Comparative Anticonvulsant Study of Epoxycarvone Stereoisomers
Abstract
:1. Introduction
2. Results and Discussion
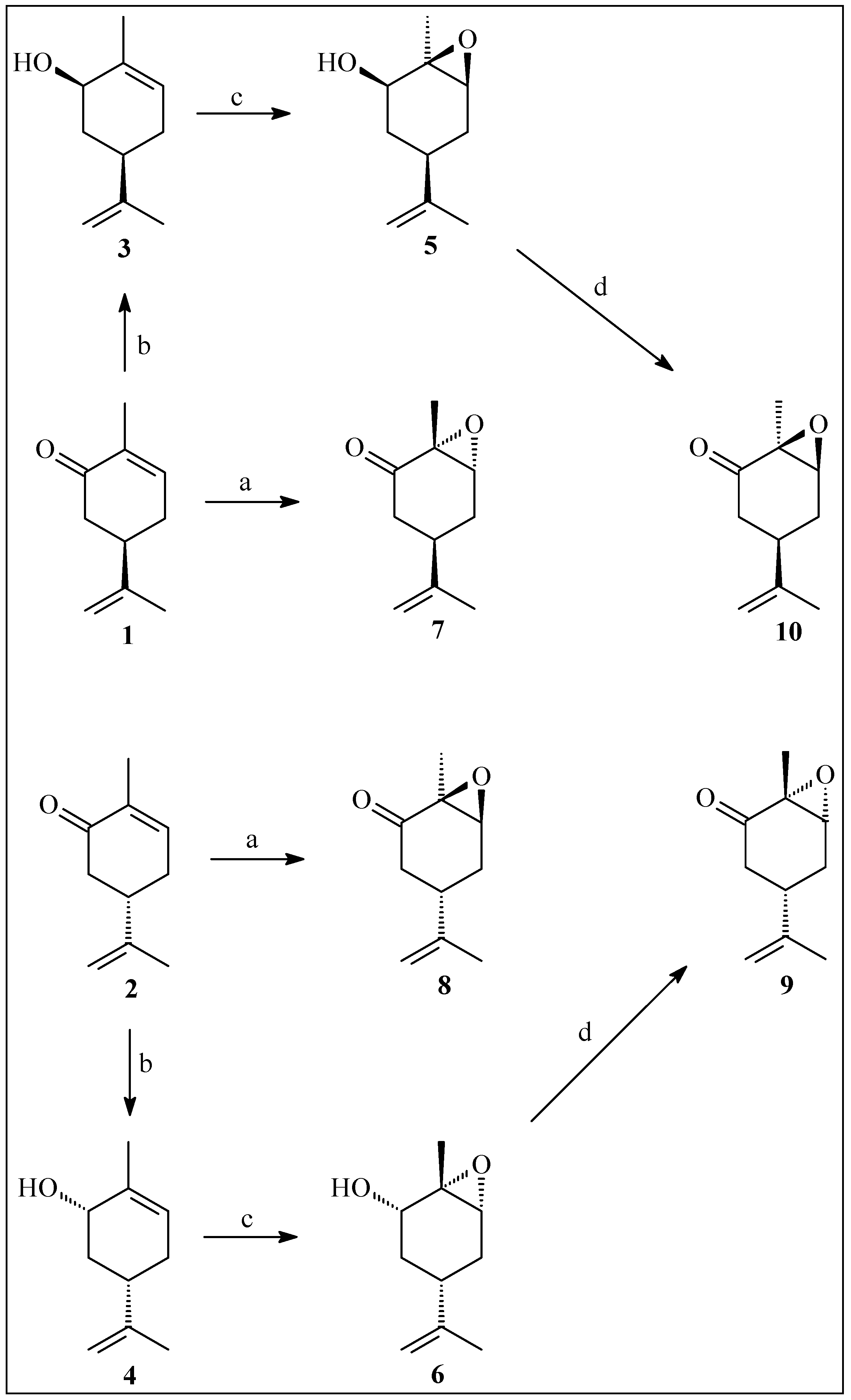
2.1. Preparation of Epoxycarvone Stereoisomers
2.2. Pentylenetetrazole-Induced Seizure Test
| Experimental Groups | Latency (s) | Mortality (%) |
|---|---|---|
| Control (Tween 80 5%) | 109.9 ± 13.0 | 50 |
| Diazepam (standard) | 900.0 ± 0.0 a | 0 |
| (+)-cis-EC (7) | 900.0 ± 0.0 a | 0 |
| (−)-cis-EC (8) | 763.3 ± 69.3 a | 0 |
| (+)-trans-EC (9) | 791.1 ± 108.9 a | 0 |
| (−)-trans-EC (10) | 743.0 ± 83.7 a | 0 |
2.3. Maximal Electroshock-Induced Seizure (MES)
| Experimental Groups | Seizures Duration (s) | Tonic Seizures (%) | Mortality (%) |
|---|---|---|---|
| Control (Tween 80 5%) | 16.3 ± 0.9 | 100 | 37.5 |
| Phenytoin (standard) | 0.0 ± 0.0 a | 0 | 0 |
| (+)-cis-EC (7) | 9.8 ± 0.5 a | 100 | 0 |
| (−)-cis-EC (8) | 8.3 ± 1.4 a | 87.5 | 0 |
| (+)-trans-EC (9) | 0.7 ± 0.7 a | 25 | 0 |
| (−)-trans-EC (10) | 0.0 ± 0.0 a | 12.5 | 0 |
2.4. Pilocarpine-Induced Seizures Test
| Experimental Group | Latency to Convulsions (s) | Latency to Death (s) | Peripheral Cholinergic Signs (%) | Stereotyped Movements (%) | Tremors (%) | Seizures (%) | Status Epilepticus (%) | Mortality (%) |
|---|---|---|---|---|---|---|---|---|
| Control (Tween 80 5%) | 429.3 ± 25.4 | 817.6 ± 22.3 | 100 | 100 | 100 | 100 | 100 | 100 |
| Diazepam (standard) | 3600.0 ± 0.0 b | 3600.0 ± 0.0 b | 100 | 0 | 50 | 12.5 | 0 | 0 |
| (+)-cis-EC (7) | 862.6 ± 36.6 b | 1845.9 ± 453.1 | 100 | 50 | 100 | 100 | 0 | 75 |
| (−)-cis-EC (8) | 890.8 ± 76.2 b | 1135.9 ± 68.1 | 100 | 100 | 100 | 100 | 50 | 100 |
| (+)-trans-EC (9) | 1044.0 ± 49.1 b | 2895.7 ± 343.3 b | 50 | 100 | 50 | 100 | 50 | 50 |
| (−)-trans-EC (10) | 888.4 ± 78.3 b | 2345.0 ± 477.9 a | 50 | 100 | 50 | 100 | 50 | 50 |
2.5. Strychnine-Induced Seizure Test
| Experimental Group | Latency to Convulsions (s) | Latency to Death (s) | Seizures (%) | Mortality (%) |
|---|---|---|---|---|
| Control (Tween 80 5%) | 51.1 ± 0.5 | 53.0 ± 0.6 | 100 | 100 |
| Diazepam (standard) | 144.3 ± 29.4 a | 435.8 ± 93.2 b | 100 | 0 |
| (+)-cis-EC (7) | 79.2 ± 20.8 | 306.5 ± 55.3 | 100 | 100 |
| (−)-cis-EC (8) | 89.2 ± 9.1 | 169.3 ± 17.8 | 100 | 100 |
| (+)-trans-EC (9) | 111.8 ± 21.8 | 396.5 ± 61.5 c | 100 | 100 |
| (−)-trans-EC (10) | 65.0 ± 4.9 | 273.8 ± 51.4 | 100 | 100 |
3. Experimental Section
3.1. Reagents
3.2. Preparation of Epoxycarvone Stereoisomers
3.2.1. Reduction of (−)-Carvone (1) and (+)-Carvone (2)
3.2.2. Epoxidation of (−)-cis-Carveol (3) and (+)-cis-Carveol (4)
3.2.3. Oxidation of (−)-Carveol Epoxide (5) and (+)-Carveol Epoxide (6)
3.2.4. Epoxidation of (−)-Carvone (1) and (+)-Carvone (2)
3.3. Experimental Section
3.3.1. Animals
3.3.2. Behavioral Experiments
Pentylenetetrazole-induced Seizure Test
Maximal Electroshock-induced Seizure (MES)
Pilocarpine-induced Seizure Test
Strychnine-induced Seizure Test
3.3.3. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Engel, J. Concepts of epilepsy. Epilepsia 1995, 36, 23–30. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Refractory epilepsy: Mechanisms and solutions. Expert Rev. Neurother. 2006, 6, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, G.; Meador, K. Epilepsy and cognition. Epilepsy Behav. 2003, 4, 25–38. [Google Scholar] [CrossRef]
- Fortini, S.; Corredera, L.; Pastrana, A.L.; Reyes, G.; Fasulo, L.; Caraballo, R.H. Encephalopathy with hemi-status epilepticus during sleep or hemi-continuous spikes and waves during slow sleep syndrome: A study of 21 patients. Seizure 2013, 22, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Galanopoulou, A.S.; Buckmaster, P.S.; Staley, K.J.; Moshe, S.L.; Perucca, E.; Engel, J., Jr.; Loscher, W.; Noebels, J.L.; Pitkanen, A.; et al. Identification of new epilepsy treatments: Issues in preclinical methodology. Epilepsia 2012, 53, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.T. Understanding the delay before epilepsy surgery: Who develops intractable focal epilepsy and when? CNS Spectrums 2004, 9, 136–144. [Google Scholar] [PubMed]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of newdrugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Craveiro, A.A.; Fernandes, A.G.; Andrade, C.H.S.; Matos, F.J.A.; Alencar, J.W.; Machado, M.I.L. Óleos Essenciais de Plantas do Nordeste; Edições UFC: Fortaleza, Brazil, 1981. [Google Scholar]
- Pultrini, A.M.; Galindo, L.A.; Costa, M. Effects of the essential oil from Citrus aurantium L. in experimental anxiety models in mice. Life Sci. J. 2006, 78, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Lis-Balchin, M.; Hart, S. Studies on the mode of action of the essential oil of lavender (Lavandula angustifolia P. Miller). Phytother. Res. 1999, 13, 540–542. [Google Scholar] [CrossRef]
- Santos, F.A.; Jeferson, F.A.; Santos, C.C.; Silveira, E.R.; Rao, V.S.N. Antinociceptive effect of leaf essential oil from Croton sonderianus in mice. Life Sci. J. 2005, 77, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.N.; Agra, M.F.; Souto Maior, F.N.; Sousa, D.P. Essential Oils and Their Constituents: Anticonvulsant Activity. Molecules 2011, 16, 2726–2742. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, F.F.F.; Salvadori, M.G.S.S.; Masson, C.J.; Mello, C.F.; Nascimento, T.S.; Leal-Cardoso, J.H.; Sousa, D.P.; Almeida, R.N. Monoterpenoid Terpinen-4-ol Exhibits Anticonvulsant Activity in Behavioural and Electrophysiological Studies. Oxid. Med. Cell. Longev. 2014, 2014, 703848. [Google Scholar] [CrossRef] [PubMed]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Vokou, D.; Douvli, P.; Blionis, G.J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef] [PubMed]
- Zunino, M.P.; Turina, A.V.; Zygadlo, J.A.; Perillo, M.A. Stereoselective effects of monoterpenes on the microviscosity and curvature of model membranes assessed by DPH steady-state fluorescence anisotropy and light scattering analysis. Chirality 2011, 23, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, N.S.; Lo Cantore, P.; Capasso, F.; Senatore, F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J. Agric. Food Chem. 2005, 53, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Shafi, P.M.; Abraham, G.T. Analysis of the essential oil of the roots of the medicinal plant Kaempferia galanga L. (Zingiberaceae) from South India. Acta Pharm. Turc. 2001, 43, 107–110. [Google Scholar]
- Kaiser, R. New or uncommon volatile components in the most diverse natural scents. Rev. Ital. Eppos 1997, 18, 18–47. [Google Scholar]
- Klein, E.; Ohloff, G. Der stereochemische verlauf der alkalischen epoxydation von α,β-ungesättigten carbonylverbindungen der cyclischen monoterpenreihe. Tetrahedron 1963, 19, 1091–1099. [Google Scholar] [CrossRef]
- Arruda, T.A.; Antunes, R.M.P.; Catão, R.M.R.; Lima, E.O.; de Sousa, D.P.; Nunes, X.P.; Pereira, M.S.V.; Barbosa-Filho, J.M.; da Cunha, E.V.L. Preliminary study of the antimicrobial activity of Mentha x villosa Hudson essential oil, rotundifolone and its analogues. Rev. Bras. Farmacogn. 2006, 16, 307–311. [Google Scholar] [CrossRef]
- De Sousa, D.P.; Nóbrega, F.F.F.; Claudino, F.S.; Almeida, R.N.; Leite, J.R.; Mattei, R. Pharmacological effects of the monoterpene α,β-epoxy-carvone in mice. Rev. Bras. Farmacogn. 2007, 17, 170–175. [Google Scholar] [CrossRef]
- Rocha, M.L.; Oliveira, L.E.G.; Santos, C.C.M.P.; De Sousa, D.P.; Almeida, R.N.; Araújo, D.A.M. Antinociceptive and anti-inflammatory effects of the monoterpene α,β-epoxy-carvone in mice. J. Nat. Med. 2013, 67, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.N.; De Sousa, D.P.; Nóbrega, F.F.F.; Claudino, F.S.; Araújo, D.A.M.; Leite, J.R.; Mattei, R. Anticonvulsant effect of a natural compound α,β-epoxy-carvone and its action on the nerve excitability. Neurosci. Lett. 2008, 443, 51–55. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; Nobrega, F.F.F.; de Almeida, R.N. Influence of the chirality of (R)-(–)- and (S)-(+)-carvone in the central nervous system: A comparative study. Chirality 2007, 19, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Valeev, R.F.; Vostrikov, N.S.; Miftakhov, M.S. Synthesis and Some Transformations of (–)-Carveol. Russ. J. Org. Chem. 2009, 45, 810–814. [Google Scholar] [CrossRef]
- Lindquist, N.; Battiste, M.A.; Whitten, W.M.; Williams, N.H.; Strekowski, L. Trans-carvone Oxide, A Monoterpene Epoxide from the Fragrance of Catasetum. Phytochemistry 1985, 24, 863–865. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Hetényi, A.; Fulöp, F. Synthesis and application of monoterpene-based chiral aminodiols. Tetrahedron 2008, 64, 1034–1039. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Csillag, K.; Fülöp, F. Stereoselective synthesis of carane-based aminodiols as chiral ligands for the catalytic addition of diethylzinc to aldehydes. Tetrahedron Asymmetry 2011, 22, 1021–1027. [Google Scholar] [CrossRef]
- Rogawski, M.A. Diverse mechanisms of antiepileptic drugs in development. Epilepsy Res. 2006, 69, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Praveen, K.U.; Naga, P.K.; Murali, K.B.; Swarnalatha, M. Evaluation of antiepileptic activity of methanolic extract of Brassica nigra seeds in mice. Int. J. Pharm. Innov. 2013, 3, 73–84. [Google Scholar]
- Zhou, C.; Li, C.; Yu, H.M.; Zhang, F.; Han, D.; Zhang, G.Y. Neuroprotection of gamma-aminobutyric acid receptor agonists via enhancing neuronal nitric oxide synthase (Ser847) phosphorylation through increased neuronal nitric oxide synthase and PSD95 interaction and inhibited protein phosphatase activity in cerebral ischemia. J. Neurosci. Res. 2008, 13, 2973–2983. [Google Scholar]
- Shin, E.J.; Bach, J.H.; Nguyen, T.T.L.; Jung, B.D.; Oh, K.W.; Kim, M.J.; Jang, C.G.; Ali, S.F.; Ko, S.K.; Yang, C.H.; et al. Gastrodia elata Bl attenuates cocaine-induced conditioned place preference and convulsion, but not behavioral sensitization in mice: Importance of GABAA receptors. Curr. Neuropharmacol. 2011, 9, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.Y.; Sule, M.I.; Sallau, M.S. Synthesis and anticonvulsant studies of isomeric N-benzyl-3-[(methylphenyl)amino]propanamides. Niger. J. Pharm. Sci. 2010, 9, 10–20. [Google Scholar]
- Wieland, S.; Belluzzi, J.D.; Stein, L.; Lan, N.C. Comparative behavioral characterization of the neuroactive steroids 3α-OH,5α-pregnan-20-one and 3α-OH,5β-pregnan-20-one in rodents. Psychopharmacology 1995, 118, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Krall, R.L.; Penry, J.K.; White, B.G.; Kupferberg, H.J.; Swinyard, E.A. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978, 19, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Fassbender, C.P.; Nolting, B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res. 1991, 8, 79–94. [Google Scholar] [CrossRef]
- Mawasi, H.; Shekh-Ahmad, T.; Finnell, R.H.; Wlodarczyk, B.J.; Bialer, M. Pharmacodynamic and pharmacokinetic analysis of CNS-active constitutional isomers of valnoctamide and sec-butylpropylacetamide—Amide derivatives of valproic acid. Epilepsy Behav. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-X.; Miao, J.-K.; Li, C.; Li, X.-W.; Wu, X.-M.; Zhangc, X.-P. Anticonvulsant Activity of Acute and Chronic Treatment with a-Asarone from Acorus gramineus in Seizure Models. Biol. Pharm. Bull. 2013, 36, 26–30. [Google Scholar] [CrossRef]
- Zolkowska, D.; Dhir, A.; Krishnan, K.; Covey, D.F.; Rogawski, M.A. Anticonvulsant potencies of the enantiomers of the neurosteroids androsterone and etiocholanolone exceed those of the natural forms. Psychopharmacology 2014, 231, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Kayal, A.R.; Shumate, M.D.; Jin, H.; Rikhter, T.Y.; Coulter, D.A. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat. Med. 1998, 4, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Loup, F.; Wieser, H.G.; Yonekawa, Y.; Aguzzi, A.; Fritschy, J.M. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. Neuron 2000, 20, 5401–5419. [Google Scholar]
- Zgrajka, W.; Nieoczym, D.; Czuczwar, M.; Kiís, J.; Brzana, W.; Wlaíz, P.; Turski, W.A. Evidences for pharmacokinetic interaction of riluzole and topiramate with pilocarpine in pilocarpine-induced seizures in rats. Epilepsy Res. 2010, 88, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.B.; Olney, J.W.; Maniotis, A.; Collins, R.C.; Zorumski, C.F. The functional anatomy and pathology of lithium-pilocarpine and high-dose pilocarpine seizures. Neuroscience 1987, 23, 953–968. [Google Scholar] [CrossRef]
- Schauwecker, P.E. Strain differences in seizure-induced cell death following pilocarpine-induced status epilepticus. Neurobiol. Dis. 2012, 45, 297–304. [Google Scholar] [CrossRef] [PubMed]
- White, H.S.; Alex, A.B.; Pollock, A.; Hen, N.; Shekh-Ahmad, T.; Wilcox, K.S.; McDonough, J.H.; Stables, J.P.; Kaufmann, D.; Yagen, B.; et al. A new derivative of valproic acid amide possesses a broad-spectrum antiseizure profile and unique activity against status epilepticus and organophosphate neuronal damage. Epilepsia 2012, 53, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Dawidowski, M.; Wilczek, M.; Kubica, K.; Skolmowski, M.; Turło, J. Structure–activity relationships of the aromatic site in novel anticonvulsant pyrrolo[1,2-a]pyrazine derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 6106–6110. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.G.; Vogel, W.H. Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: New York, NY, USA, 2002; pp. 421–424. [Google Scholar]
- Kuno, M.; Weakly, J.N. Quantal components of the inhibitory synaptic potentials in spinal motor neurons of cat. J. Physiol. 1972, 224, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Areco, V.A.; Figueroa, S.; Cosa, M.T.; Dambolena, J.S.; Zygadlo, J.A.; Zunino, M.P. Effect of pinene isomers on germination and growth of maize. Biochem. Syst. Ecol. 2014, 55, 27–33. [Google Scholar] [CrossRef]
- Okoye, T.C.; Akah, P.A.; Omeje, E.O.; Okoye, F.B.; Nworu, C.S. Anticonvulsant effect of kaurenoic acid isolated from the root bark of annona senegalensis. Pharmacol. Biochem. Behav. 2013, 109, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Sadeghnia, H.R. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of gabaergic and opioids systems. Phytomedicine 2007, 14, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Sadeghi Shakib, S.; Khadem Sameni, A.; Taghiabadi, E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran. J. Pharm. Res. 2013, 12, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, J.J.; Włodarczyk, M.; Gleńsk, M.; Marzęda, E.; Durmowicz, D.; Florek-Łuszczki, M.J. Effects of alizarin, betulin, curcumin, diosmin, linalool, menthofuran, α-terpineol, theobromine, β-thujaplicin and vanillin against maximal electroshock-induced seizures in mice. Pre-Clin. Clin. Res. 2013, 7, 40–42. [Google Scholar]
- Viana, G.S.; do Vale, T.G.; Silva, C.M.; Matos, F.J. Anticonvulsant activity of essential oils and active principles from chemotypes of Lippia alba (Mill.) N.E. Brown. Biol. Pharm. Bull. 2000, 23, 1314–1317. [Google Scholar] [PubMed]
- De Almeida, A.A.; Costa, J.P.; de Carvalho, R.B.; de Sousa, D.P.; de Freitas, R.M. Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res. 2012, 1448, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Czuczwar, S.J.; Frey, H.H. Effect of morphine and morphine-like analgesics on susceptibility to seizures in mice. Neuropharmacology 1986, 25, 465–469. [Google Scholar] [CrossRef]
- Tortoriello, J.; Ortega, A. Sedative effect of galphimine b, a nor-seco-triterpenoid from Galphimia glauca. Planta Med. 1993, 59, 398–400. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.C.; Cavalcanti, I.M.; Satyal, P.; Santos-Magalhaes, N.S.; Rolim, H.M.; Freitas, R.M. Acute toxicity and anticonvulsant activity of liposomes containing nimodipine on pilocarpine-induced seizures in mice. Neurosci. Lett. 2015, 585, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, E.A.; Santos, N.F.; Priel, M.R. The pilocarpine model of epilepsy in mice. Epilepsia 1996, 37, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the epoxycarvone stereoisomers are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado, P.R.R.; Da Fonsêca, D.V.; Braga, R.M.; De Melo, C.G.F.; Andrade, L.N.; De Almeida, R.N.; De Sousa, D.P. Comparative Anticonvulsant Study of Epoxycarvone Stereoisomers. Molecules 2015, 20, 19660-19673. https://doi.org/10.3390/molecules201119649
Salgado PRR, Da Fonsêca DV, Braga RM, De Melo CGF, Andrade LN, De Almeida RN, De Sousa DP. Comparative Anticonvulsant Study of Epoxycarvone Stereoisomers. Molecules. 2015; 20(11):19660-19673. https://doi.org/10.3390/molecules201119649
Chicago/Turabian StyleSalgado, Paula Regina Rodrigues, Diogo Vilar Da Fonsêca, Renan Marinho Braga, Cynthia Germoglio Farias De Melo, Luciana Nalone Andrade, Reinaldo Nóbrega De Almeida, and Damião Pergentino De Sousa. 2015. "Comparative Anticonvulsant Study of Epoxycarvone Stereoisomers" Molecules 20, no. 11: 19660-19673. https://doi.org/10.3390/molecules201119649
APA StyleSalgado, P. R. R., Da Fonsêca, D. V., Braga, R. M., De Melo, C. G. F., Andrade, L. N., De Almeida, R. N., & De Sousa, D. P. (2015). Comparative Anticonvulsant Study of Epoxycarvone Stereoisomers. Molecules, 20(11), 19660-19673. https://doi.org/10.3390/molecules201119649





