An Effective Vacuum Assisted Extraction Method for the Optimization of Labdane Diterpenoids from Andrographis paniculata by Response Surface Methodology
Abstract
:1. Introduction

2. Results and Discussion
2.1. Actualization of the Vacuum Assisted Heat Reflux Extraction
| Ethanol Concentration | Boiling Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | |||||
| Saturated Vapor Pressure (MPa) | Vacuum (MPa) | Saturated Vapor Pressure (MPa) | Vacuum (MPa) | Saturated Vapor Pressure (MPa) | Vacuum (MPa) | Saturated Vapor Pressure (MPa) | Vacuum (MPa) | |
| 50% | 0.0231 | 0.0769 | 0.0366 | 0.0634 | 0.0571 | 0.0429 | 0.0847 | 0.0153 |
| 60% | 0.0243 | 0.0757 | 0.0389 | 0.0611 | 0.0607 | 0.0393 | 0.0894 | 0.0106 |
| 70% | 0.0248 | 0.0752 | 0.0407 | 0.0593 | 0.0636 | 0.0364 | 0.0935 | 0.0065 |
| 80% | 0.0247 | 0.0753 | 0.0417 | 0.0583 | 0.0659 | 0.0341 | 0.0970 | 0.0030 |
| 90% | 0.0239 | 0.0761 | 0.0422 | 0.0578 | 0.0676 | 0.0324 | 0.0999 | 0.0001 |
2.2. Key Factors Screening through Placket-Burman Design
| Run | Factors | Response Variables | ||||||
|---|---|---|---|---|---|---|---|---|
| A (Boiling Temperature) | B (Ethanol Concentration) | C (Extraction Time) | D (Extraction Cycles) | E (Ratio of Liquid to Solid) | AP1 (%) | AP3 (%) | ES (%) | |
| 1 | 80 (+1) | 90 (+1) | 40 (+1) | 1 (−1) | 12 (+1) | 81.40 | 84.71 | 5.82 |
| 2 | 40 (−1) | 90 (+1) | 10 (−1) | 1 (−1) | 6 (−1) | 58.24 | 52.37 | 3.84 |
| 3 | 80 (+1) | 90 (+1) | 40 (+1) | 3 (+1) | 6 (−1) | 75.41 | 70.77 | 7.82 |
| 4 | 40 (−1) | 90 (+1) | 40 (+1) | 1 (−1) | 6 (−1) | 52.42 | 47.89 | 3.96 |
| 5 | 40 (−1) | 50 (−1) | 40 (+1) | 3 (+1) | 12 (+1) | 58.70 | 55.51 | 8.78 |
| 6 | 80 (+1) | 50 (−1) | 40 (+1) | 1 (−1) | 12 (+1) | 107.48 | 105.83 | 8.36 |
| 7 | 80 (+1) | 50 (−1) | 10 (−1) | 3 (+1) | 6 (−1) | 107.22 | 106.07 | 9.44 |
| 8 | 40 (−1) | 90 (−1) | 10 (−1) | 3 (+1) | 12 (+1) | 52.24 | 45.37 | 5.54 |
| 9 | 40 (−1) | 50 (−1) | 10 (−1) | 1 (−1) | 12 (+1) | 62.08 | 55.87 | 6.42 |
| 10 | 40 (−1) | 50 (−1) | 40 (+1) | 3 (+1) | 6 (−1) | 58.71 | 55.51 | 8.78 |
| 11 | 80 (+1) | 50 (−1) | 10 (−1) | 1 (−1) | 6 (−1) | 113.22 | 108.07 | 8.44 |
| 12 | 80 (+1) | 90 (+1) | 10 (−1) | 3 (+1) | 12 (+1) | 89.58 | 85.13 | 5.14 |
| AP1 (%) | AP3 (%) | ES (%) | ||||||
| Source | F-value | p-value | F-value | p-value | F-value | p-value | ||
| Model | 32.77 | 0.0003 | 31.11 | 0.0003 | 19.90 | 0.0011 | ||
| A (boiling temperature) | 131.44 | 0.0001 | 129.12 | 0.0001 | 12.04 | 0.0133 | ||
| B (ethanol concentration) | 23.53 | 0.0029 | 21.25 | 0.0037 | 66.68 | 0.0002 | ||
| C (extraction time) | 5.74 | 0.0535 | 2.24 | 0.1851 | 4.50 | 0.0782 | ||
| D (extraction cycles) | 2.66 | 0.1539 | 2.78 | 0.1467 | 15.29 | 0.0079 | ||
| E (ratio of liquid to solid) | 0.46 | 0.5229 | 0.14 | 0.7178 | 1.00 | 0.3568 | ||
2.3. Optimization of Screened Variables for the Diterpenoids Production Using Response Surface Methodology
| Run | Factors | Response Variables | ||||
|---|---|---|---|---|---|---|
| A (Boiling Temperature) | B (Ethanol Concentration) | C (Extraction Time) | AP1 (%) | AP3 (%) | ES (%) | |
| 1 | 40 | 50 | 25 | 95.20 | 93.94 | 7.39 |
| 2 | 80 | 70 | 10 | 125.89 | 111.12 | 8.14 |
| 3 | 80 | 70 | 40 | 109.07 | 106.97 | 8.94 |
| 4 | 80 | 90 | 25 | 91.81 | 85.2 | 4.95 |
| 5 | 40 | 90 | 25 | 59.85 | 56.39 | 1.79 |
| 6 | 60 | 70 | 25 | 96.45 | 92.53 | 6.25 |
| 7 | 60 | 70 | 25 | 90.52 | 85.59 | 6.87 |
| 8 | 60 | 90 | 10 | 75.40 | 72.48 | 2.62 |
| 9 | 80 | 50 | 25 | 148.37 | 132.37 | 9.34 |
| 10 | 60 | 50 | 10 | 136.76 | 120.42 | 7.99 |
| 11 | 60 | 70 | 25 | 109.92 | 106.27 | 5.74 |
| 12 | 40 | 70 | 40 | 65.77 | 74.03 | 5.85 |
| 13 | 60 | 90 | 40 | 65.89 | 67.94 | 3.62 |
| 14 | 40 | 70 | 10 | 65.03 | 66.27 | 4.50 |
| 15 | 60 | 50 | 40 | 129.69 | 127.99 | 9.24 |
| 16 | 60 | 70 | 25 | 103.18 | 100.97 | 6.10 |
| 17 | 60 | 70 | 25 | 98.39 | 95.32 | 5.62 |
| Response | Source | Sum of Square | D.f. | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|---|
| AP1 (%) | Model | 10944.05 | 9 | 1216.01 | 21.46 | 0.0003 | significant |
| A | 4478.84 | 1 | 4478.84 | 79.05 | 0.0001 | ||
| B | 5889.92 | 1 | 5889.92 | 103.96 | 0.0001 | ||
| C | 133.33 | 1 | 133.33 | 2.35 | 0.1689 | ||
| AB | 112.47 | 1 | 112.47 | 1.99 | 0.2017 | ||
| AC | 77.09 | 1 | 77.09 | 1.36 | 0.2816 | ||
| BC | 1.49 | 1 | 1.49 | 0.026 | 0.8758 | ||
| A2 | 136.31 | 1 | 136.31 | 2.41 | 0.1648 | ||
| B2 | 97.22 | 1 | 97.22 | 1.72 | 0.2316 | ||
| C2 | 27.64 | 1 | 27.64 | 0.49 | 0.5074 | ||
| Residual | 396.6 | 7 | 56.66 | ||||
| Lack of fit | 183.49 | 3 | 61.16 | 1.15 | 0.4316 | not significant | |
| Pure error | 213.11 | 4 | 53.28 | ||||
| Total | 11340.65 | 16 | |||||
| AP3 (%) | Model | 7529.74 | 9 | 836.64 | 17.61 | 0.0005 | significant |
| A | 2629.61 | 1 | 2629.61 | 55.34 | 0.0001 | ||
| B | 4642.14 | 1 | 4642.14 | 97.70 | 0.0001 | ||
| C | 5.51 | 1 | 5.51 | 0.12 | 0.7434 | ||
| AB | 23.14 | 1 | 23.14 | 0.49 | 0.5078 | ||
| AC | 35.46 | 1 | 35.46 | 0.75 | 0.4162 | ||
| BC | 36.66 | 1 | 36.66 | 0.77 | 0.4088 | ||
| A2 | 145.85 | 1 | 145.85 | 3.07 | 0.1232 | ||
| B2 | 12.52 | 1 | 12.52 | 0.26 | 0.6235 | ||
| C2 | 1.80 | 1 | 1.80 | 0.038 | 0.8514 | ||
| Residual | 332.59 | 7 | 47.51 | ||||
| Lack of fit | 81.64 | 3 | 27.21 | 0.43 | 0.7407 | not significant | |
| Pure error | 250.95 | 4 | 62.74 | ||||
| Total | 7862.33 | 16 | |||||
| ES (%) | Model | 78.06 | 9 | 8.67 | 42.50 | 0.0001 | significant |
| A | 17.52 | 1 | 17.52 | 85.87 | 0.0001 | ||
| B | 55.02 | 1 | 55.02 | 269.63 | 0.0001 | ||
| C | 2.42 | 1 | 2.42 | 11.86 | 0.0108 | ||
| AB | 0.37 | 1 | 0.37 | 1.79 | 0.2223 | ||
| AC | 0.076 | 1 | 0.076 | 0.37 | 0.5619 | ||
| BC | 0.016 | 1 | 0.016 | 0.077 | 0.7900 | ||
| A2 | 0.58 | 1 | 0.58 | 2.84 | 0.1360 | ||
| B2 | 1.61 | 1 | 1.61 | 7.91 | 0.0260 | ||
| C2 | 0.58 | 1 | 0.58 | 2.84 | 0.1360 | ||
| Residual | 1.43 | 7 | 0.20 | ||||
| Lack of fit | 0.45 | 3 | 0.15 | 0.62 | 0.6372 | not significant | |
| Pure error | 0.97 | 4 | 0.24 | ||||
| Total | 79.49 | 16 |
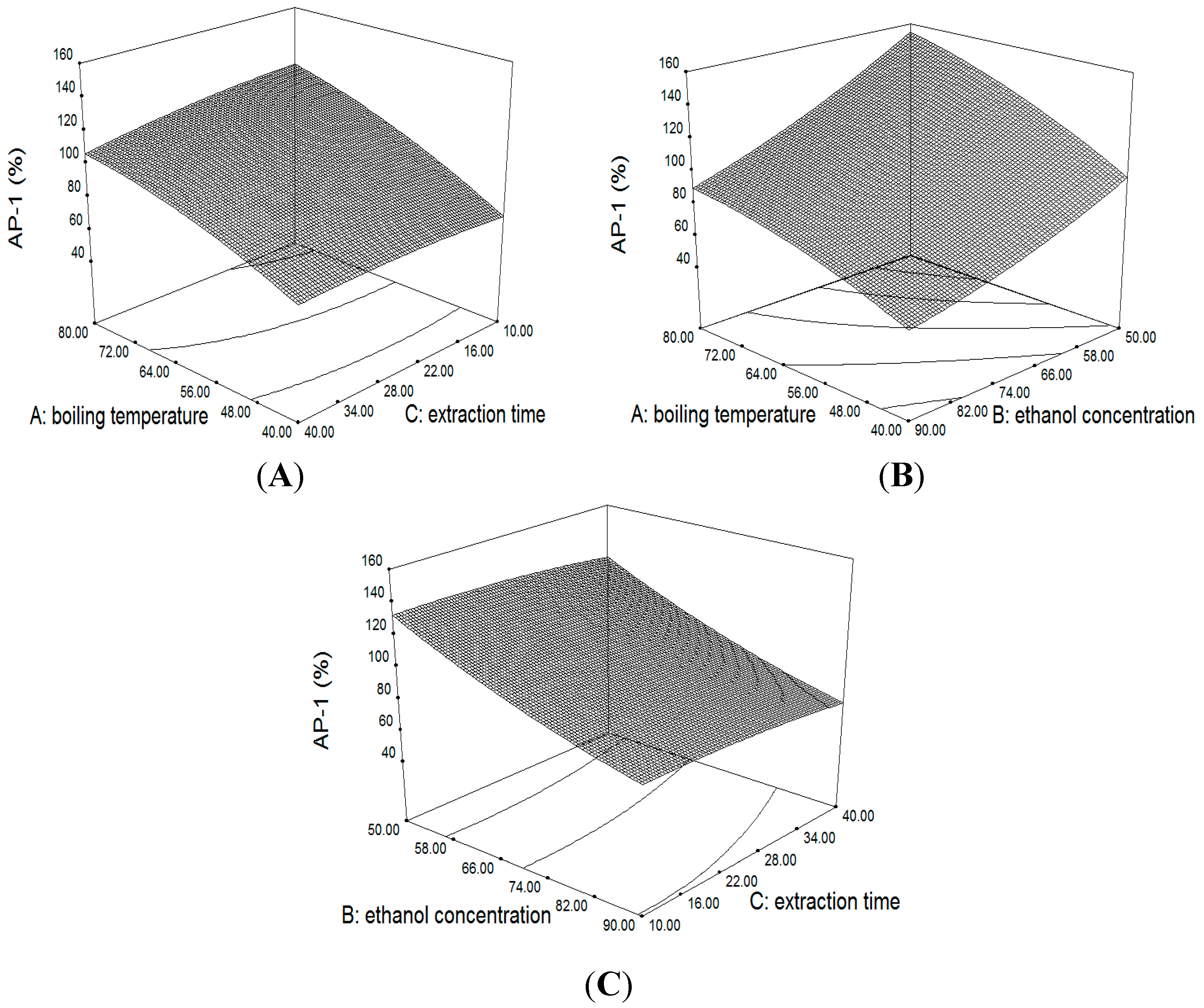
2.3.1. Response Surface of the Extraction Yield of AP1 and AP3

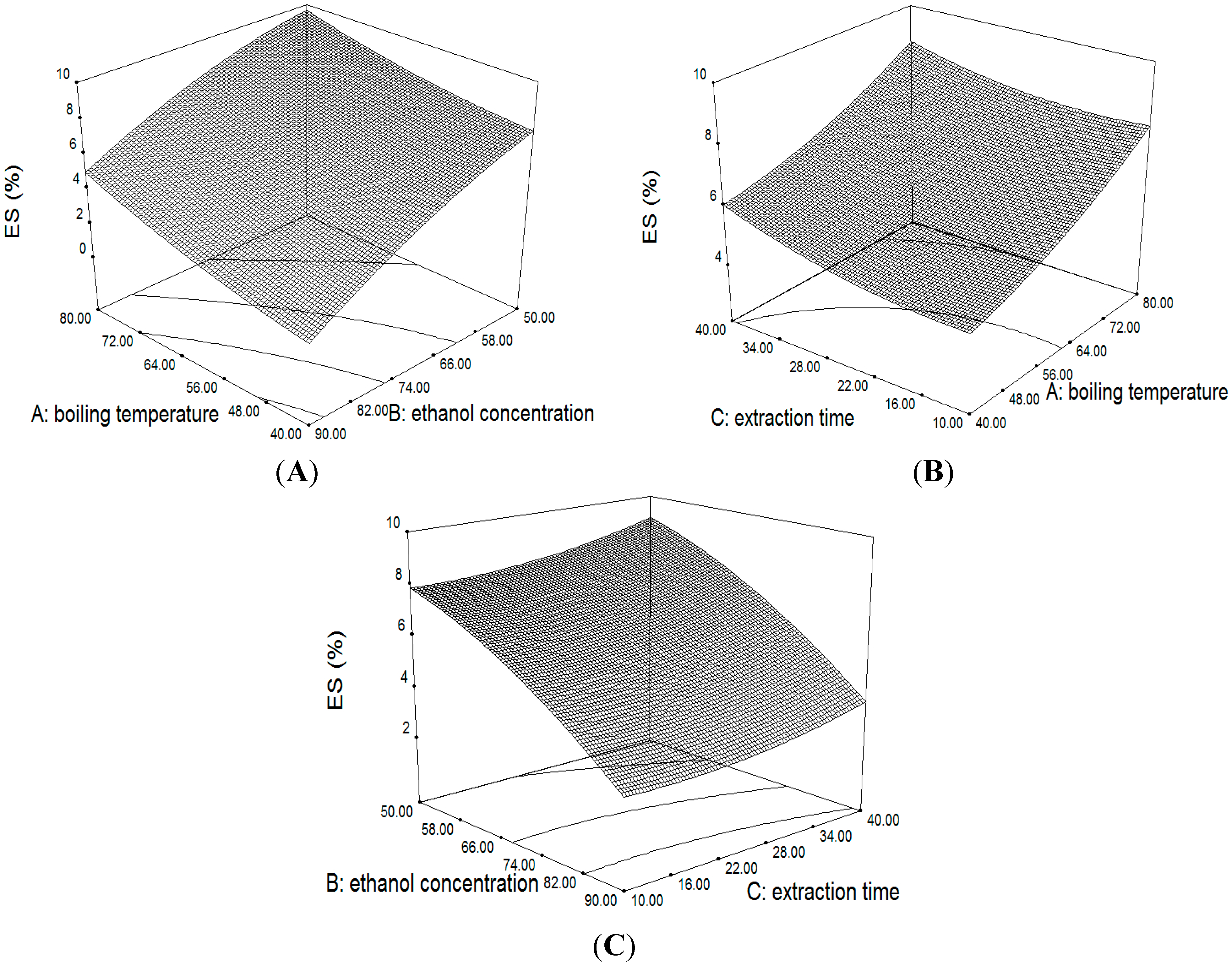
2.3.2. Response Surface of the Yield of ES
2.3.3. Optimization of the Extraction Process
| Response | Goal | Importance | Replicate 1 | Replicate 2 | Replicate 3 | Predicted Value |
|---|---|---|---|---|---|---|
| AP1 (%) | Maximize | 5 | 135.3 | 139.6 | 138.3 | 138.3 |
| AP3 (%) | Maximize | 5 | 120.4 | 123.3 | 122.6 | 123.0 |
| ES (%) | Minimize | 3 | 7.9 | 8.0 | 8.1 | 8.12 |
2.4. Verification
2.5. Comparison
| Extraction Method | Ethanol Concentration (%) | Extraction Time (min) | AP1 (%) | AP3 (%) | ES (%) |
|---|---|---|---|---|---|
| RE1 | 85 | 240 | 100.00 | 100.00 | 10.0 |
| RE2 | 40 | 30 | 129.26 | 120.74 | 16.5 |
| RE3 | 50 | 16 | 89.63 | 92.11 | 9.0 |
| VAE | 50 | 16 | 137.7 | 122.1 | 8.0 |
3. Experimental Section
3.1. Materials
3.2. Apparatus
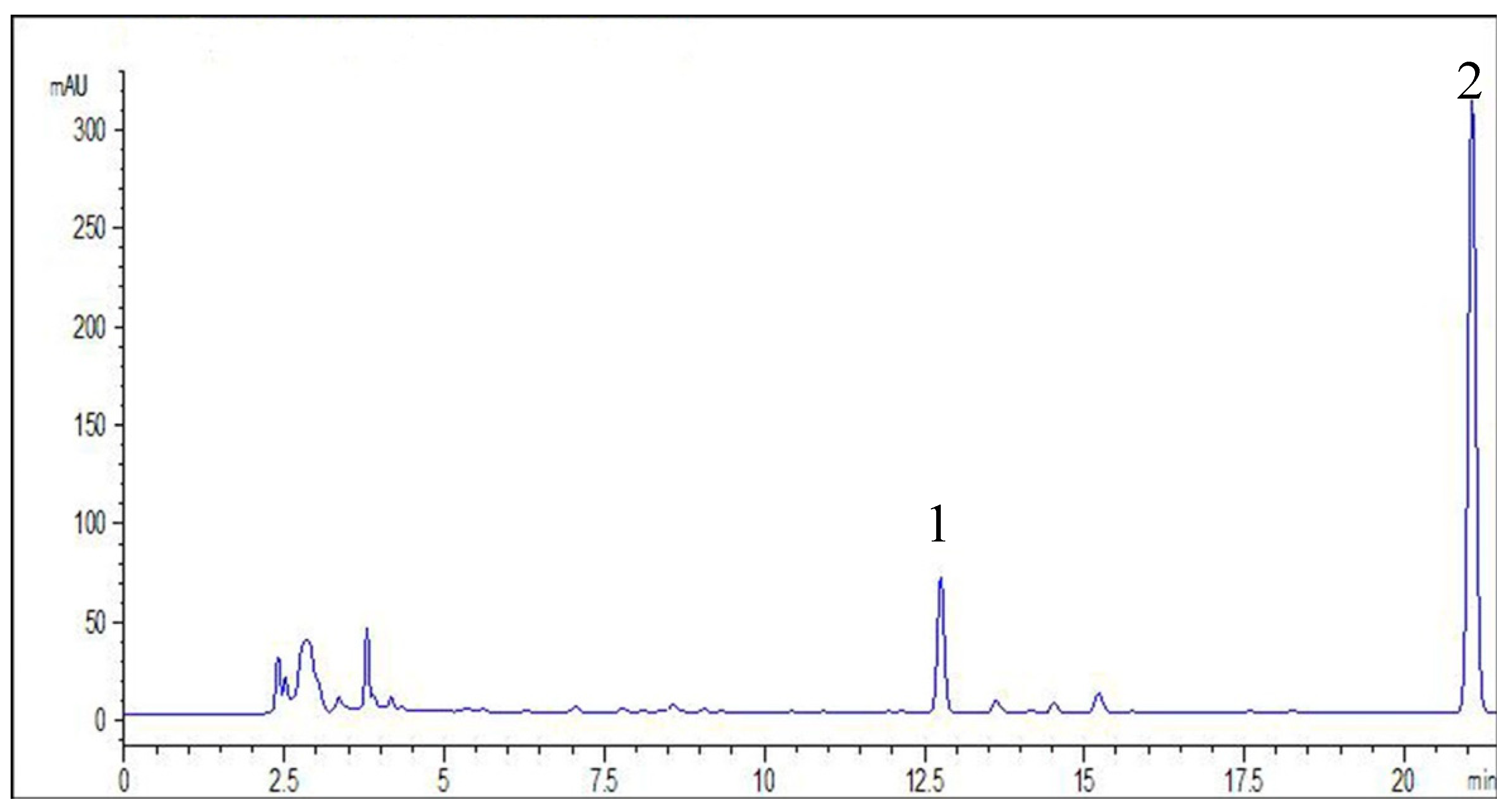
3.3. High Performance Liquid Chromatograph Analysis
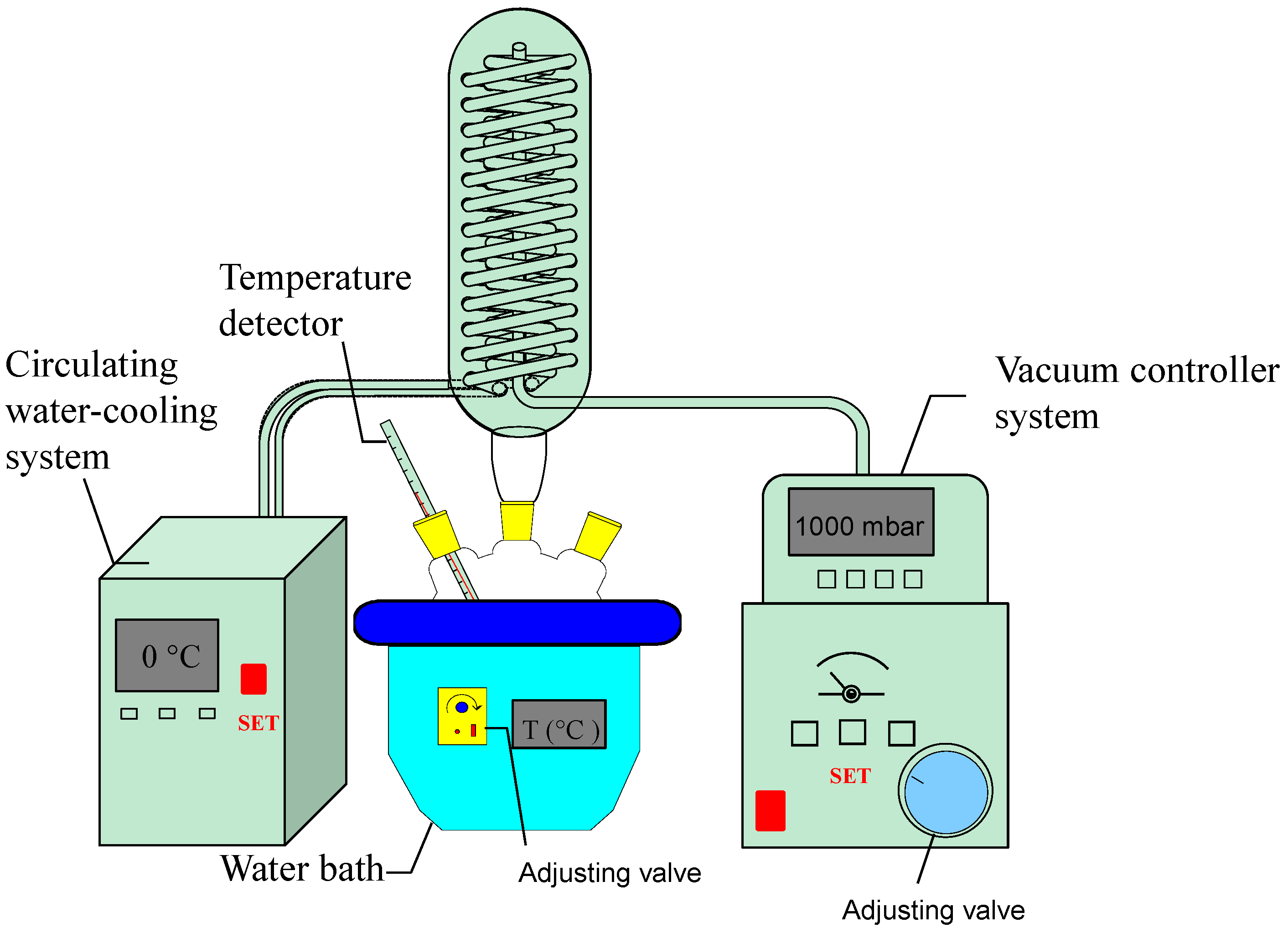
3.4. Vacuum Assisted Extraction (VAE)
3.5. Response Surface Methodology (RSM)
3.6. Optimization
3.7. Reference Extraction Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kumoro, A.C.; Hasan, M. Supercritical carbon dioxide extraction of andrographolide from Andrographis paniculata: Effect of the solvent flow rate, pressure, and temperature. Chin. J. Chem. Eng. 2007, 15, 877–883. [Google Scholar] [CrossRef]
- Wongkittipong, R.; Prat, L.; Damronglerd, S.; Gourdon, C. Solid-liquid extraction of andrographolide from plants-experimental study, kinetic reaction and model. Sep. Purif. Technol. 2000, 40, 147–154. [Google Scholar] [CrossRef]
- Calabrese, C.; Berman, S.H.; Babish, J.G.; Ma, X.F.; Shinto, L.; Dorr, M.; Wells, K.; Wenner, C.A.; Standish, L.J. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 2000, 14, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, R.; Vijayakumar, M.; Pushpangadan, P. Antioxidant approach to disease management and the role of ‘Rasayana’ herbs of Ayurveda. J. Ethnopharmacol. 2005, 99, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wang, D.X.; Zhang, W.; Liao, X.Q.; Guan, X.; Bo, H.; Sun, J.Y.; Huang, N.W.; He, J.; Zhang, Y.K.; et al. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-kappa B. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.P.; Gupta, V.; Sivaraj, N.; Dutta, M. Andrographis paniculata (Burm. f.) Wall. ex Nees (kalmegh), a traditional hepatoprotective drug from India. Genet. Resour. Crop Evol. 2013, 60, 1181–1189. [Google Scholar] [CrossRef]
- Ajaya Kumar, R.; Sridevi, K.; Vijaya Kumar, N.; Nanduri, S.; Rajagopal, S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sheeja, K.; Guruvayoorappan, C.; Kuttan, G. Antiangiogenic activity of Andrographis paniculata extract and andrographolide. Int. Immunopharmacol. 2007, 7, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Tan, B.K. Antihyperglycaemic and anti-oxidant properties of Andrographis paniculata in normal and diabetic rats. Clin. Exp. Pharmacol. Physiol. 2000, 27, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kumoro, A.C.; Hasan, M. Modeling of supercritical carbon dioxide extraction of andrographolide from Andrographis paniculata leaves. Chem. Eng. Commun. 2008, 195, 72–80. [Google Scholar] [CrossRef]
- Trivedi, P.; Singh, R.; Shanker, K. Micellar extraction and cloud-point preconcentration of labdane diterpenoids from Andrographis paniculata. J. Liquid Chromatogr. Relat. Technol. 2011, 34, 2085–2102. [Google Scholar] [CrossRef]
- Vasu, S.; Palaniyappan, V.; Badami, S. A novel microwave-assisted extraction for the isolation of andrographolide from Andrographis paniculata and its in vitro antioxidant activity. Nat. Prod. Res. 2010, 24, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Gu, C.B.; Wang, W.; Yao, X.H. Negative pressure cavitation extraction and antioxidant activity of biochanin A and genistein from the leaves of Dalbergia odorifera T. Chen. Sep. Purif. Technol. 2011, 83, 91–99. [Google Scholar] [CrossRef]
- Cheong, M.W.; Tan, A.A.A.; Liu, S.Q.; Curran, P.; Yu, B. Pressurised liquid extraction of volatile compounds in coffee bean. Talanta 2013, 115, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D. An Vacuum Assisted Extraction Device for Traditional Chinese Medicine. China Patent ZL200420024138.7, 2 February 2005. [Google Scholar]
- Wang, J.X.; Xiao, X.H.; Li, G.K. Study of vacuum microwave-assisted extraction of polyphenolic compounds and pigment from Chinese herbs. J. Chromatogr. A 2008, 1198–1199, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Li, Y.W.; Zhang, Y.; Li, G.K.; Chen, Y.Q. Development of sample preparation method for auxin analysis in plants by vacuum microwave-assisted extraction combined with molecularly imprinted clean-up procedure. Anal. Bioanal. Chem. 2011, 399, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Holt, B.D.; Sturchio, N.C.; Arehart, G.B.; Bakel, A.J. Ultrasonic vacuum extraction of gases from water for chemical and isotopic analysis. Chem. Geol. 1995, 122, 275–284. [Google Scholar] [CrossRef]
- Li, H.Y.; Deng, Z.Y.; Wu, T.; Liu, R.H.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Pholphana, N.; Rangkadilok, N.; Thongnest, S.; Ruchirawat, S.; Ruchirawat, M.; Satayavivad, J. Determination and variation of three active diterpenoids in Andrographis paniculata (Burm.f.) Nees. Phytochem. Anal. 2004, 15, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.D.; Su, Z.R.; Lai, X.P.; Lin, S.H.; Dong, X.B.; Liu, Z.Q.; Xie, P.S. Changes of dehydroandrographolide’s contents of andrographis tablet in the process of production. China J. Chin. Mater. Med. 2002, 27, 911–913. [Google Scholar]
- Nie, L.Y.; Luo, X.P. Study on the extractive process of Kalmegh and its heat stability. Pharm. J. Chin. PLA 2005, 21, 32–34. [Google Scholar]
- Zhang, H.F.; Zhang, X.; Yang, X.H.; Qiu, N.X.; Wang, Y.; Wang, Z.Z. Microwave assisted extraction of flavonoids from cultivated Epimedium sagittatum: Extraction yield and mechanism, antioxidant activity and chemical composition. Ind. Crops Prod. 2013, 50, 857–865. [Google Scholar] [CrossRef]
- Liu, J.L.; Zheng, S.L.; Fan, Q.J.; Yuan, J.C.; Yang, S.M.; Kong, F.L. Optimization of high-pressure ultrasonic-assisted simultaneous extraction of six major constituents from Ligusticum chuanxiong Rhizome using response surface methodology. Molecules 2014, 19, 1887–1911. [Google Scholar] [CrossRef] [PubMed]
- Giergielewicz-Mozajska, H.; Dabrowski, L.; Namiesnik, J. Accelerate solvent extraction (ASE) in the analysis of environmental solid samples-some aspects of theory and practice. Crit. Rev. Anal. Chem. 2001, 31, 149–165. [Google Scholar] [CrossRef]
- The State Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2010; Volume I, p. 964. [Google Scholar]
- Sample Availability: Samples of the Andrographis paniculata are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-Q.; Wu, Z.-F.; Ke, G.; Yang, M. An Effective Vacuum Assisted Extraction Method for the Optimization of Labdane Diterpenoids from Andrographis paniculata by Response Surface Methodology. Molecules 2015, 20, 430-445. https://doi.org/10.3390/molecules20010430
Wang Y-Q, Wu Z-F, Ke G, Yang M. An Effective Vacuum Assisted Extraction Method for the Optimization of Labdane Diterpenoids from Andrographis paniculata by Response Surface Methodology. Molecules. 2015; 20(1):430-445. https://doi.org/10.3390/molecules20010430
Chicago/Turabian StyleWang, Ya-Qi, Zhen-Feng Wu, Gang Ke, and Ming Yang. 2015. "An Effective Vacuum Assisted Extraction Method for the Optimization of Labdane Diterpenoids from Andrographis paniculata by Response Surface Methodology" Molecules 20, no. 1: 430-445. https://doi.org/10.3390/molecules20010430
APA StyleWang, Y.-Q., Wu, Z.-F., Ke, G., & Yang, M. (2015). An Effective Vacuum Assisted Extraction Method for the Optimization of Labdane Diterpenoids from Andrographis paniculata by Response Surface Methodology. Molecules, 20(1), 430-445. https://doi.org/10.3390/molecules20010430





