On the Antimicrobial Activity of Various Peptide-Based Dendrimers of Similar Architecture
Abstract
:1. Introduction
2. Results and Discussion
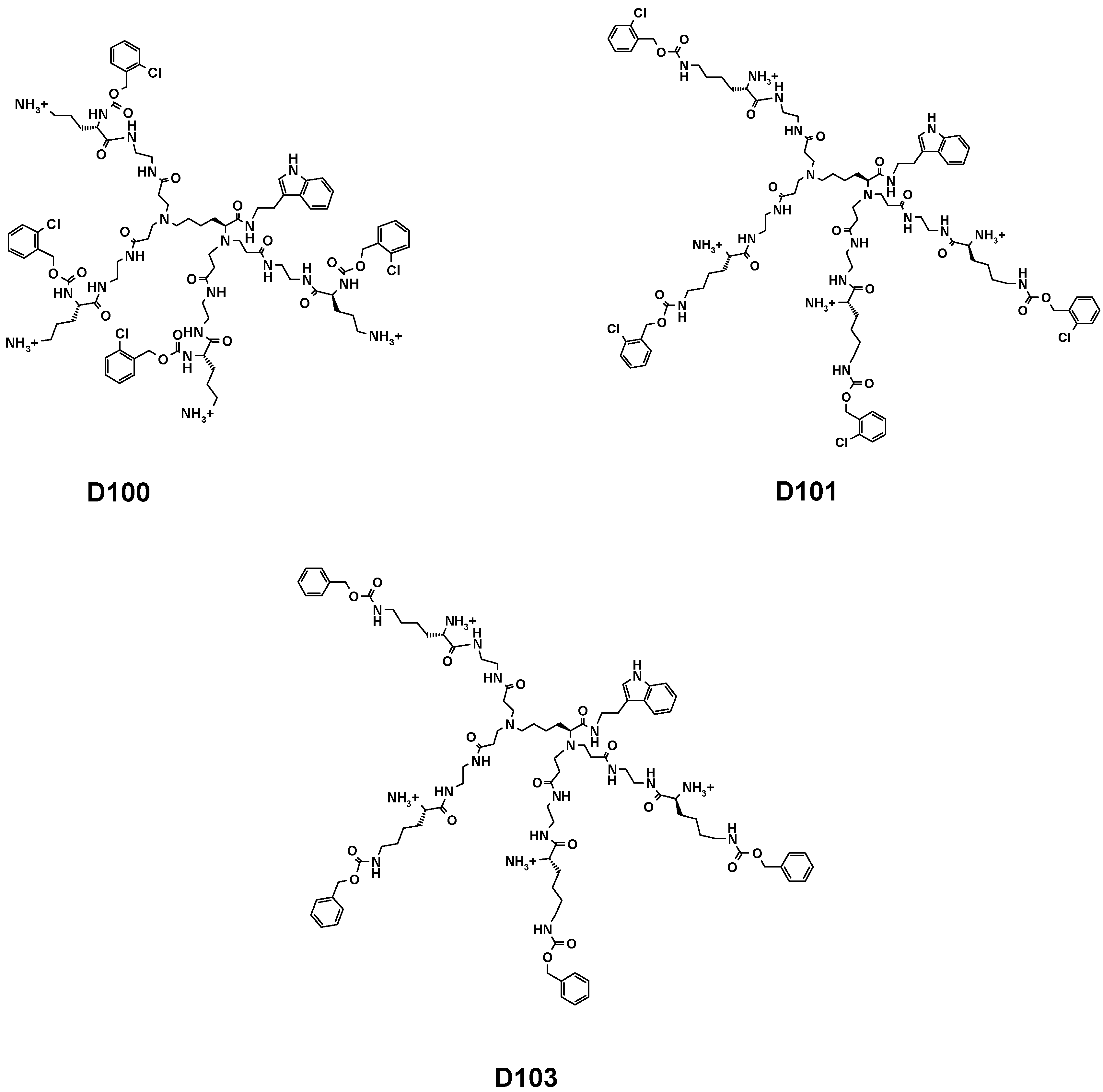
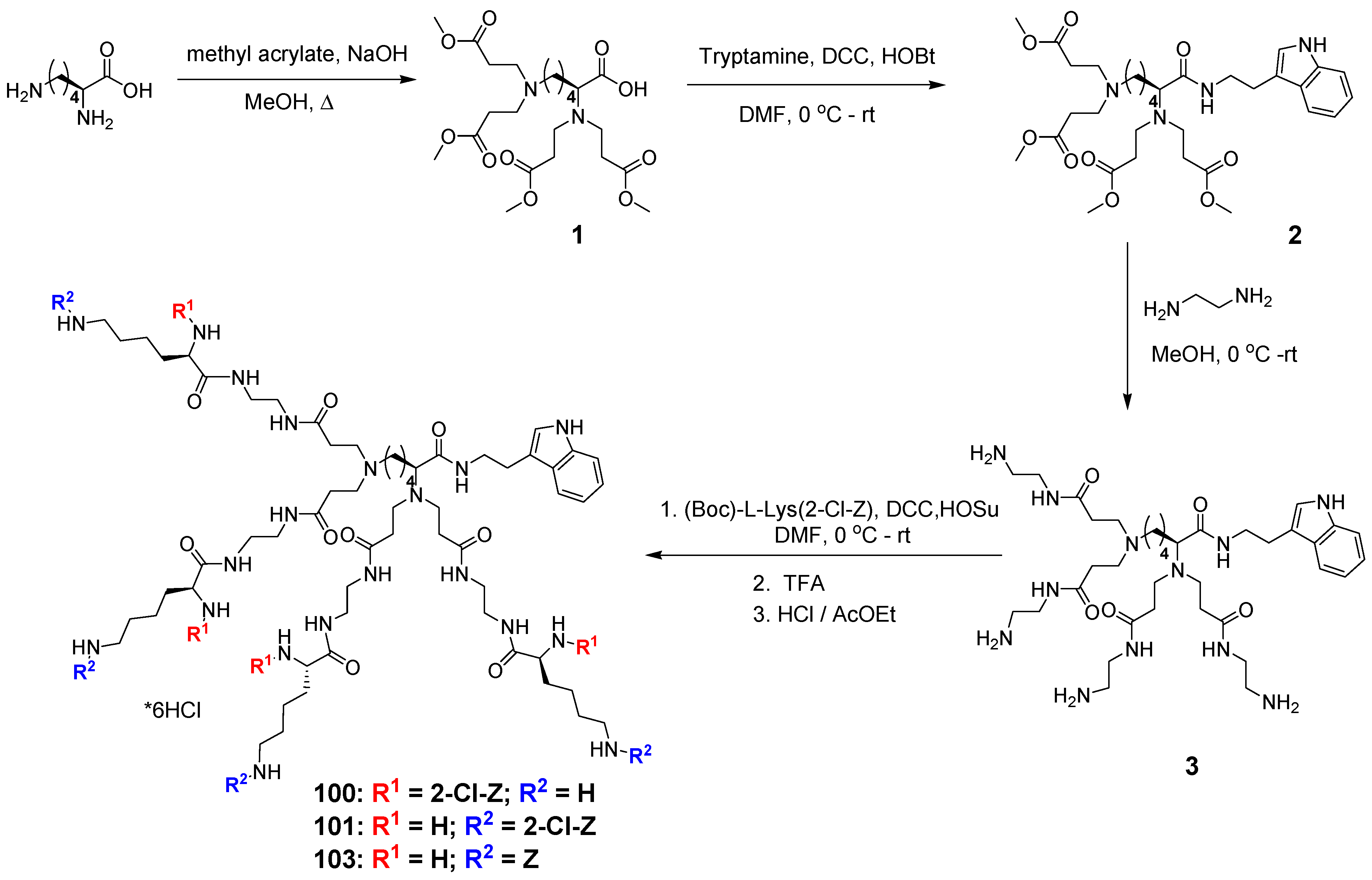
2.1. Organic Synthesis
2.2. Antimicrobial and Hemolytic Activity of Dendrimers
| Strain | MIC in μM (1) | ||
|---|---|---|---|
| 100 (2-Cl-Z)-Lys | 101 Lys(2-Cl-Z) | 103 Lys(Z) | |
| S. aureus, ATCC 25923 | 0.93 | 0.93 | 12.9 |
| S. aureus, ATTC 43300 | 5.81 | 10.0 | 74 |
| E. coli, ATTC 25922 | 3.71 | 12.0 | 111 |
| P. aeruginosa, ATTC 27853 | 41 | 51 | 149 |
| HEM (µM) | 64 | 32 | ≫128 (2) |
2.3. Interfacial Behavior at Silica Surfaces

2.4. Interfacial Behavior at Model Cell Membranes
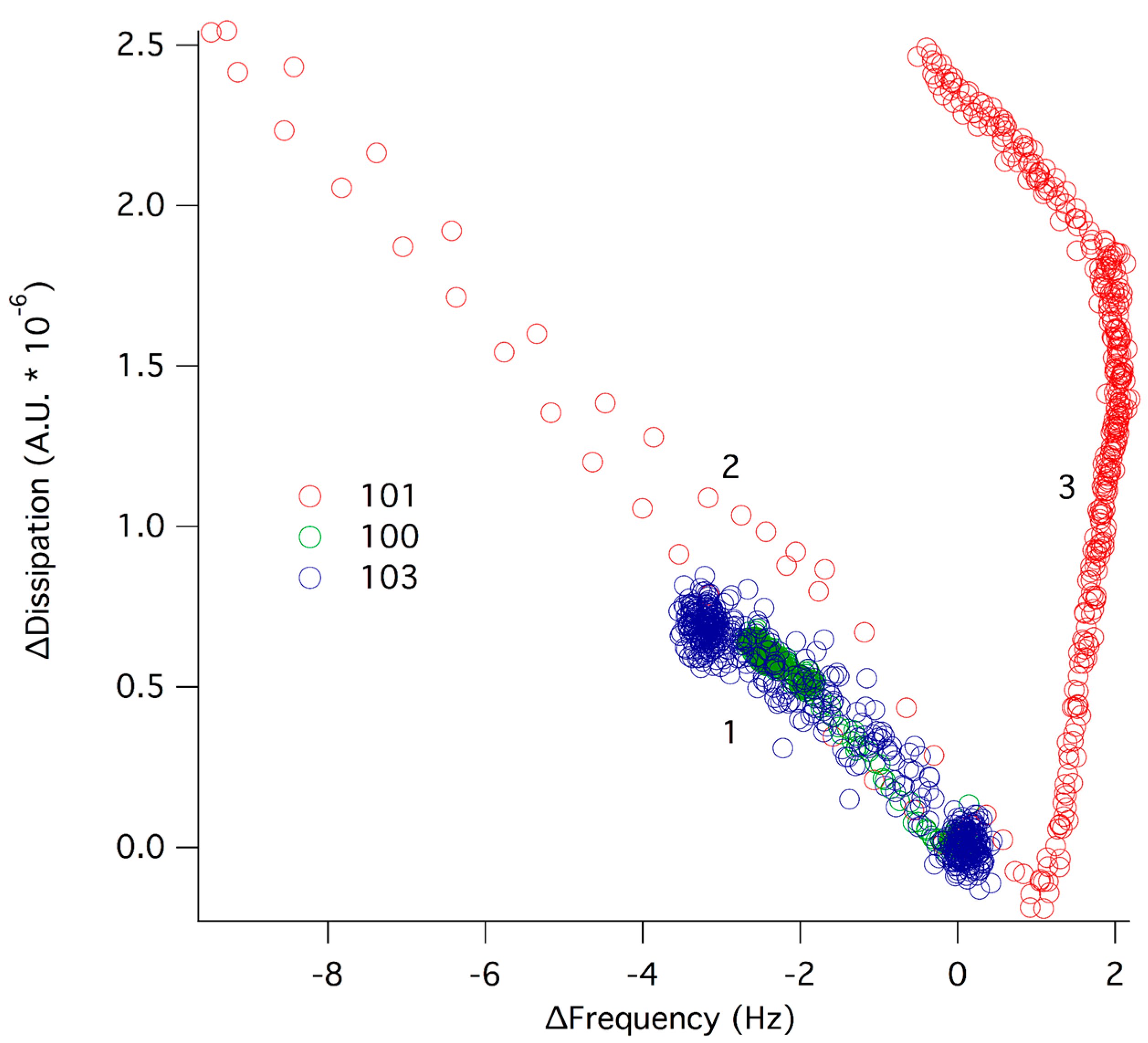
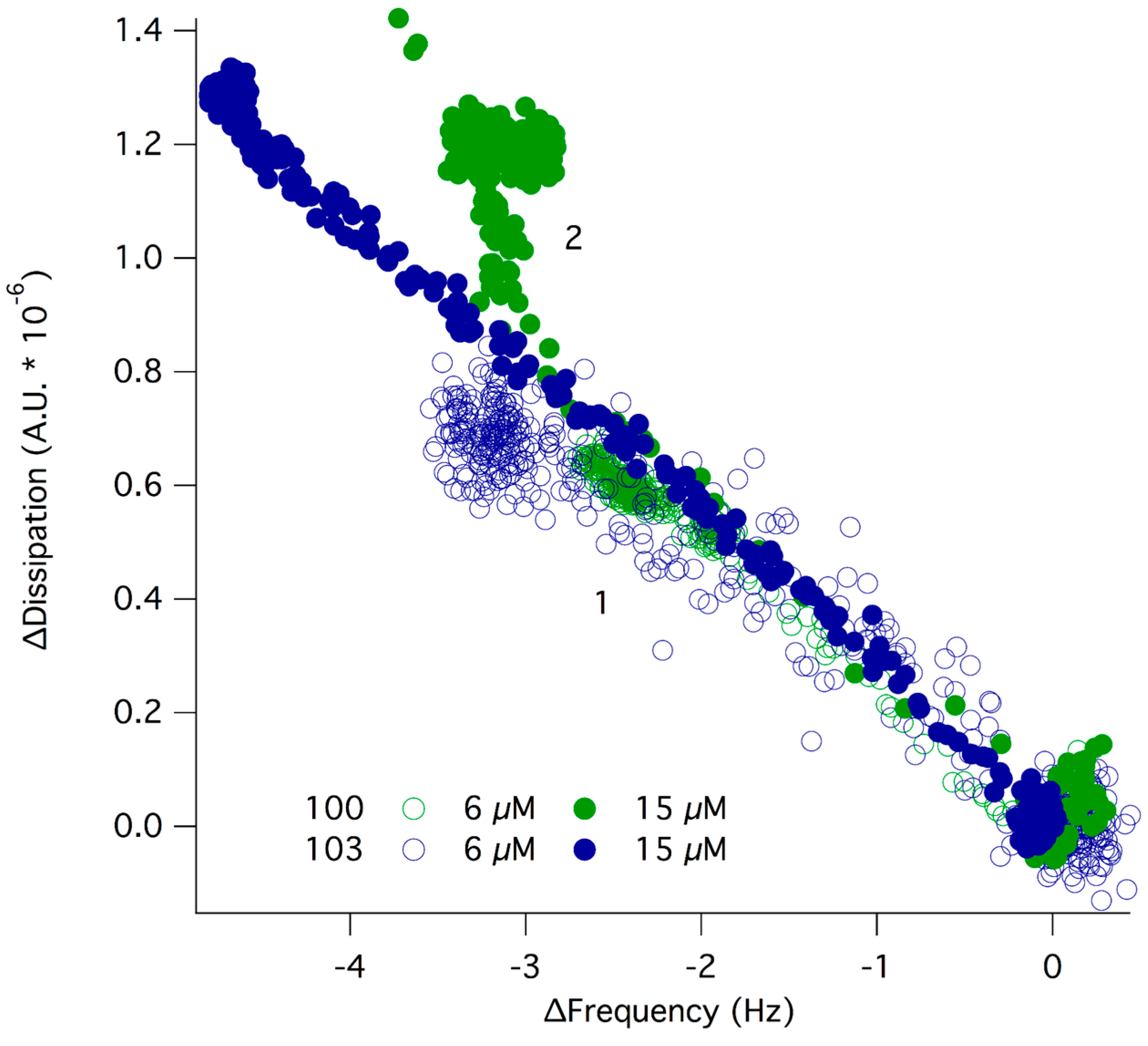
2.5. Antimicrobial Activity, Hemotoxicity, Affinity for Lipid Membranes and Chemical Architecture
3. Experimental Section
3.1. Materials
3.2. Dendrimer Synthesis
3.3. Dendrimer Solutions
3.4. Small Unilamellar Vesicles (SUVs)
3.5. Dissipation-Enhanced Quartz Crystal Microbalance (QCM-D)
3.6. Determination of Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmidtchen, A.; Pasupuleti, M.; Malmsten, M. Effect of hydrophobic modifications in antimicrobial peptides. Adv. Colloid Interface Sci. 2014, 205, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Antimicrobial peptides. Upsala J. Med. Sci. 2014, 119, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef] [PubMed]
- Stromstedt, A.A.; Ringstad, L.; Schmidtchen, A.; Malmsten, M. Interaction between amphiphilic peptides and phospholipid membranes. Curr. Opin. Colloid Interface Sci. 2010, 15, 467–478. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Reymond, J.L.; Darbre, T. Peptide and glycopeptide dendrimer apple trees as enzyme models and for biomedical applications. Org. Biomol. Chem. 2012, 10, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Sadler, K.; Tam, J.P. Peptide dendrimers: Applications and synthesis. Rev. Mol. Biotechnol. 2002, 90, 195–229. [Google Scholar] [CrossRef]
- Crespo, L.; Sanclimens, G.R.; Pons, M.; Giralt, E.; Royo, M.; Albericio, F. Peptide and amide bond-containing dendrimers. Chem. Rev. 2005, 105, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, J.; Swieton, J.; Lipkowski, A.W.; Urbanczyk-Lipkowska, Z. Low molecular mass peptide dendrimers that express antimicrobial properties. Bioorg. Med. Chem. Lett. 2003, 13, 3711–3713. [Google Scholar] [CrossRef] [PubMed]
- Polcyn, P.; Zielinska, P.; Zimnicka, M.; Troć, A.; Kalicki, P.; Solecka, J.; Laskowska, A.; Urbanczyk-Lipkowska, Z. Novel antimicrobial peptide dendrimers with amphiphilic surface and their interactions with phospholipids—Insights from mass spectrometry. Molecules 2013, 18, 7120–7144. [Google Scholar] [CrossRef] [PubMed]
- Lind, T.K.; Zielinska, P.; Wacklin, H.P.; Urbanczyk-Lipkowska, Z.; Cardenas, M. Continuous flow atomic force microscopy imaging reveals fluidity and time-dependent interactions of antimicrobial dendrimer with model lipid membranes. ACS Nano 2014, 8, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Verwendung von schwingquarzen zur wagung dunner schichten und zur mikrowagung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Holmberg, K.J.B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution, 2nd ed.; John Wiley & Sons, Ltd: Chichester, UK, 2012; p. 562. [Google Scholar]
- Lind, T.K.; Cardenas, M.; Wacklin, H.P. Formation of supported lipid bilayers by vesicle fusion: Effect of deposition temperature. Langmuir 2014, 30, 7259–7263. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, M.; Schillen, K.; Alfredsson, V.; Duan, R.D.; Nyberg, L.; Arnebrant, T. Solubilization of sphingomyelin vesicles by addition of a bile salt. Chem. Phys. Lipids 2008, 151, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Palma, C.-A.; Bonini, M.; Samorì, P. Towards supramolecular engineering of functional nanomaterials: Pre-programming multi-component 2D self-assembly at solid-liquid interfaces. Adv. Mater. 2010, 22, 3506–3520. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Lu, Y.A.; Yang, J.L. Antimicrobial dendrimeric peptides. Eur. J. Biochem. 2002, 269, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.P.; Zhou, L.; Lakshminarayanan, R.; Beuerman, R.W. Multivalent antimicrobial peptides as therapeutics: Design principles and structural diversities. Int. J. Pept. Res. Ther. 2010, 16, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, A.; Lundgaard, C.V.; Ehrlich, N.; Pomorski, T.G.; Stamou, D.; Cárdenas, M. Induced dye leakage by pamam g6 does not imply dendrimer entry into vesicle lumen. Soft Matter 2012, 8, 8972–8980. [Google Scholar] [CrossRef]
- Cho, N.-J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Lind, T.K.; Wacklin, H.P.; Ghellinck, A.D.; Moulin, M.; Heartlein, M.; Günther-Pomorski, T.; Cárdenas, M. Formation and characterization of supported lipid bilayers composed of lipids extracted from Escherichia coli. PLoS One 2015. pending submission. [Google Scholar]
- Wilker, M.A.; Cockerill, F.R.; Bush, K.; Dudley, M.N.; Eliopoulos, G.M.; Hardy, D.J.; Hecht, D.W.; Hindler, J.F.; Patel, J.B.; Powell, M.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard—Eighth Edition; CLSI document M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; Volume 29. [Google Scholar]
- Knopik-Skrocka, A.; Bielawski, J. Differences in amphotericin B-induced hemolysis between human erythrocytes obtained from male and female donors. Biol. Lett. 2005, 42, 49–60. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lind, T.K.; Polcyn, P.; Zielinska, P.; Cárdenas, M.; Urbanczyk-Lipkowska, Z. On the Antimicrobial Activity of Various Peptide-Based Dendrimers of Similar Architecture. Molecules 2015, 20, 738-753. https://doi.org/10.3390/molecules20010738
Lind TK, Polcyn P, Zielinska P, Cárdenas M, Urbanczyk-Lipkowska Z. On the Antimicrobial Activity of Various Peptide-Based Dendrimers of Similar Architecture. Molecules. 2015; 20(1):738-753. https://doi.org/10.3390/molecules20010738
Chicago/Turabian StyleLind, Tania K., Piotr Polcyn, Paulina Zielinska, Marité Cárdenas, and Zofia Urbanczyk-Lipkowska. 2015. "On the Antimicrobial Activity of Various Peptide-Based Dendrimers of Similar Architecture" Molecules 20, no. 1: 738-753. https://doi.org/10.3390/molecules20010738
APA StyleLind, T. K., Polcyn, P., Zielinska, P., Cárdenas, M., & Urbanczyk-Lipkowska, Z. (2015). On the Antimicrobial Activity of Various Peptide-Based Dendrimers of Similar Architecture. Molecules, 20(1), 738-753. https://doi.org/10.3390/molecules20010738





