Antiproliferative and Apoptotic Activity of Chamaecyparis obtusa Leaf Extract against the HCT116 Human Colorectal Cancer Cell Line and Investigation of the Bioactive Compound by Gas Chromatography-Mass Spectrometry-Based Metabolomics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antiproliferative Activity of Various Extracts
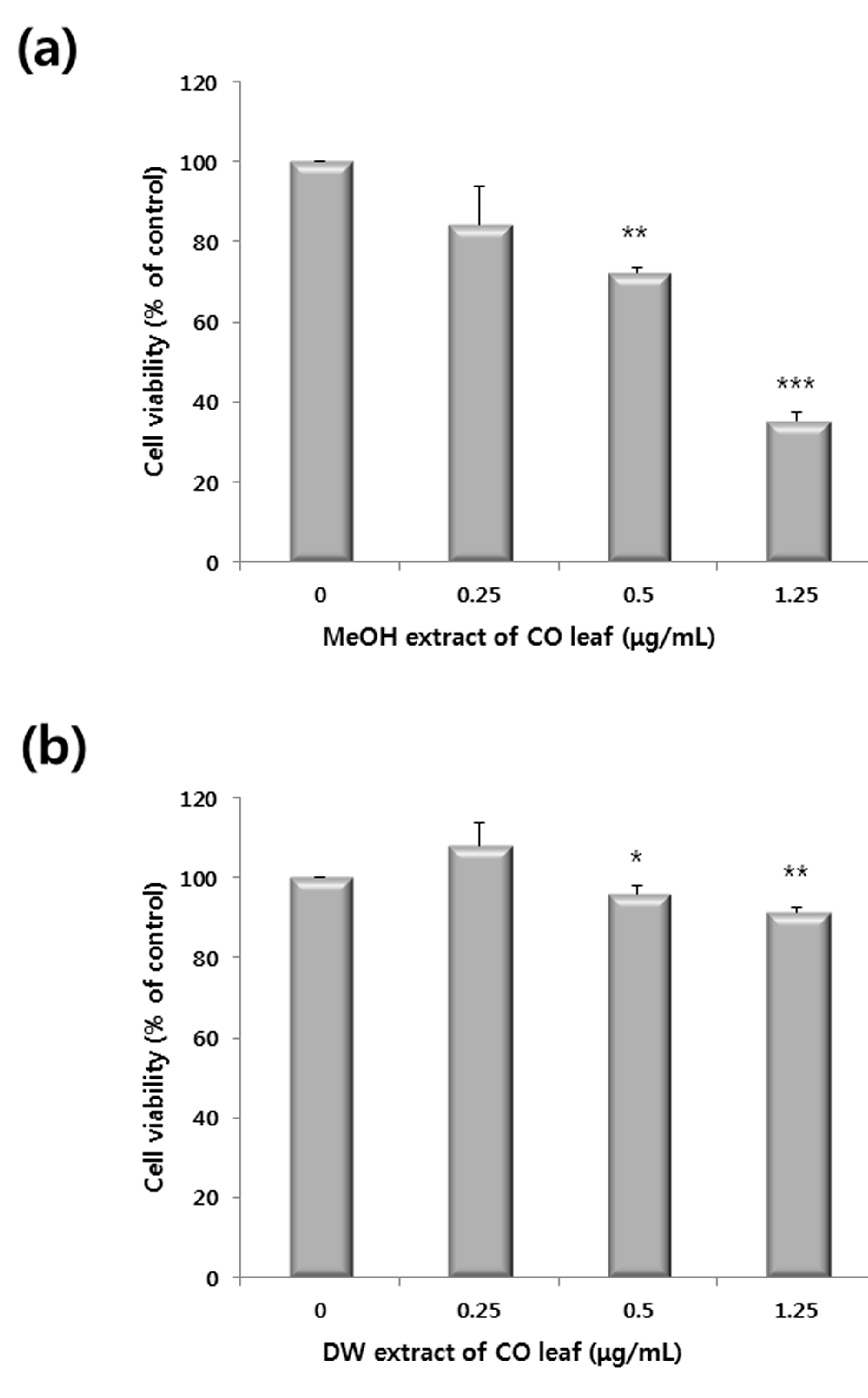
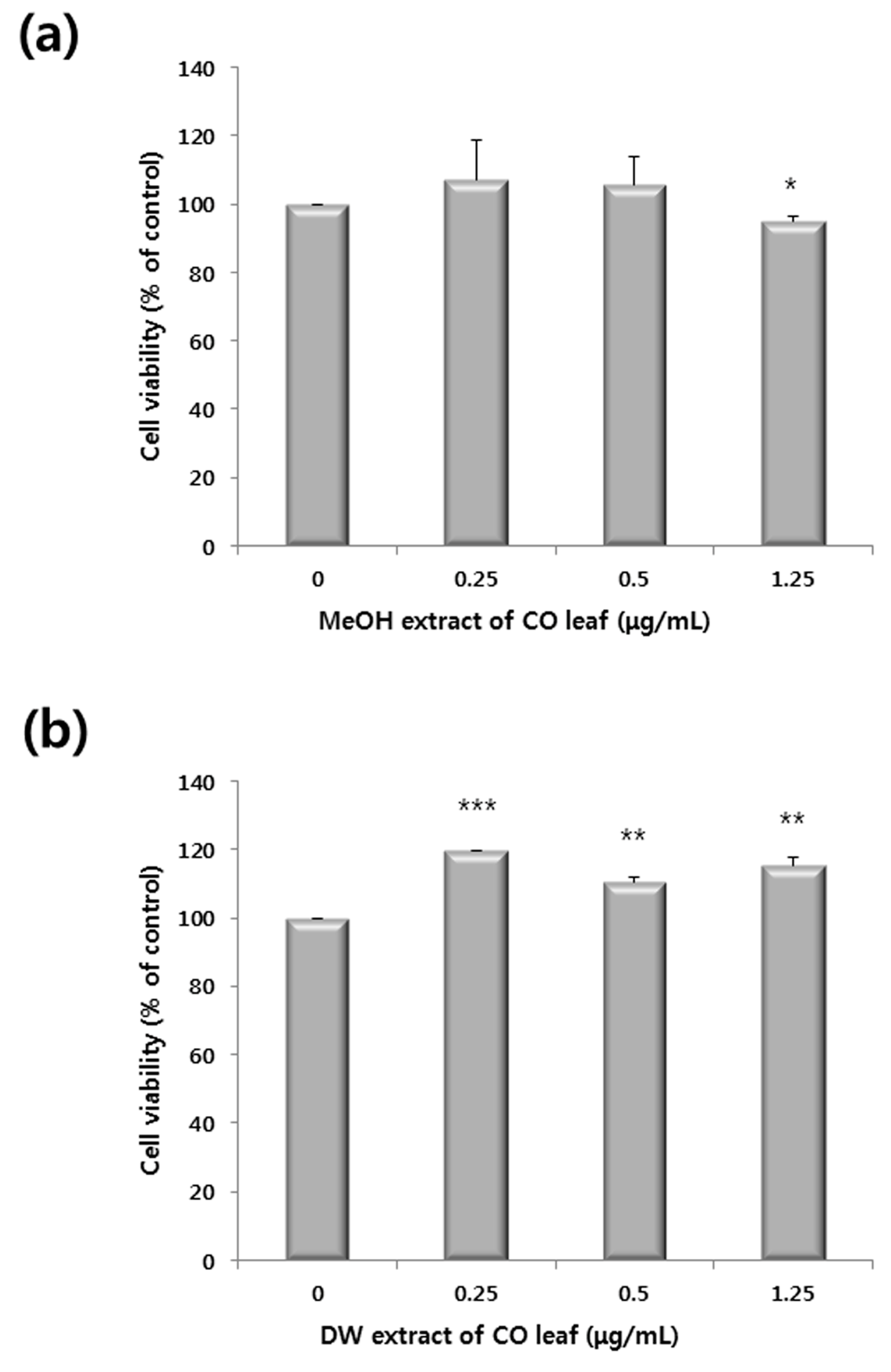
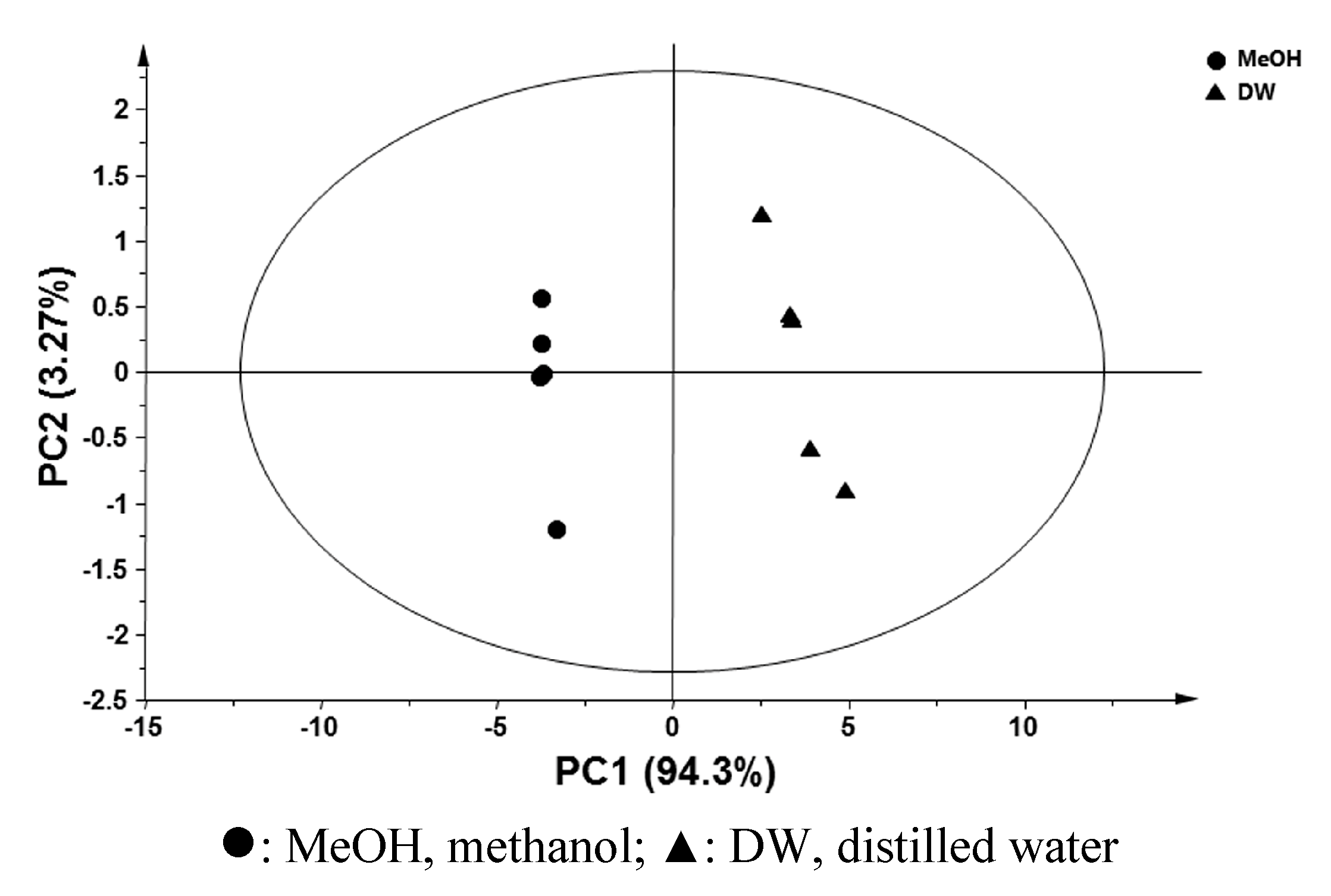
2.2. Comparative Global Metabolite Profiling of Methanol and Water Extracts Using GC-MS
| Compound | RT (min) | Relative Intensity | |
|---|---|---|---|
| DW | MeOH | ||
| Alcohols | |||
| Glycerol * | 10.23 | 1.90 ± 0.23 | 1.19 ± 0.12 |
| Mannitol * | 28.56 | 0.51 ± 0.07 | 0.28 ± 0.04 |
| Myo-inositol * | 33.37 | 2.16 ± 0.23 | 1.12 ± 0.11 |
| Amino acids | |||
| Aspartic acid ** | 16.58 | 0.06 ± 0.01 | ND |
| Glutamic acid ** | 19.30 | 0.14 ± 0.02 | ND |
| Serine ** | 12.47 | 0.05 ± 0.00 | ND |
| Fatty acids | |||
| Palmitic acid ** | 32.25 | ND | 0.09 ± 0.01 |
| Lignan | |||
| Anthricin ** | 57.14 | ND | 0.21 ± 0.02 |
| Organic acids | |||
| Succinic acid * | 11.27 | 0.13 ± 0.02 | 0.08 ± 0.01 |
| Malic acid ** | 15.80 | 4.61 ± 0.51 | 0.76 ± 0.08 |
| Xylonic acid ** | 22.53 | 1.05 ± 0.10 | 0.25 ± 0.03 |
| Phenolic acids | |||
| Shikimic acid * | 25.21 | 9.28 ± 1.03 | 5.73 ± 0.54 |
| Sterol | |||
| β-Sitosterol ** | 57.92 | ND | 0.33 ± 0.03 |
| Sesquiterpenes | |||
| Thujopsene ** | 14.44 | ND | 0.06 ± 0.01 |
| β-Eudesmol ** | 22.64, 22.88 | ND | 4.03 ± 0.46 |
| Sugar | |||
| Glucose * | 30.44 | 40.36 ± 11.01 | 20.15 ± 2.41 |
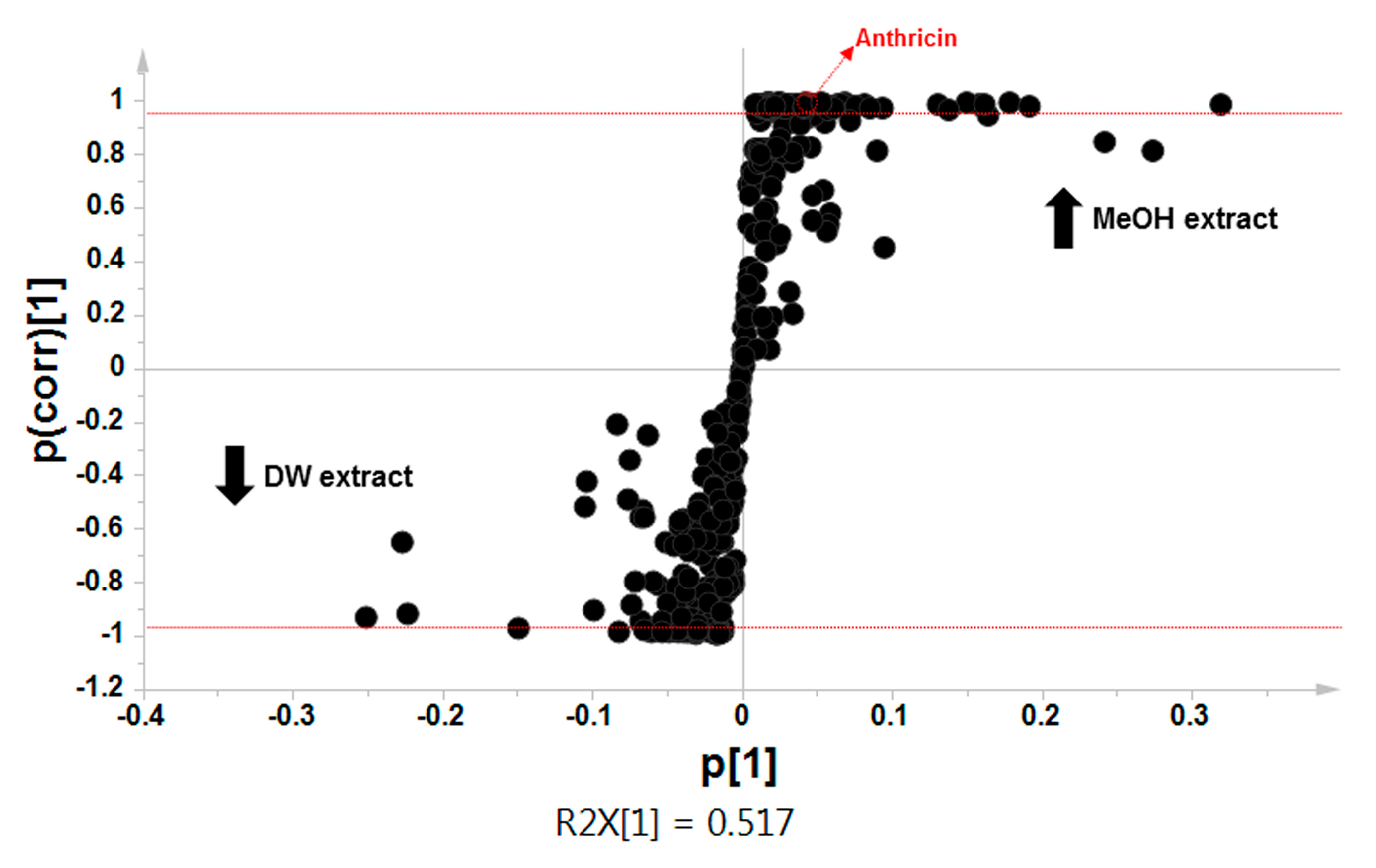
| MeOH Extract (p < 0.01) | DW Extract (p < 0.05) |
|---|---|
| Palmitic acid | Glycerol |
| Thujopsene | Mannitol |
| Anthricin | Myo-inositol |
| β-Sitosterol | Aspartic acid |
| β-Eudesmol | Glutamic acid |
| Serine | |
| Succinic acid | |
| Malic acid | |
| Xylonic acid | |
| Shikimic acid |
| Compound | Regression Equation | R2 Value | Absolute Concentration a (μg/1000 mL) |
|---|---|---|---|
| Anthricin | y = 0.0029x − 0.6787 | 0.9945 | 36.1 |
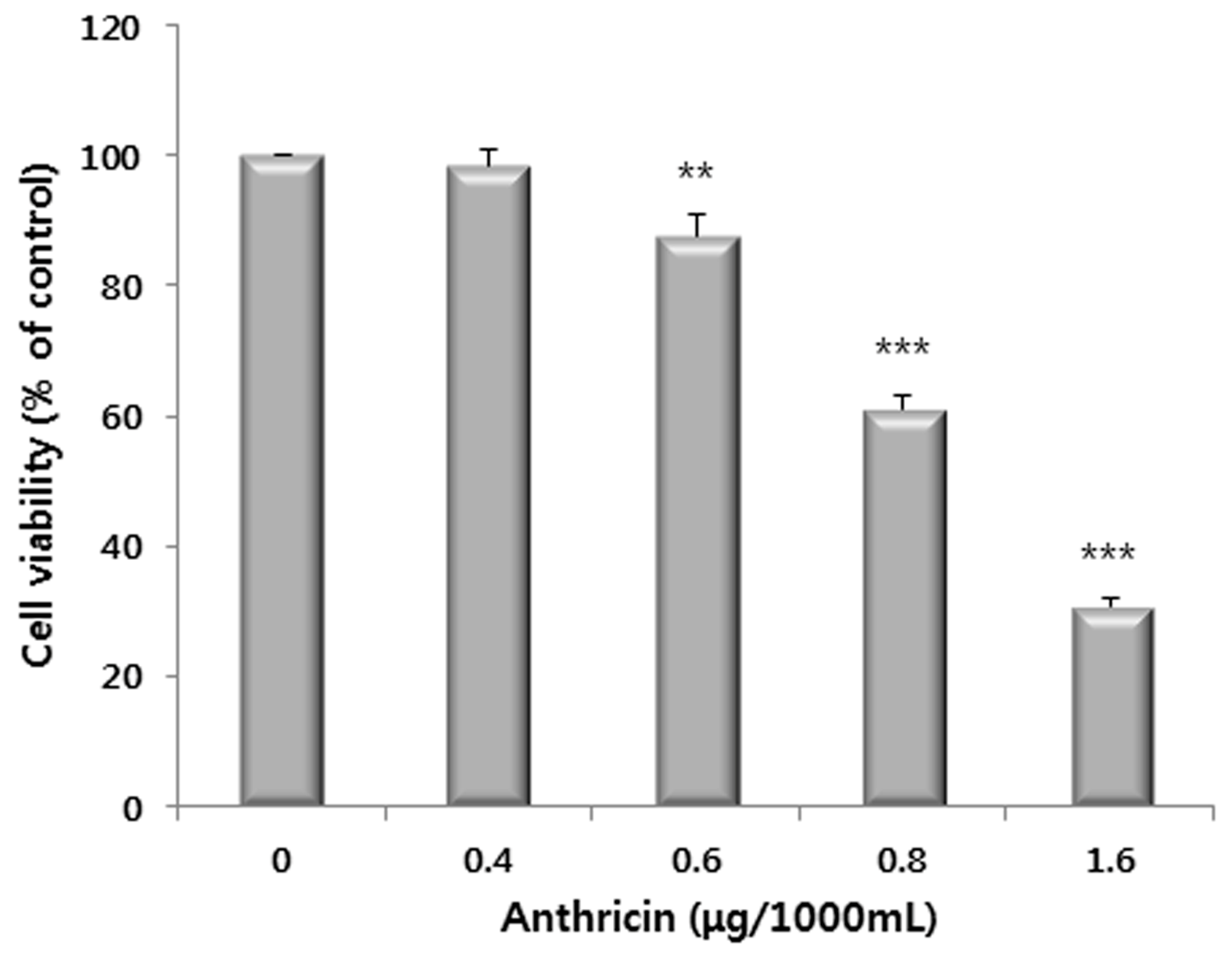
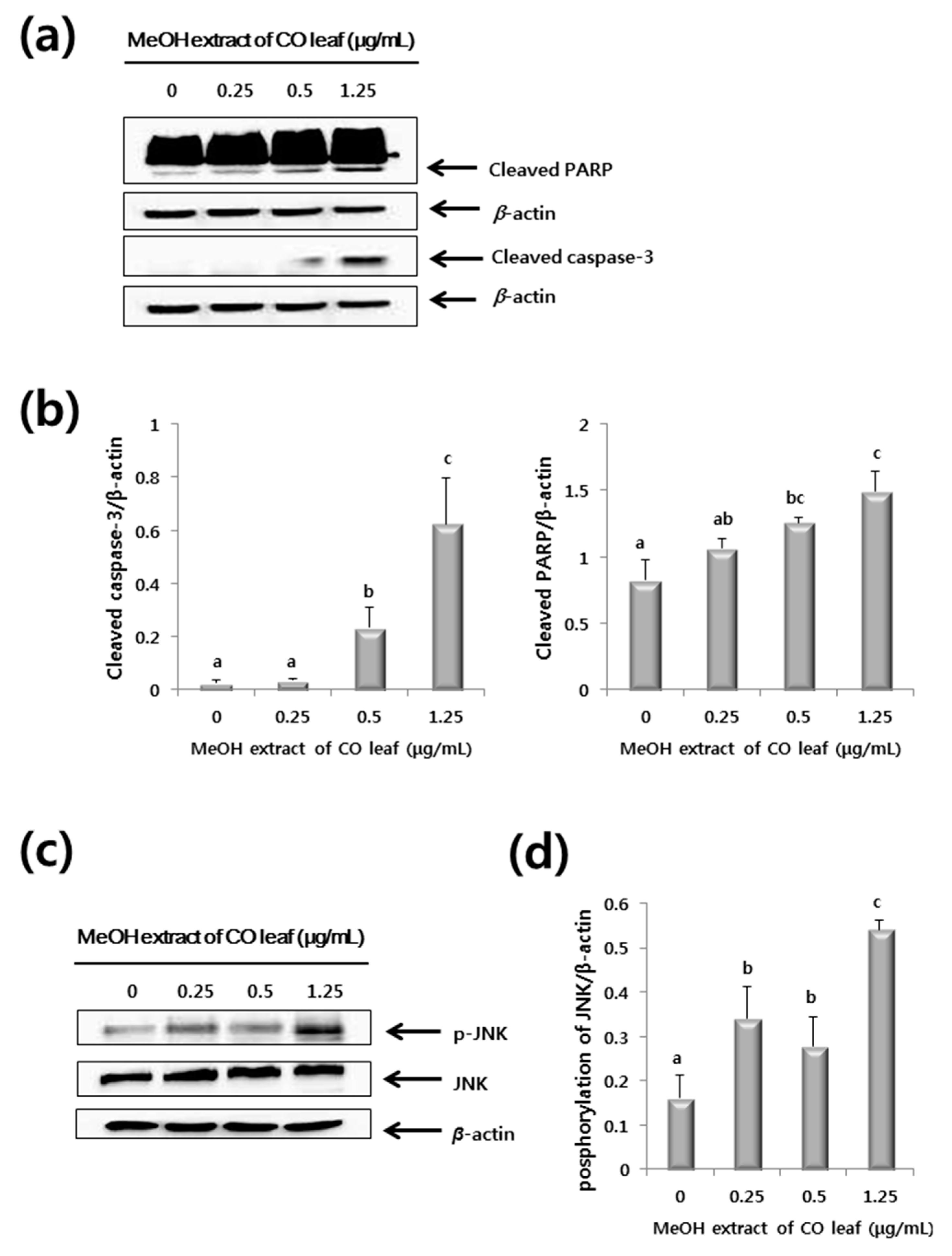
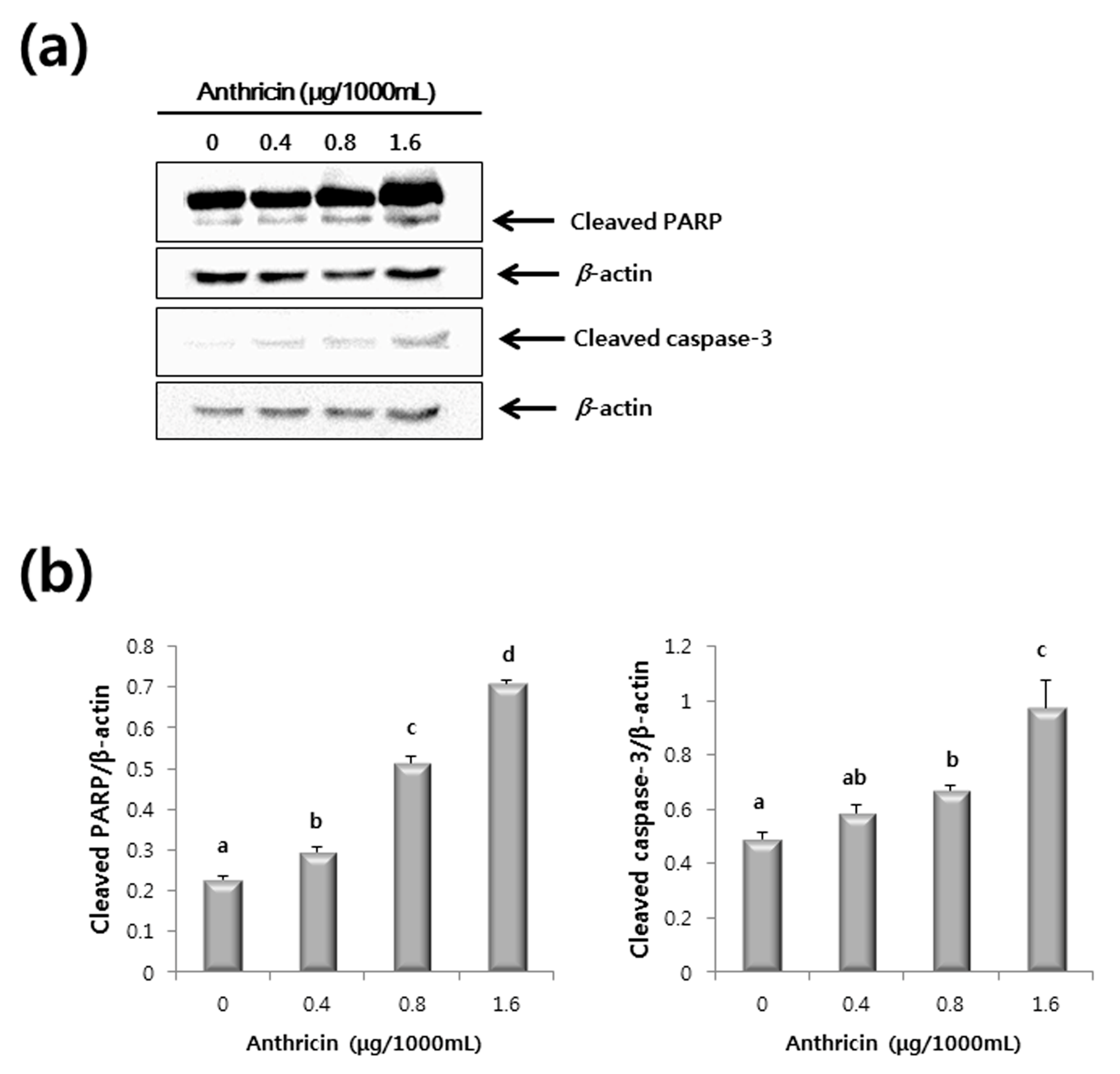
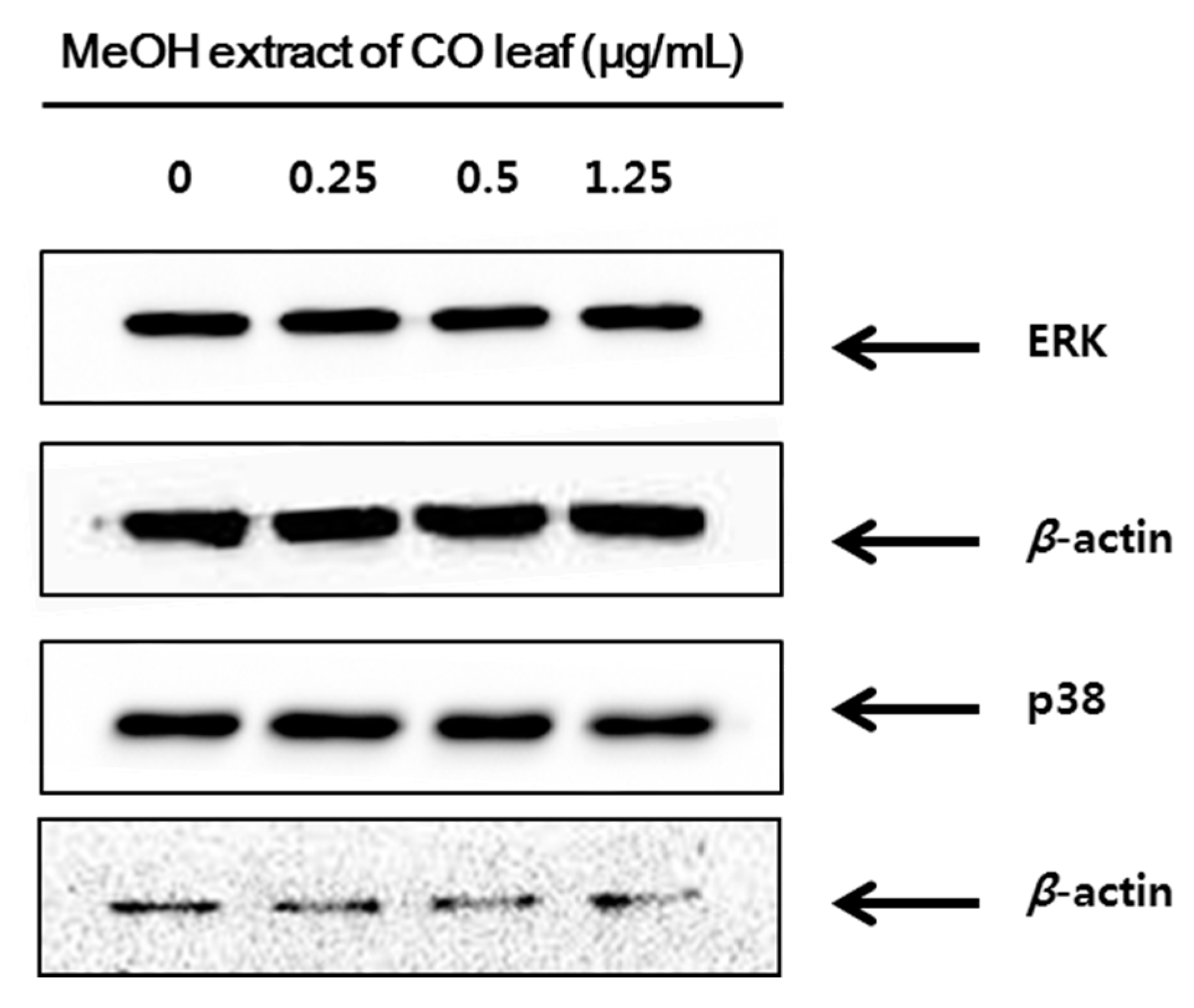
2.3. Western Blot Analysis
3. Experimental Section
3.1. Sample Preparation
3.2. Antiproliferative Activity against the HCT116 Cell Line and Viability Test Using Chang Liver Cell Line
3.3. Global Metabolite Profiling Using GC-MS
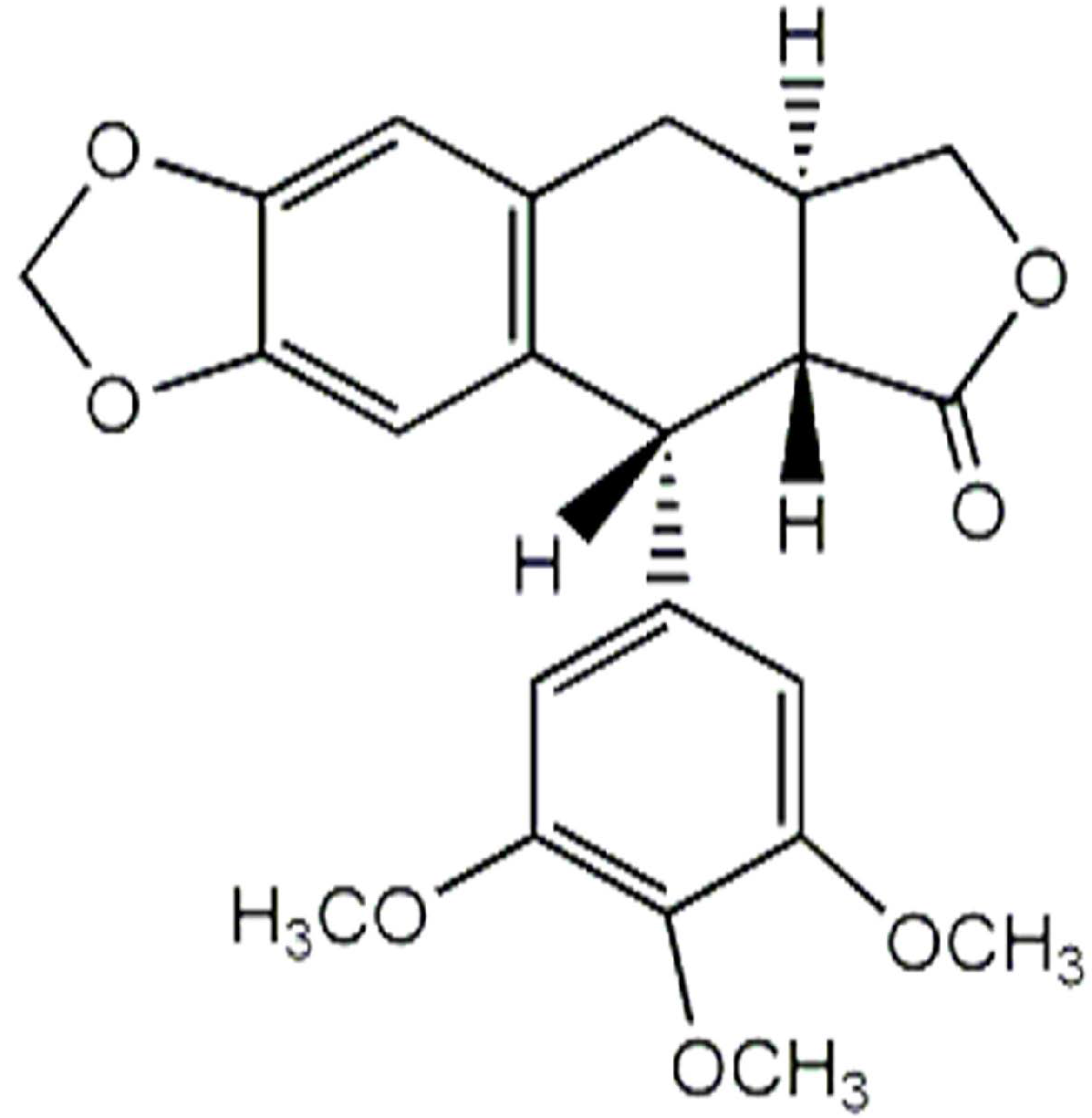
3.4. Quantification of Anthricin by GC-MS
3.5. Statistical Analysis
3.6. Western Blot Analysis
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Wang, W.; Hwang, C.; Lin, T.; Hwang, S. Historical biogeography and phylogenetic relationships of the genus Chamaecyparis (Cupressaceae) inferred from chloroplast DNA polymorphism. Plant Syst. Evol. 2003, 241, 13–28. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, C.; Cheng, S.; Chang, S. Phylogenetic relationships of the genus Chamaecyparis inferred from leaf essential oil. Chem. Biodivers. 2011, 8, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Farjon, A. A Monograph of Cupressaceae and Sciadopitys; Kew Royal Botanic Gardens: Richmond, UK, 2005; p. 643. [Google Scholar]
- Namba, T. The Encyclopedia of Wakan-Yaku (Traditional Sino-Japanese Medicines) with Color Pictures; Hoikusha: Osaka, Japan, 1993; Volume II. [Google Scholar]
- Yang, J.K.; Choi, M.S.; Seo, W.T.; Rinker, D.L.; Han, S.W.; Cheong, G.W. Chemical composition and antimicrobial activity of Chamaecyparis obtuse leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, B.K.; Kim, J.H.; Lee, S.H.; Hong, S.K. Comparison of chemical compositions and antimicrobial activities of essential oils from three conifer trees; Pinus densiflora, Cryptomeria japonica, and Chamaecyparis obtusa. J. Microbiol. Biotechnol. 2009, 19, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lee, C.; Kim, M.; Kim, J.; Lee, S.; Lee, H. Acaricidal activity of active constituent isolated in Chamaecyparis obtusa leaves against Dermatophagoides spp. J. Agric. Food Chem. 2005, 53, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Kang, J.; Yang, H.; Jung, E.; Kang, H.; Choi, I.; Park, M.; Jeung, E. Anti-inflammatory effects of essential oils from Chamaecyparis obtusa via the cyclooxygenase-2 pathway in rats. Mol. Med. Rep. 2013, 8, 255–259. [Google Scholar] [PubMed]
- Jeong, E.J.; Hwang, L.; Lee, M.; Lee, K.Y.; Ahn, M.; Sung, S.H. Neuroprotective bioflavonoid of Chamaecyparis obtusa leaves against glutamate-induced oxidative stress in HT22 hippocampal cells. Food Chem. Toxicol. 2014, 64, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, S.K.; Kang, W.S.; Woo, J.; Kim, J.W. Effects of essential oil from Chamaecyparis obtusa on cytokine genes in the hippocampus of maternal separation rats. Can. J. Physiol. Pharmacol. 2013, 92, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Lee, Y.J.; Lee, Y.R.; Row, K.H. Examination of 1-methylimidazole series ionic liquids in the extraction of flavonoids from Chamaecyparis obtusa leaves using a response surface methodology. J. Chromatogr. B 2013, 933, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Gadek, P.; Quinn, C. Biflavones of the subfamily Cupressoideae, Cupressaceae. Phytochemistry 1985, 24, 267–272. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Poblocka, L.; El Hela, A.A. Biflavones from Chamaecyparis obtusa. Z. Naturforschung C 2005, 60, 679–685. [Google Scholar] [CrossRef]
- Ulrich-Merzenich, G.; Zeitler, H.; Jobst, D.; Panek, D.; Vetter, H.; Wagner, H. Application of the “-Omic-” technologies in phytomedicine. Phytomedicine 2007, 14, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Ellis, D.I. Metabolomics: Current analytical platforms and methodologies. TrAC Trends Anal. Chem. 2005, 24, 285–294. [Google Scholar]
- Falasca, A.; Melck, D.; Paris, D.; Saviano, G.; Motta, A.; Iorizzi, M. Seasonal changes in the metabolic fingerprint of Juniperus communis L. berry extracts by 1H NMR-based metabolomics. Metabolomics 2014, 10, 165–174. [Google Scholar] [CrossRef]
- Inaba, H.; Nagaoka, Y.; Kushima, Y.; Kumagai, A.; Matsumoto, Y.; Sakaguchi, M.; Baba, K.; Uesato, S. Comparative examination of anti-proliferative activities of (−)-epigallocatechin gallate and (−)-epigallocatechin against HCT116 colorectal carcinoma cells. Biol. Pharm. Bull. 2008, 31, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Hussien, N.A.; Mohammed, N.G.; Mohammed, D.I.; El-Ghor, A.A. Antiproliferative and apoptotic effects of grape seed extract on human colon cancer cell line HCT116. Am. Eurasian J. Sustain. Agric. 2013, 7, 241–249. [Google Scholar]
- Lupidi, G.; Bramucci, M.; Quassinti, L.; Fornari, E.; Avenali, L.; Khalife, H.; Gali-Muhtasib, H.U. Antiproliferative activities of artemisia herba-alba ethanolic extract in human colon cancer cell line (HCT116). Altern. Med. Stud. 2011, 1, e14. [Google Scholar] [CrossRef]
- Aljaiyash, A.; Gonaid, M.H.; Islam, M.; Chaouch, A. Antibacterial and cytotoxic activities of some Libyan medicinal plants. J. Nat. Prod. Plant Resour. 2014, 4, 43–51. [Google Scholar]
- Lee, Y.; Choi, K.; Kim, W.; Jeon, Y.; Lee, Y.; Hong, J.; Yun, Y.; Yoo, H. Hinokitiol inhibits cell growth through induction of s-phase arrest and apoptosis in human colon cancer cells and suppresses tumor growth in a mouse xenograft experiment. J. Nat. Prod. 2013, 76, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Lee, E.; Hong, Y.; Yun, H.; Kim, B. Widdrol from Juniperus chinensis induces apoptosis in human colon adenocarcinoma HT29 cells. Biotechnol. Bioprocess. Eng. 2010, 15, 167–172. [Google Scholar] [CrossRef]
- Hendrawati, O.; Woerdenbag, H.J.; Hille, J.; Quax, W.J.; Kayser, O. Seasonal variations in the deoxypodophyllotoxin content and yield of Anthriscus sylvestris L. (Hoffm.) grown in the field and under controlled conditions. J. Agric. Food Chem. 2011, 59, 8132–8139. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.; You, Y.; Ahn, B. Deoxypodophyllotoxin; The cytotoxic and antiangiogenic component from Pulsatilla koreana. Planta Med. 2002, 68, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.; Kwon, O.; Park, B.; Oh, S.; Ahn, K.; Chang, M.; Oh, W.K.; Kim, J.; Min, B.; Kim, Y. Lignans and coumarins from the roots of Anthriscus sylvestris and their increase of caspase-3 activity in HL-60 cells. Biol. Pharm. Bull. 2007, 30, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Muto, N.; Tomokuni, T.; Haramoto, M.; Tatemoto, H.; Nakanishi, T.; Inatomi, Y.; Murata, H.; Inada, A. Isolation of apoptosis-and differentiation-inducing substances toward human promyelocytic leukemia HL-60 cells from leaves of Juniperus taxifolia. Biosci. Biotechnol. Biochem. 2008, 72, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Cho, H.; Yu, S.; Kim, S.; Yu, H.; Park, Y.; Mirkheshti, N.; Kim, S.; Song, C.; Chatterjee, B. Interplay of reactive oxygen species, intracellular Ca2+ and mitochondrial homeostasis in the apoptosis of prostate cancer cells by deoxypodophyllotoxin. J. Cell. Biochem. 2013, 114, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Yong, Y.; Kim, C.G.; Lee, Y.H.; Lim, Y. Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in HeLa cells. Cancer Lett. 2010, 287, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Yong, Y.; Lee, Y.H. Effect of deoxypodophyllotoxin isolated from Anthriscus sylvestris roots on the expression of cell cycle-regulatory proteins in hela cells. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 304–309. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, H.; Ahn, J.; Jung, S.K.; Um, M.Y.; Son, K.; Kim, T.W.; Ha, T.Y. Anthricin isolated from Anthriscus sylvestris (L.) Hoffm. inhibits the growth of breast cancer cells by inhibiting Akt/mTOR signaling, and Its apoptotic effects are enhanced by autophagy inhibition. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Murphy, B.T.; Hammond, G.B.; Vinson, J.A.; Neto, C.C. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Hwang, W.I.; Lim, S.T. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J. Ethnopharmacol. 2004, 93, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. An anti-GD2 monoclonal antibody enhances apoptotic effects of anti-cancer drugs against small cell lung cancer cells via JNK (c-Jun terminal kinase) activation. Cancer Sci. 2002, 93, 816–824. [Google Scholar] [CrossRef]
- Soldani, C.; Scovassi, A. Poly (ADP-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis 2002, 7, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Wang, W.J.; Chan, J.L.; Wang, L.H. Differential requirements of the MAP kinase and PI3 kinase signaling pathways in Src- versus insulin and IGF-1 receptors-induced growth and transformation of rat intestinal epithelial cells. Oncogene 2000, 19, 5385–5397. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T., Jr.; McCubrey, J.A. The Raf/MEK/ERK signal transduction cascade as a target for chemotherapeutic intervention in leukemia. Leukemia 2002, 16, 486–507. [Google Scholar] [PubMed]
- Sui, H.; Fan, Z.Z.; Li, Q. Signal transduction pathways and transcriptional mechanisms of ABCB1/Pgp-mediated multiple drug resistance in human cancer cells. J. Int. Med. Res. 2012, 40, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.J.; Lee, T.J.; Lee, S.H.; Lee, J.; Seo, J.; Jeong, Y.; Park, J.; Kwon, T.K. Elevated gadd153/chop expression during resveratrol-induced apoptosis in human colon cancer cells. Biochem. Pharmacol. 2007, 73, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Huang, Y.X.; Sun, Y.; Zheng, L.H.; Li, Y.X. Butyrate induces cell apoptosis through activation of JNK MAP kinase pathway in human colon cancer RKO cells. Chem. Biol. Interact. 2010, 185, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Kim, B.R.; Chen, C.; Hebbar, V.; Kong, A.N. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis 2003, 24, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Collett, G.P.; Campbell, F.C. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis 2004, 25, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Banerjee, P.; Nikolakaki, E.; Dai, T.; Rubie, E.A.; Ahmad, M.F.; Avruch, J.; Woodgett, J.R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 1994, 369, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Hibi, M.; Lin, A.; Smeal, T.; Minden, A.; Karin, M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993, 7, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Kang, P.M.; Hampe, J.; Yoshimura, K.; Noma, T.; Matsuzaki, M.; Izumo, S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J. Biol. Chem. 2002, 277, 10244–10250. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Boyd, C.; Ahmed, R.; Spencer, J.; Duncan, R.; Rice-Evans, C.; Cadenas, E. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: New target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem. J. 2003, 372, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yu, M.; Guan, D.; Gu, J.; Cao, X.; Wang, W.; Zheng, S.; Xu, Y.; Shen, Z.; Liu, X. ASK1-JNK signaling cascade mediates Ad-ST13-induced apoptosis in colorectal HCT116 cells. J. Cell. Biochem. 2010, 110, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, S.; Saxena, S.; Yoshida, K.; Pandey, P.; Kaneki, M.; Wang, Q.; Cheng, K.; Chen, Y.N.; Campbell, A.; Sudha, T.; et al. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J. Biol. Chem. 2000, 275, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Inoshita, S.; Takeda, K.; Hatai, T.; Terada, Y.; Sano, M.; Hata, J.; Umezawa, A.; Ichijo, H. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J. Biol. Chem. 2002, 277, 43730–43734. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Mishra, N.C.; Yoshida, K.; Kharbanda, S.; Saxena, S.; Kufe, D. Mitochondrial targeting of JNK/SAPK in the phorbol ester response of myeloid leukemia cells. Cell Death Differ. 2001, 8, 794–800. [Google Scholar] [CrossRef] [PubMed]
- AMDIS. Available online: http://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:amdis (accessed on 1 October 2015).
- NIH IMAGE. Available online: http://rsb.info.nih.gov/nih-image/ (accessed on 1 October 2015).
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-Y.; Lee, S.-G.; Oh, T.-J.; Lim, S.R.; Kim, S.-H.; Lee, H.J.; Kim, Y.-S.; Choi, H.-K. Antiproliferative and Apoptotic Activity of Chamaecyparis obtusa Leaf Extract against the HCT116 Human Colorectal Cancer Cell Line and Investigation of the Bioactive Compound by Gas Chromatography-Mass Spectrometry-Based Metabolomics. Molecules 2015, 20, 18066-18082. https://doi.org/10.3390/molecules201018066
Kim H-Y, Lee S-G, Oh T-J, Lim SR, Kim S-H, Lee HJ, Kim Y-S, Choi H-K. Antiproliferative and Apoptotic Activity of Chamaecyparis obtusa Leaf Extract against the HCT116 Human Colorectal Cancer Cell Line and Investigation of the Bioactive Compound by Gas Chromatography-Mass Spectrometry-Based Metabolomics. Molecules. 2015; 20(10):18066-18082. https://doi.org/10.3390/molecules201018066
Chicago/Turabian StyleKim, Hye-Youn, Seul-Gi Lee, Taek-Joo Oh, Sa Rang Lim, So-Hyun Kim, Hong Jin Lee, Young-Suk Kim, and Hyung-Kyoon Choi. 2015. "Antiproliferative and Apoptotic Activity of Chamaecyparis obtusa Leaf Extract against the HCT116 Human Colorectal Cancer Cell Line and Investigation of the Bioactive Compound by Gas Chromatography-Mass Spectrometry-Based Metabolomics" Molecules 20, no. 10: 18066-18082. https://doi.org/10.3390/molecules201018066
APA StyleKim, H.-Y., Lee, S.-G., Oh, T.-J., Lim, S. R., Kim, S.-H., Lee, H. J., Kim, Y.-S., & Choi, H.-K. (2015). Antiproliferative and Apoptotic Activity of Chamaecyparis obtusa Leaf Extract against the HCT116 Human Colorectal Cancer Cell Line and Investigation of the Bioactive Compound by Gas Chromatography-Mass Spectrometry-Based Metabolomics. Molecules, 20(10), 18066-18082. https://doi.org/10.3390/molecules201018066







