnagou, A., & Kontoghiorghes, G. J. (2015). Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules, 20(11), 20841-20872. https://doi.org/10.3390/molecules201119725







