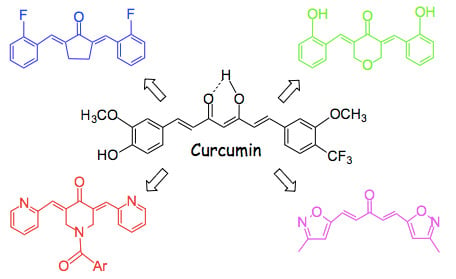
Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs)
Abstract
:1. Introduction
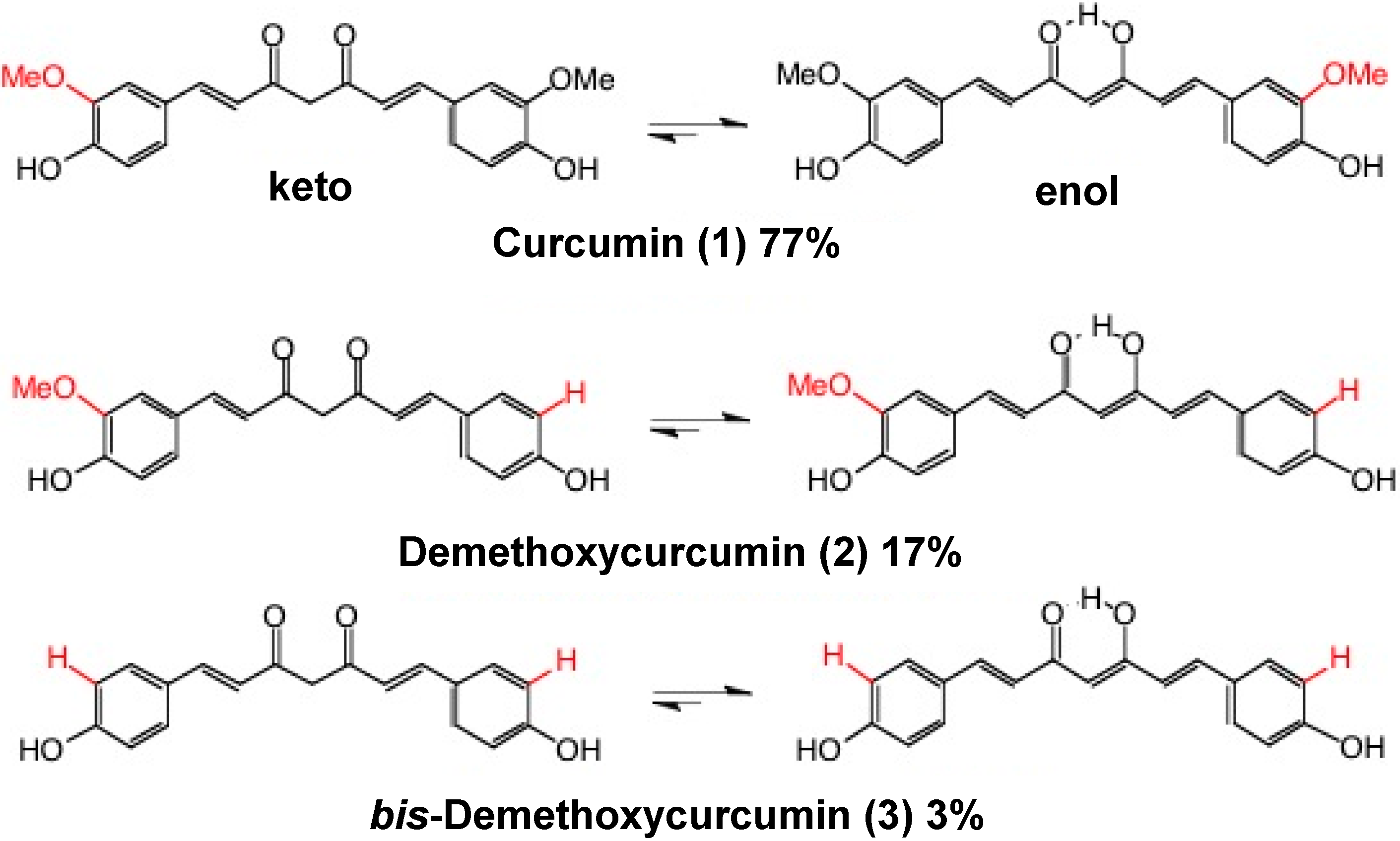
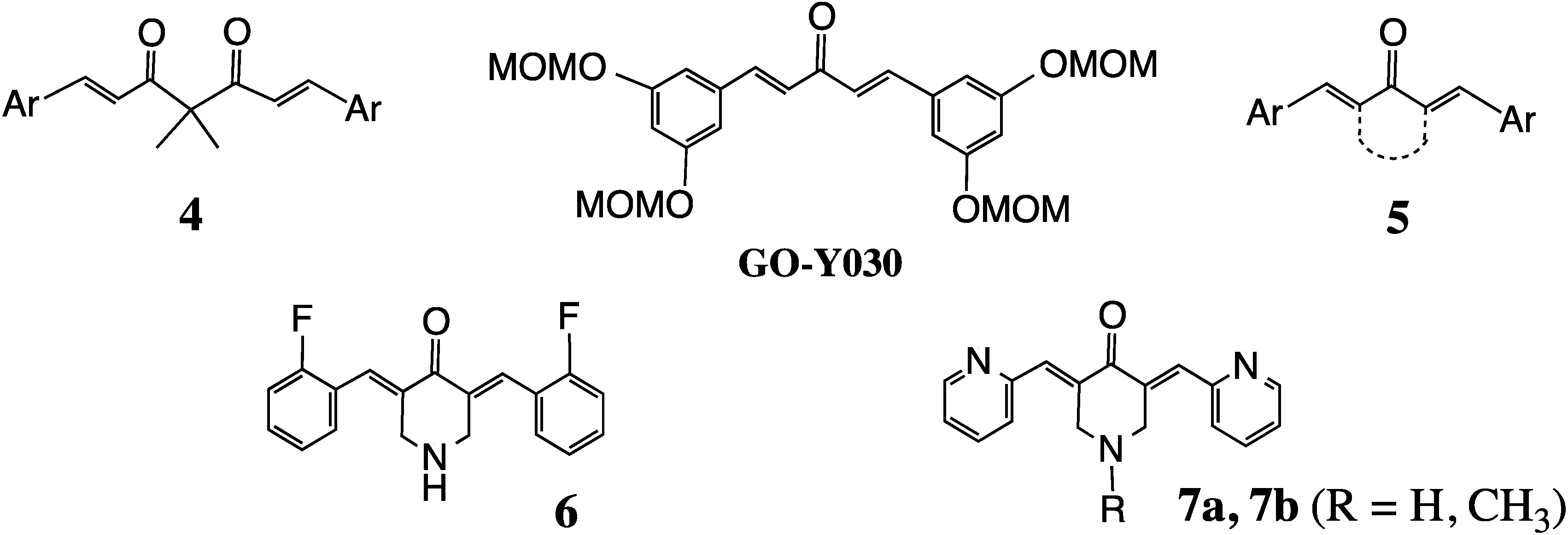
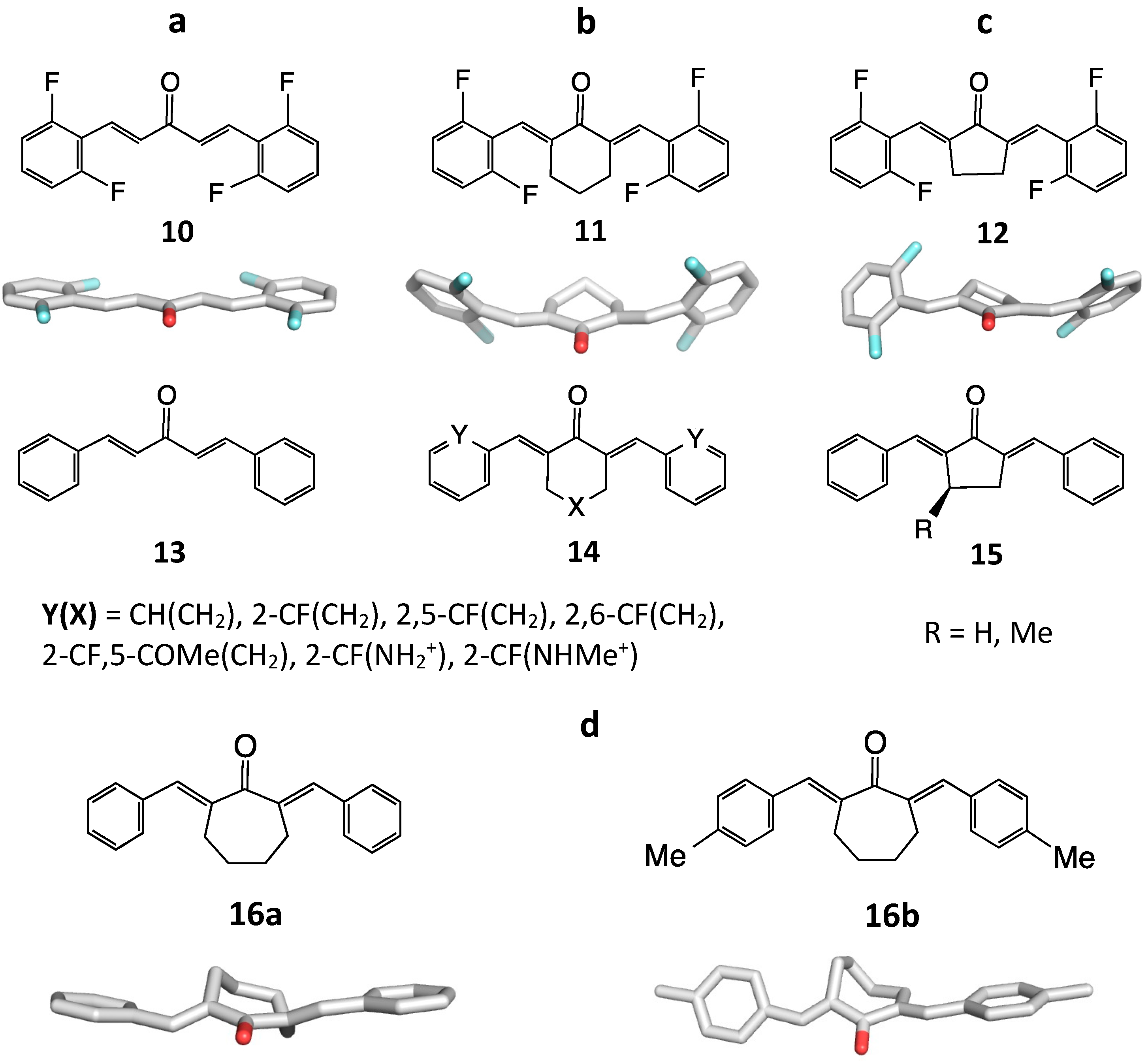
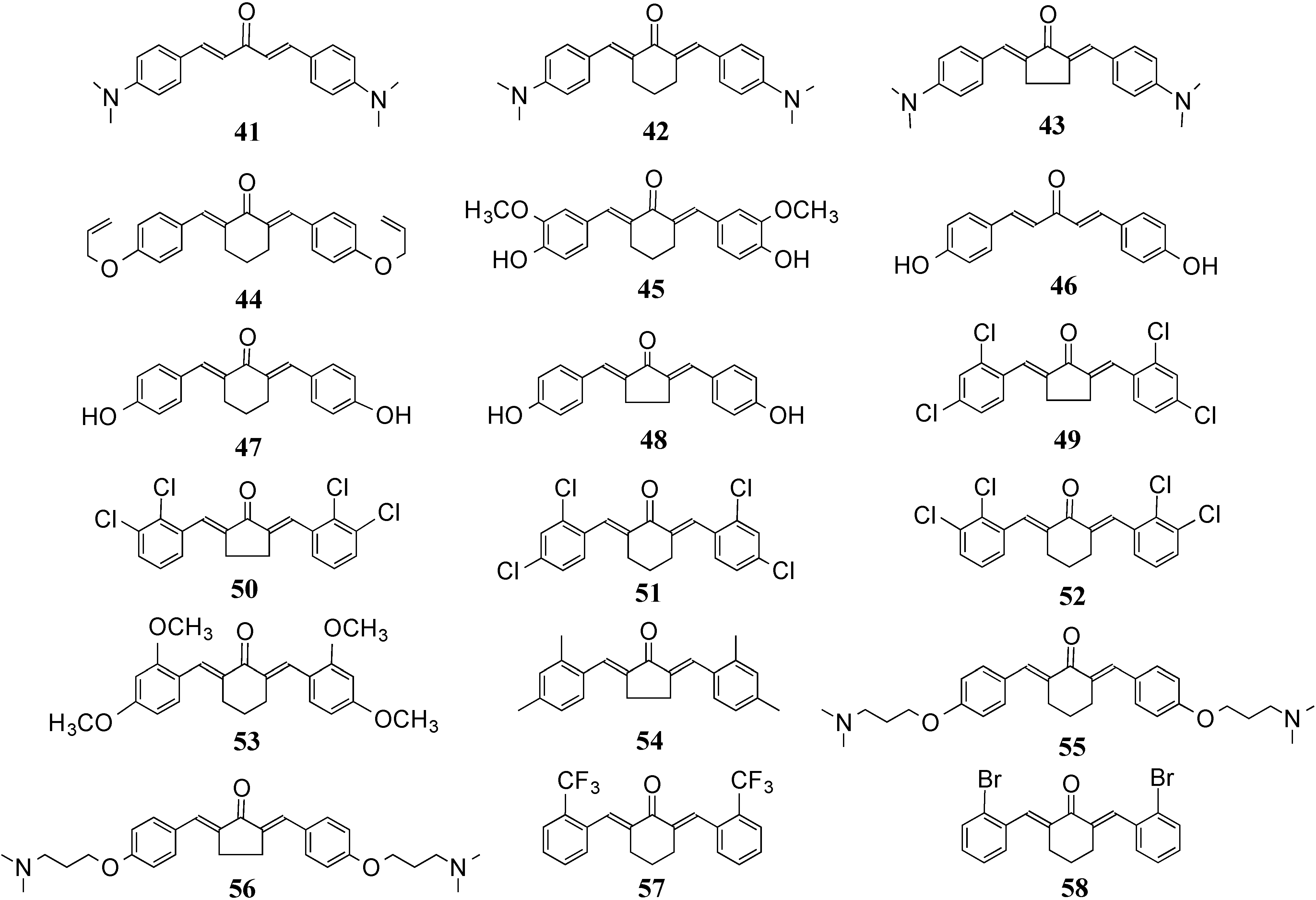
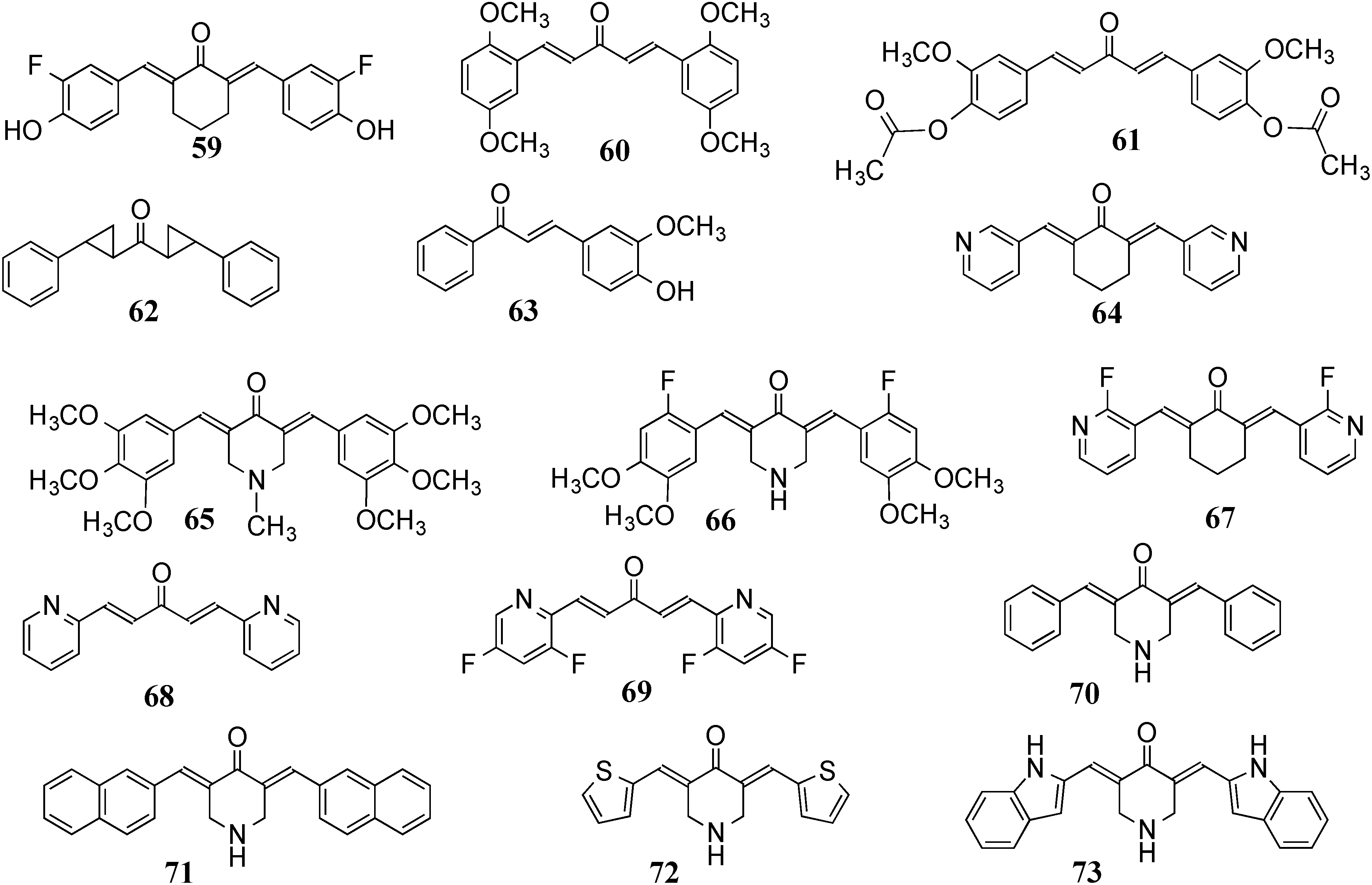
2. Structural Diversity
2.1. 2D Diversity

2.2. 3D Diversity
3. Inflammation Control in Vitro and in Vivo by MACs
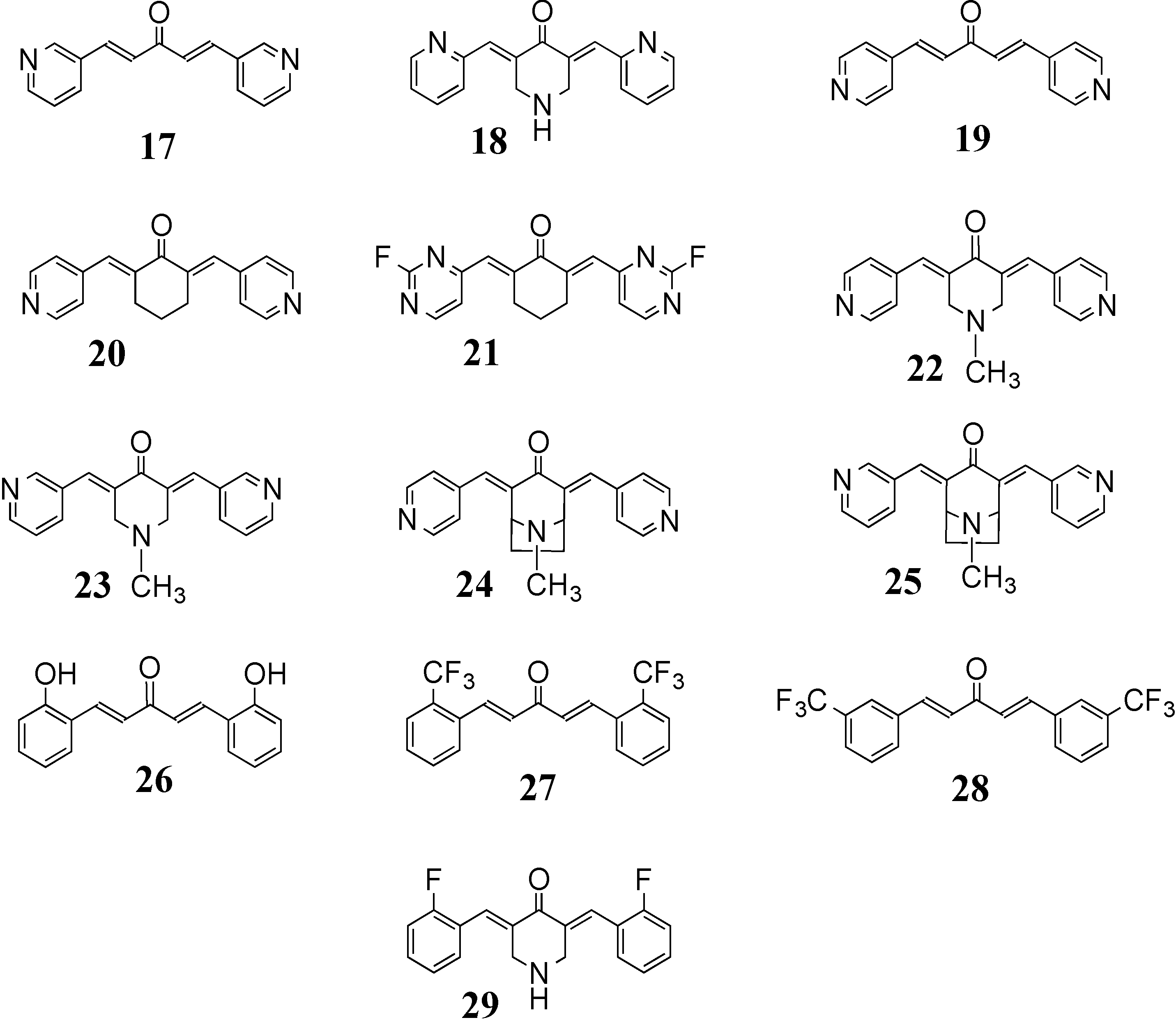
3.1. NF-κB/TNF-α
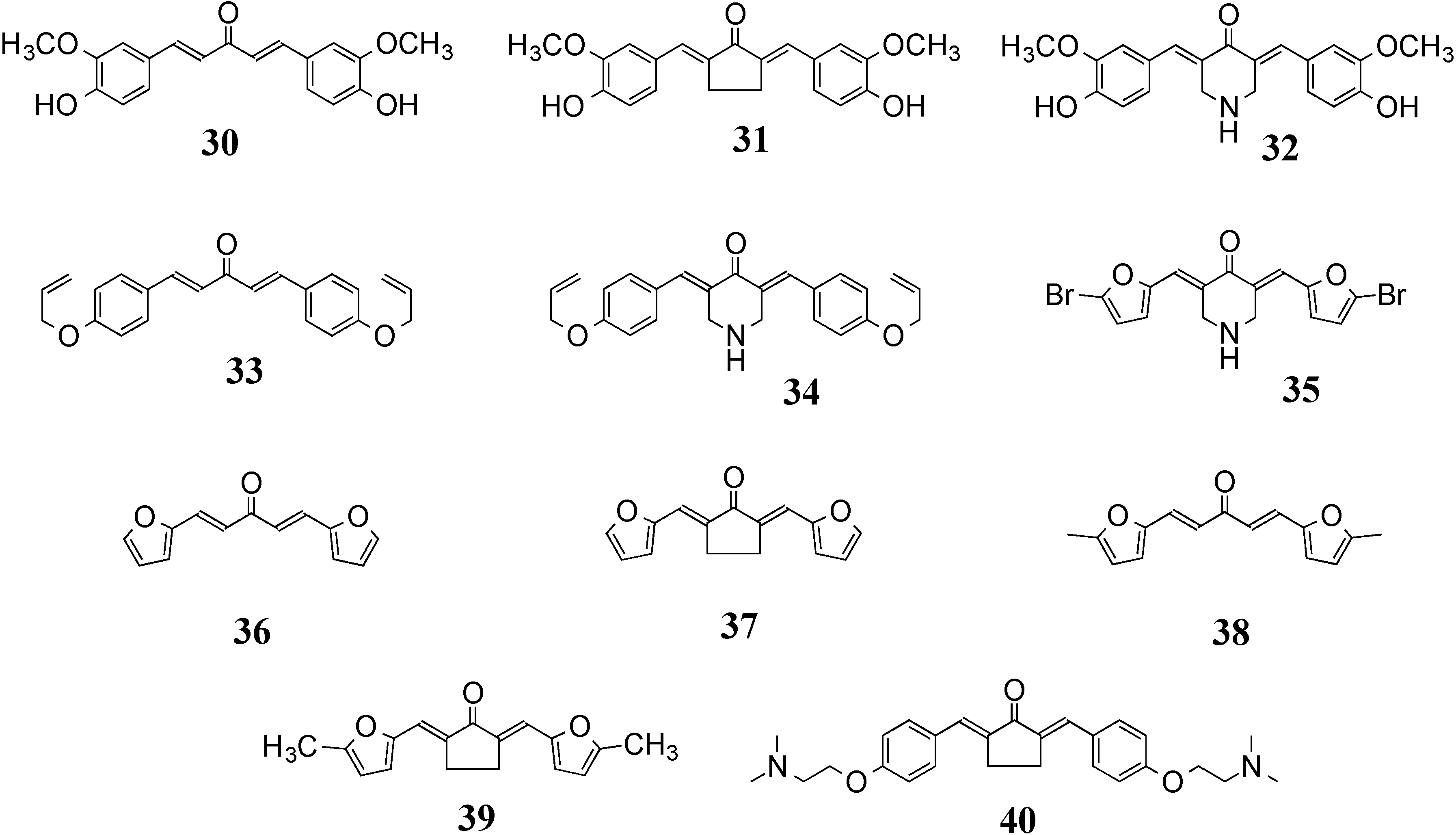
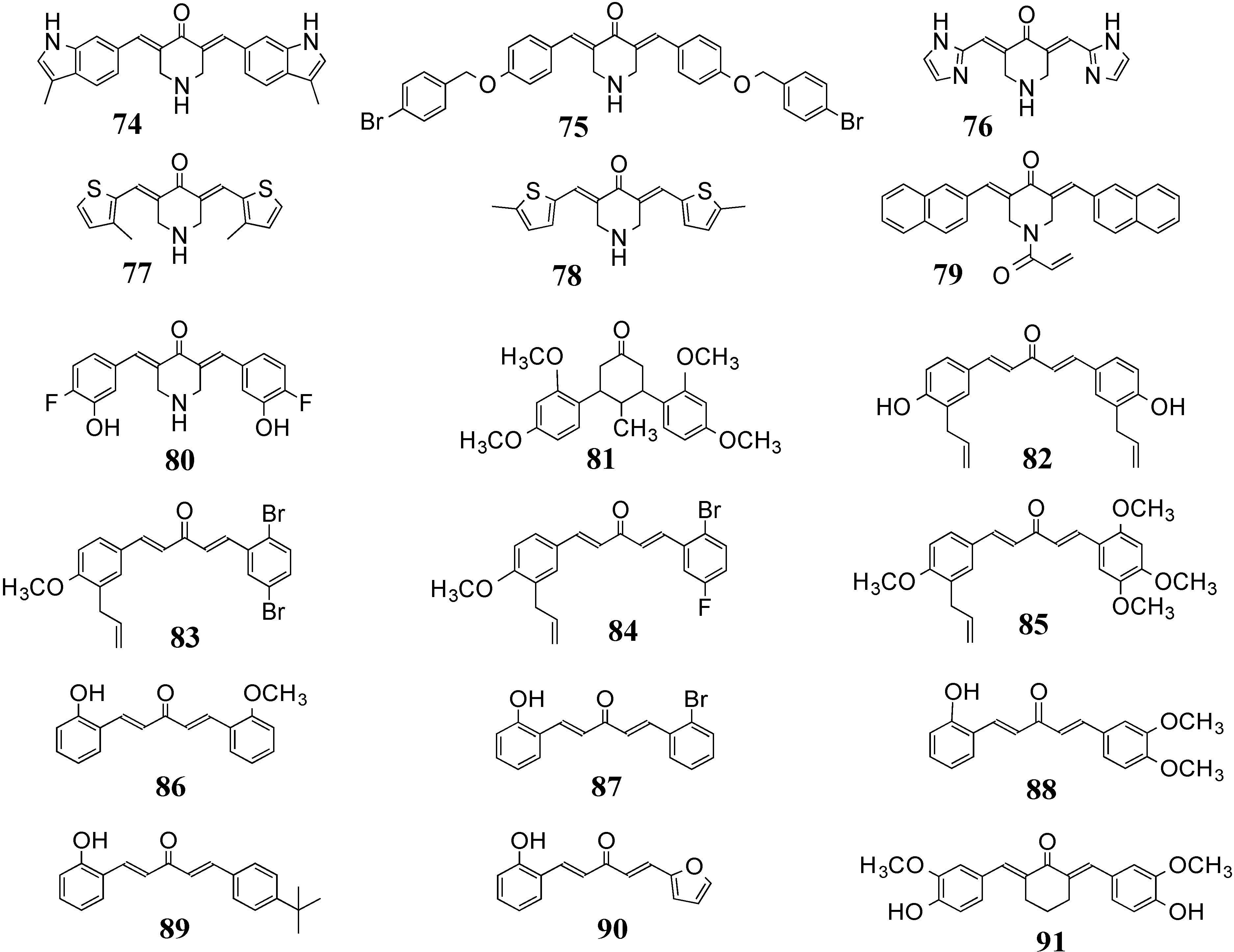
3.2. 5-LOX/COX-2
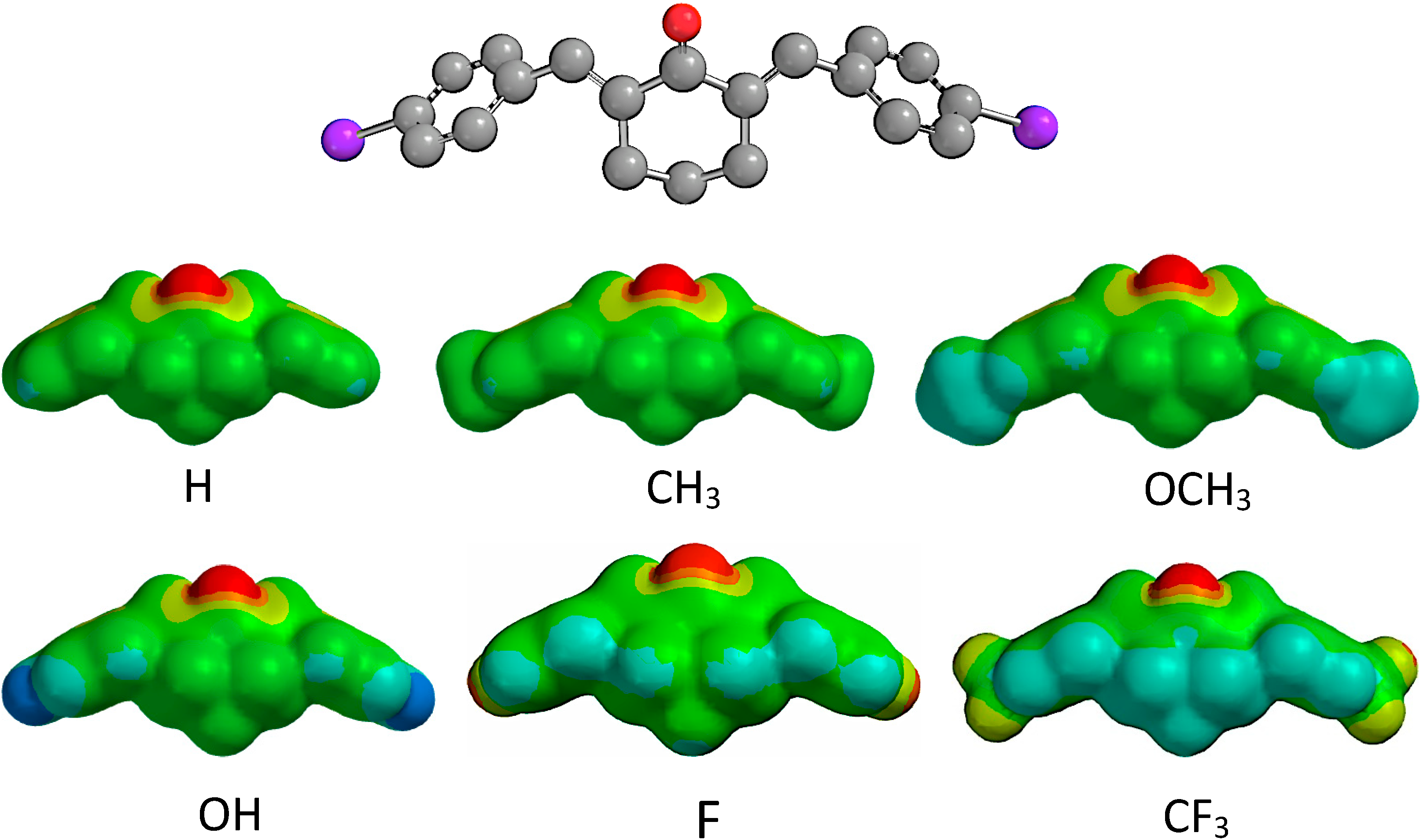
4. Cancer Mediation in Vitro and in Vivo by MACs
4.1. In Vitro Probes of Cancer Cell Lines and Signaling Factors
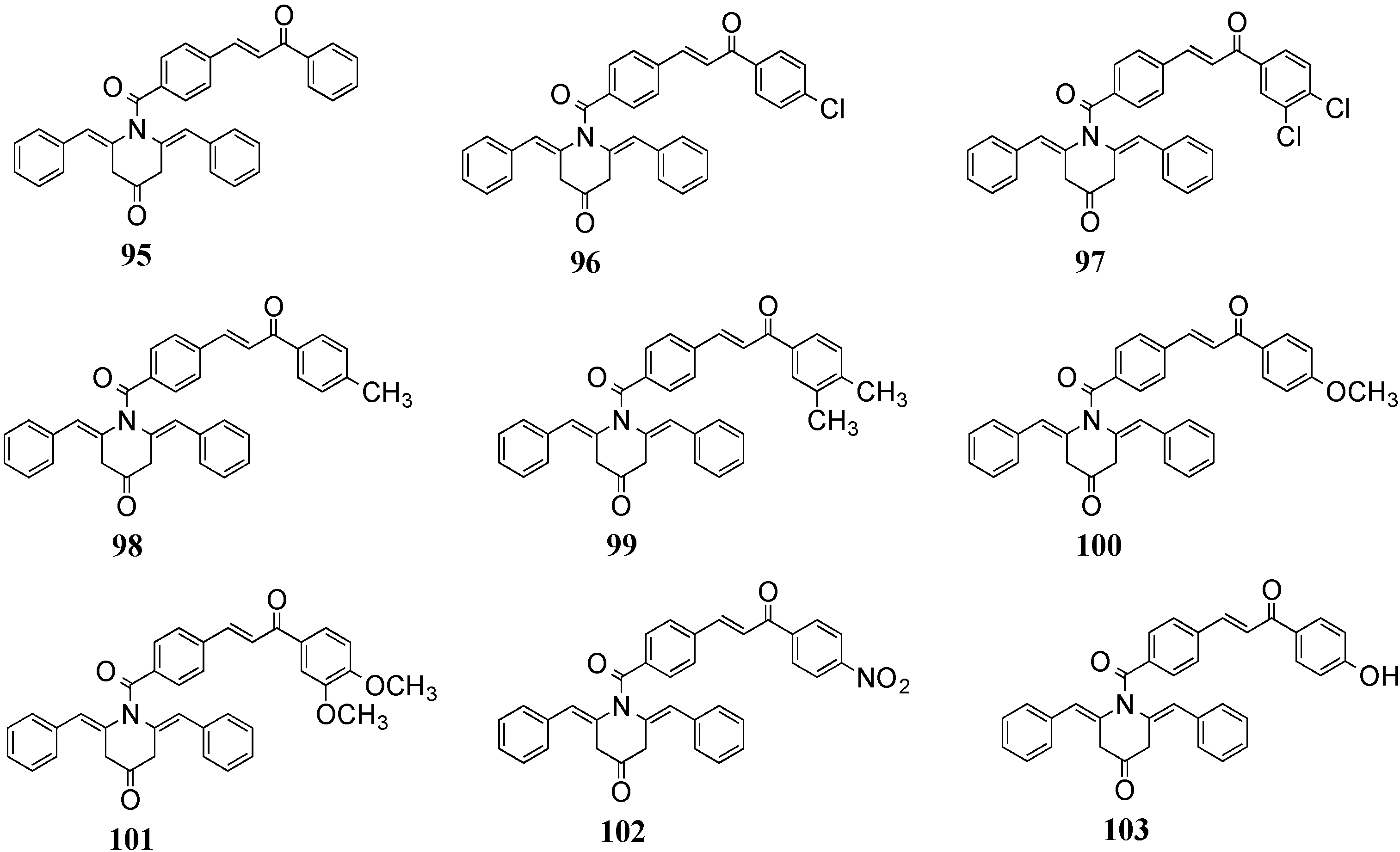
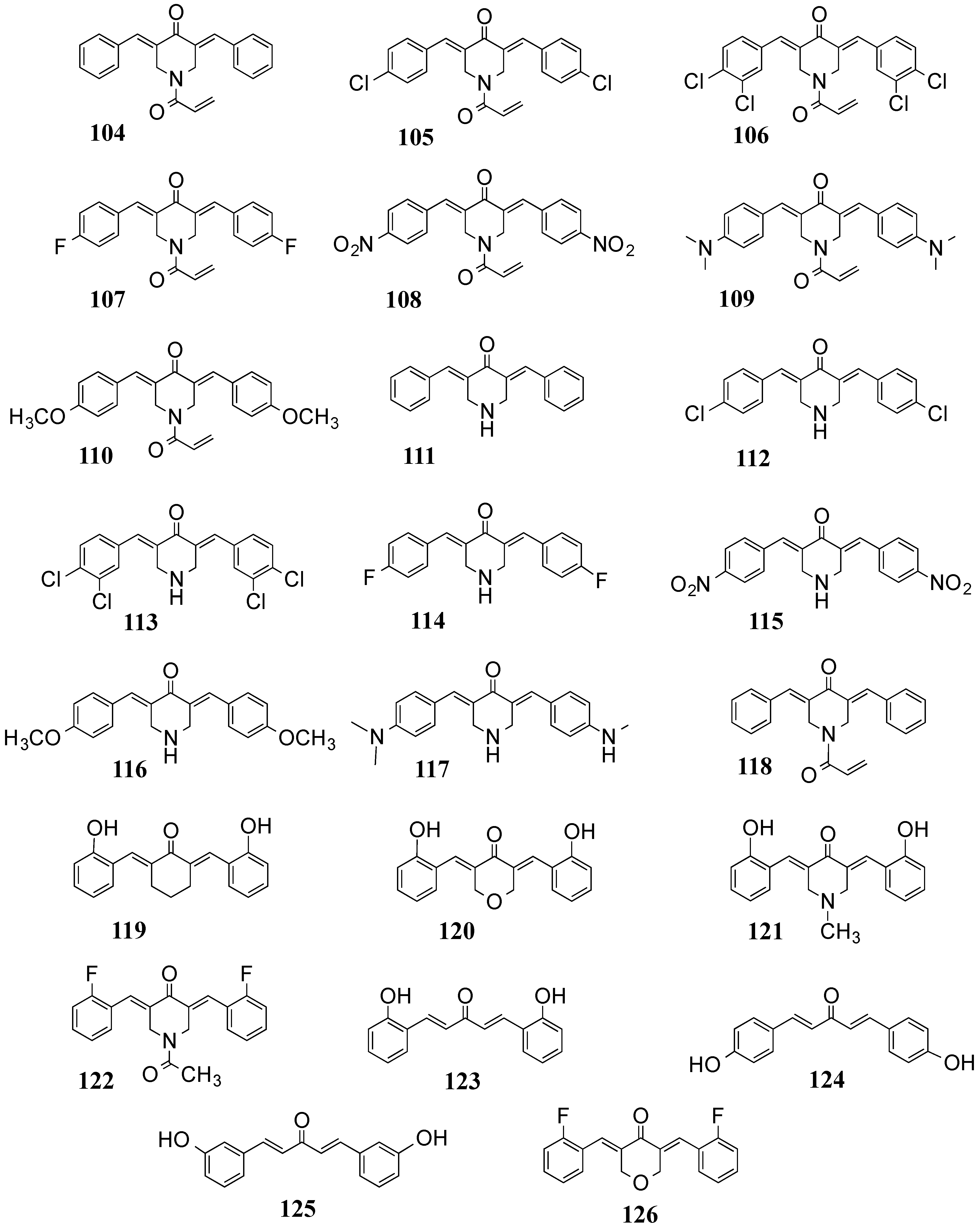
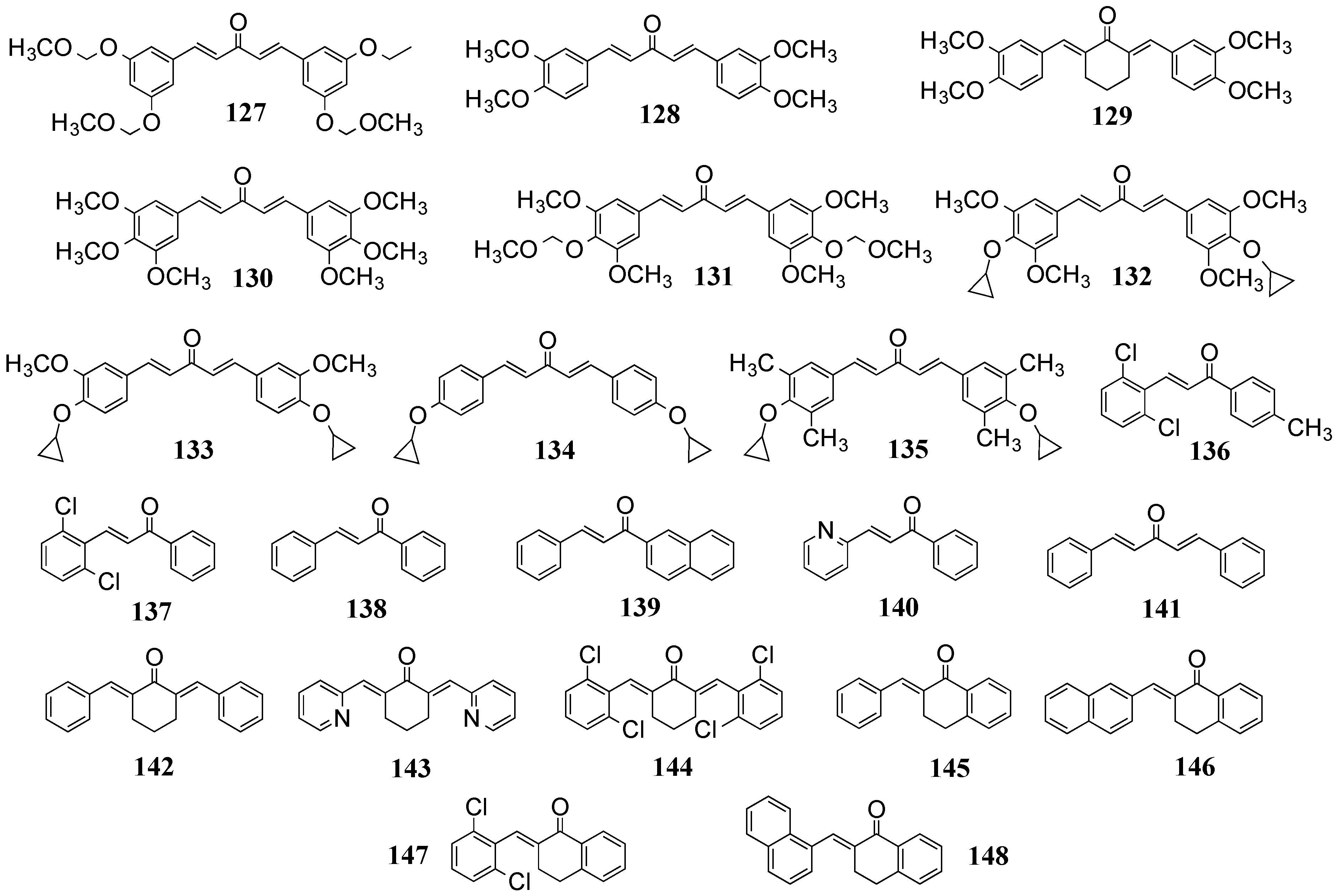
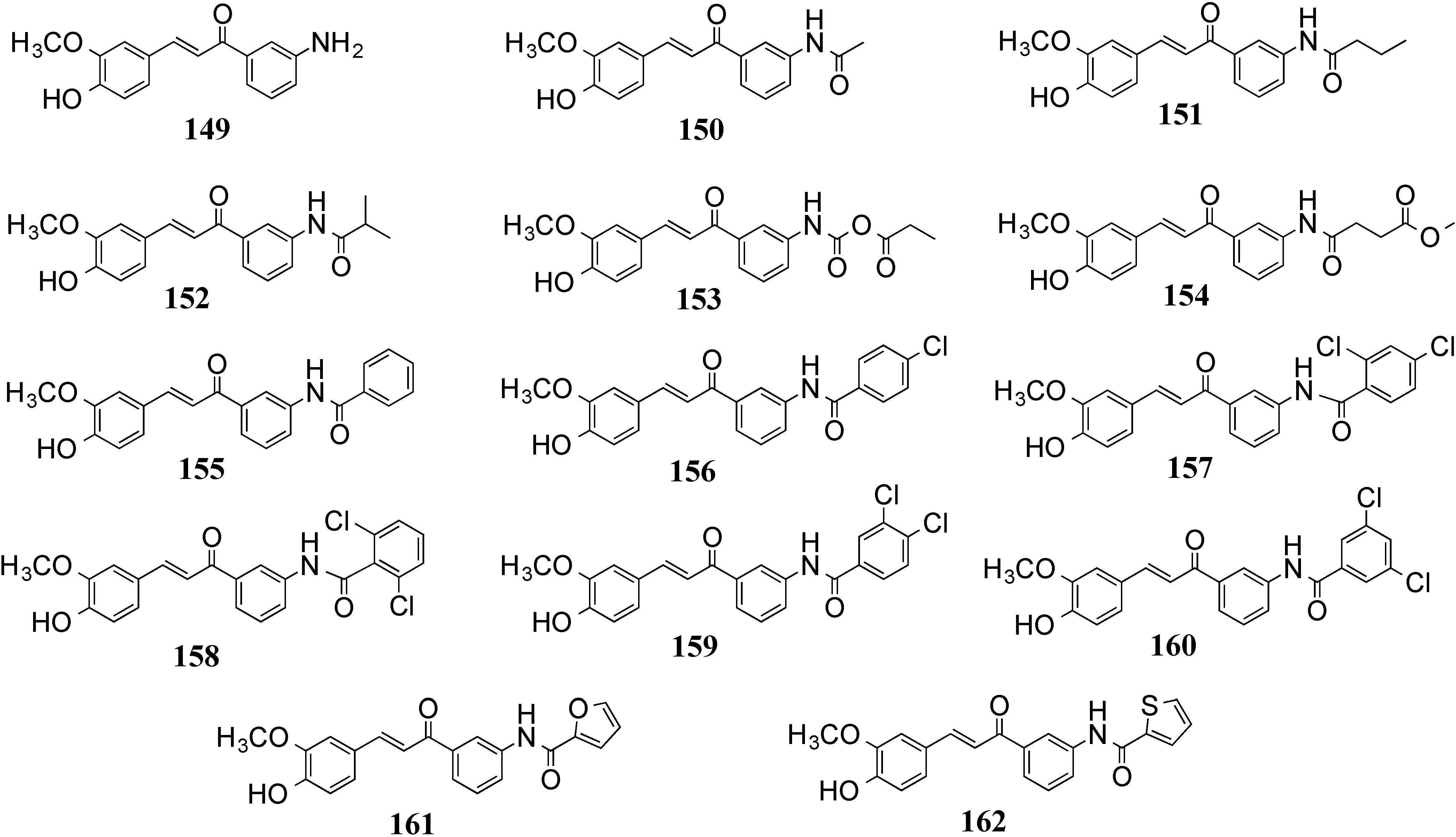
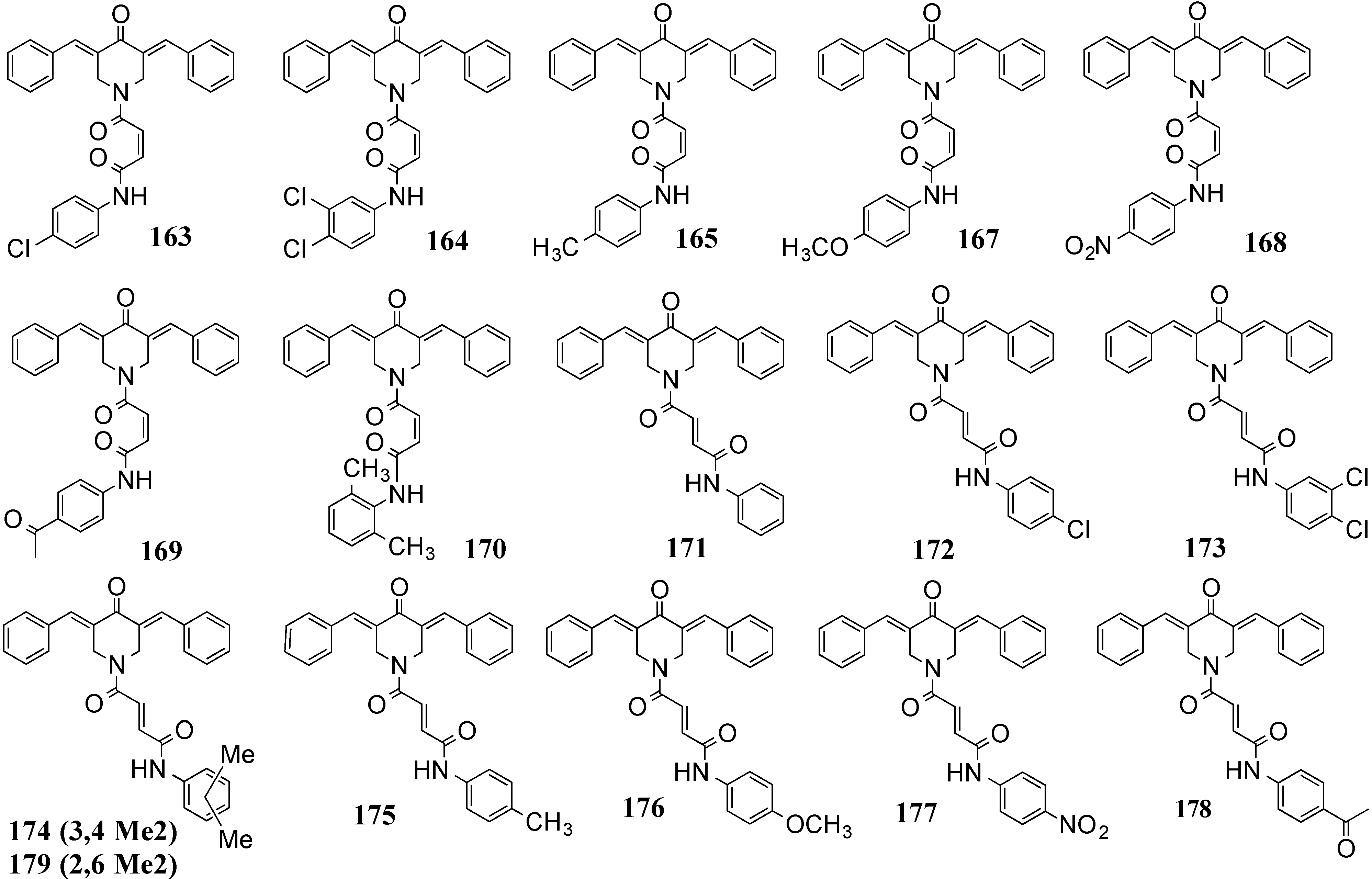
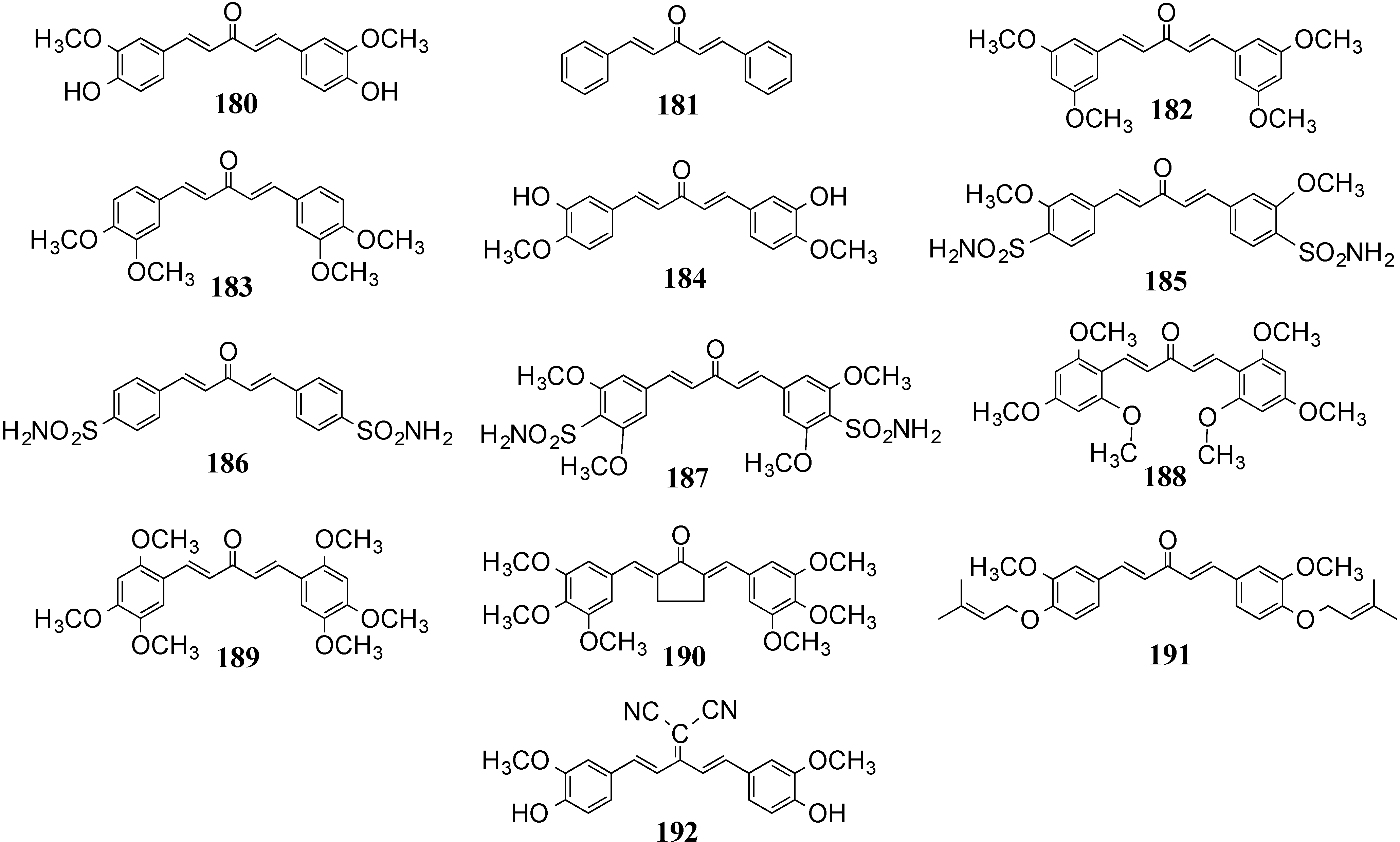
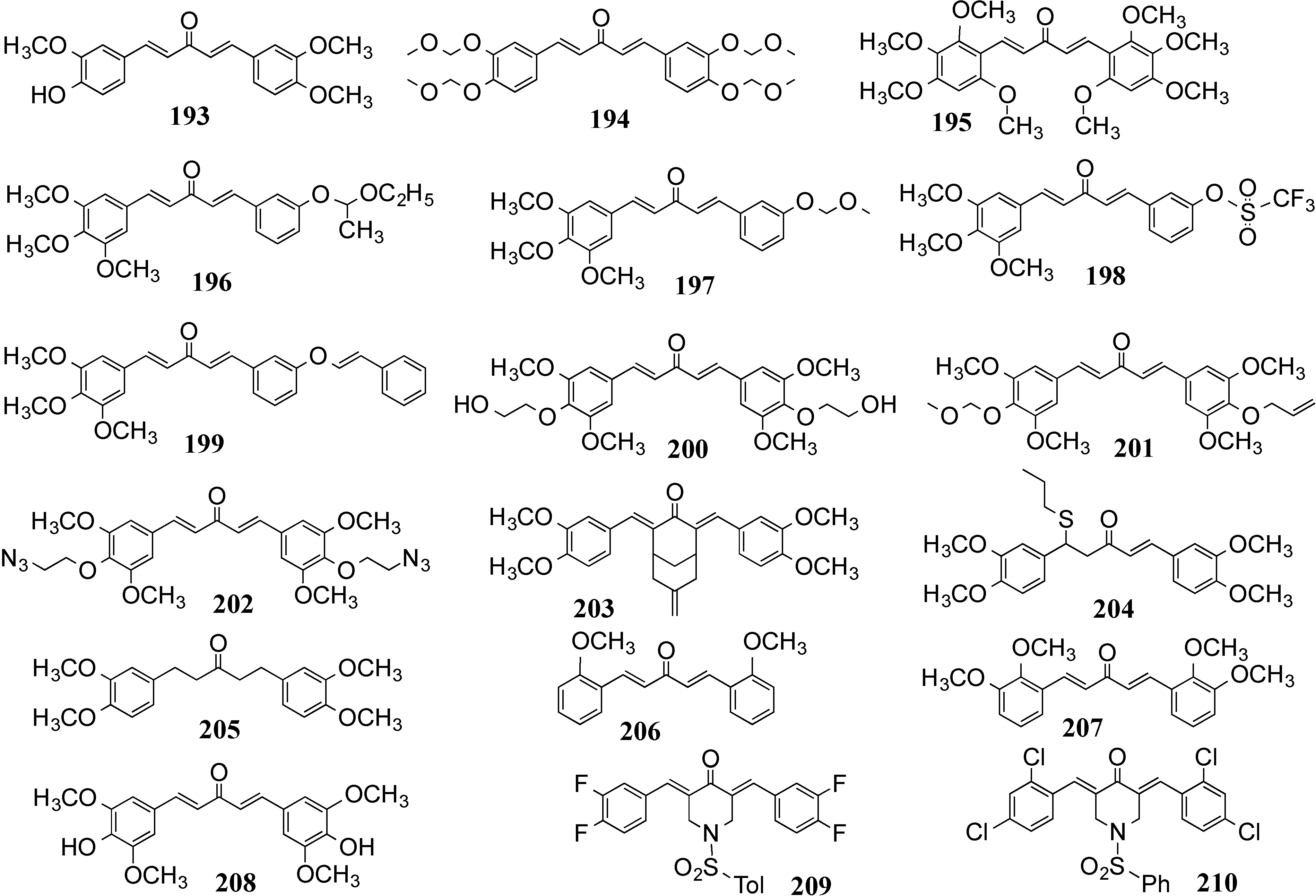
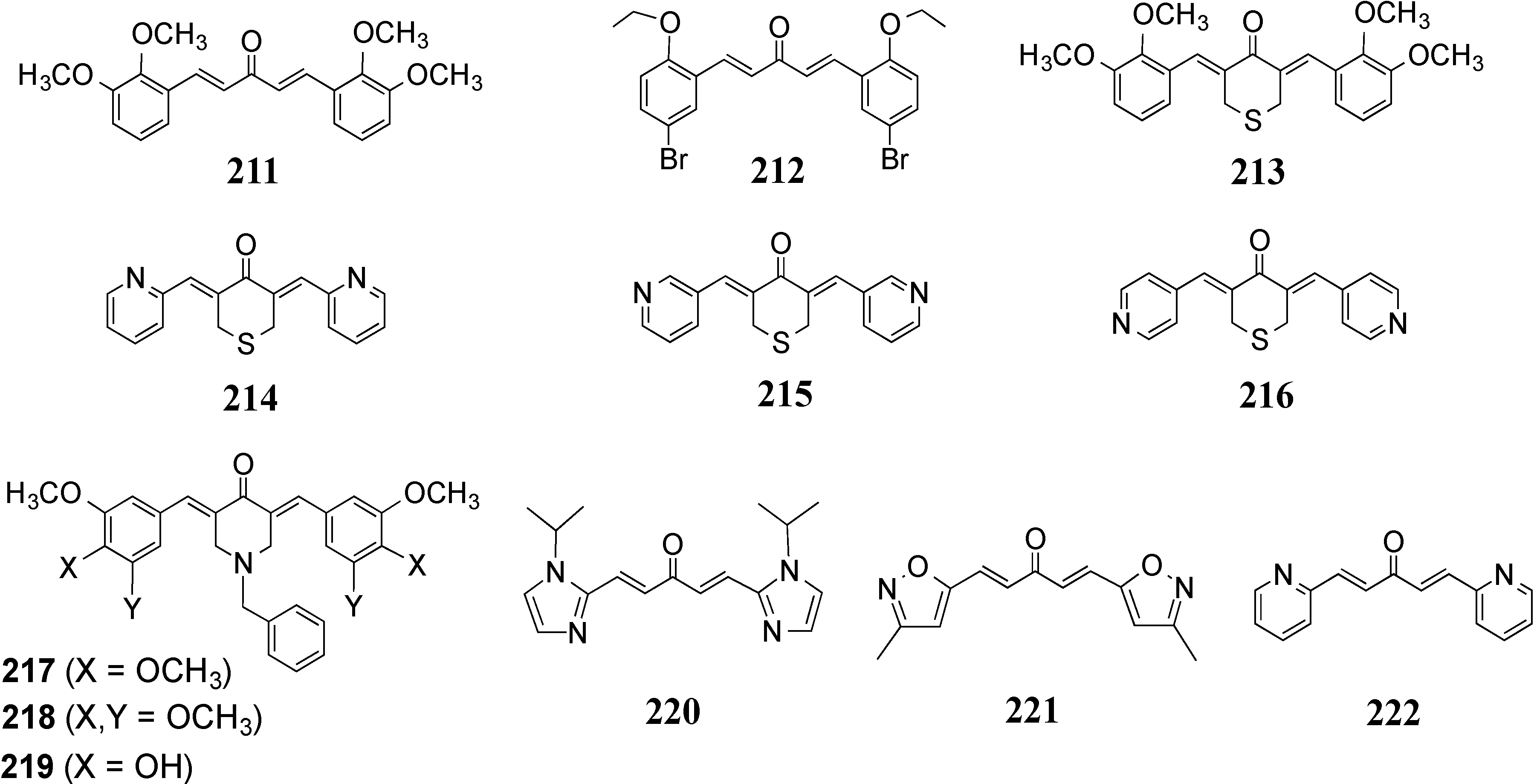
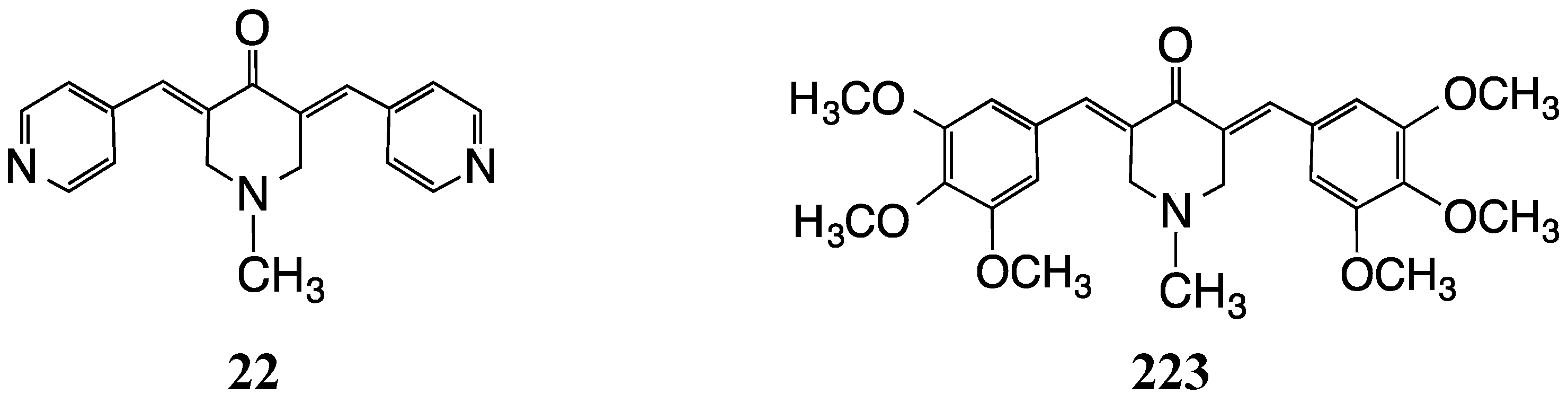
4.2. In Vivo Cancer Models, Tumor Growth and Regression: MACs
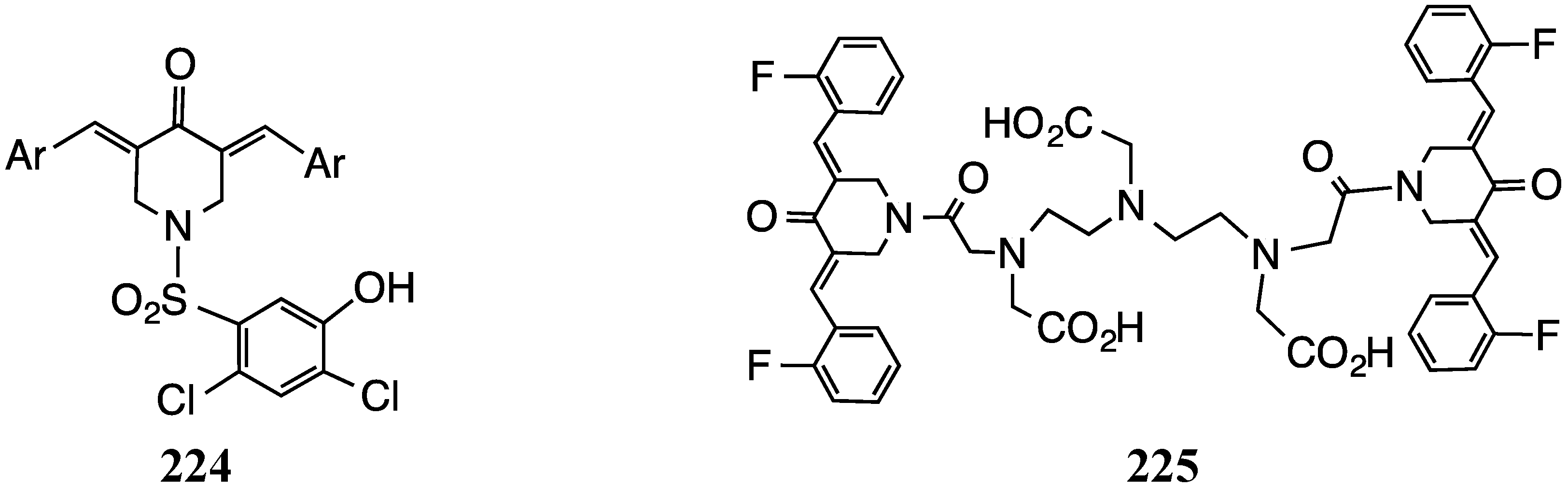
5. Bacterial Growth
6. Tuberculosis
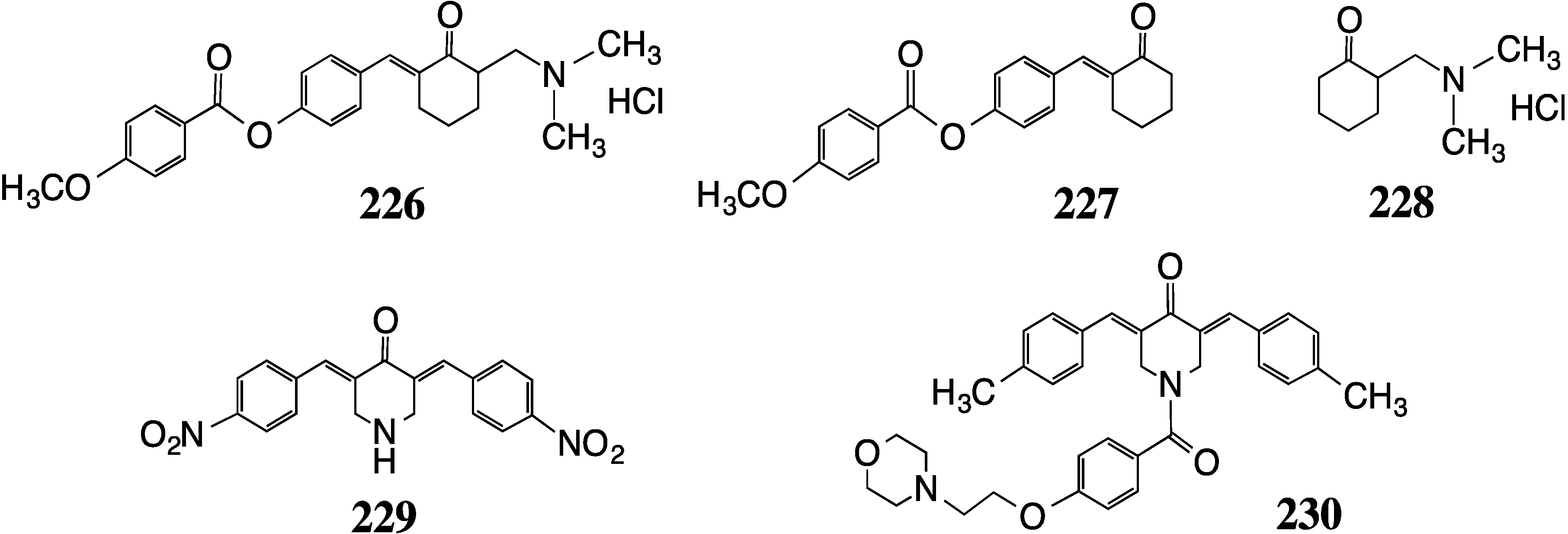
7. Alzheimer’s Disease
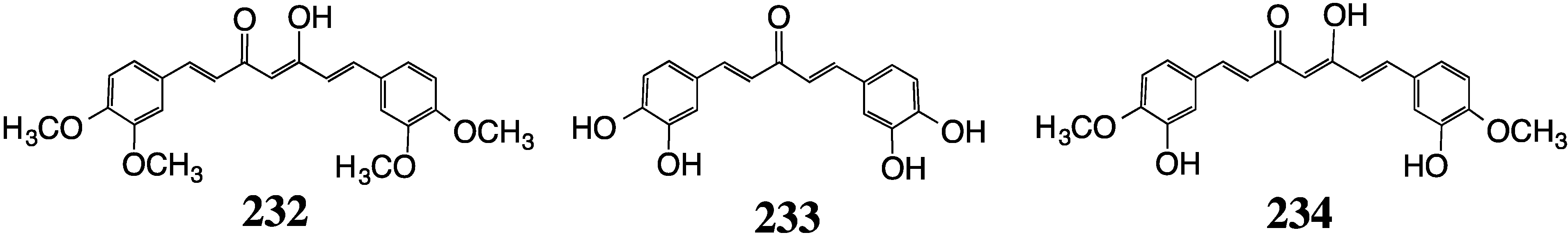
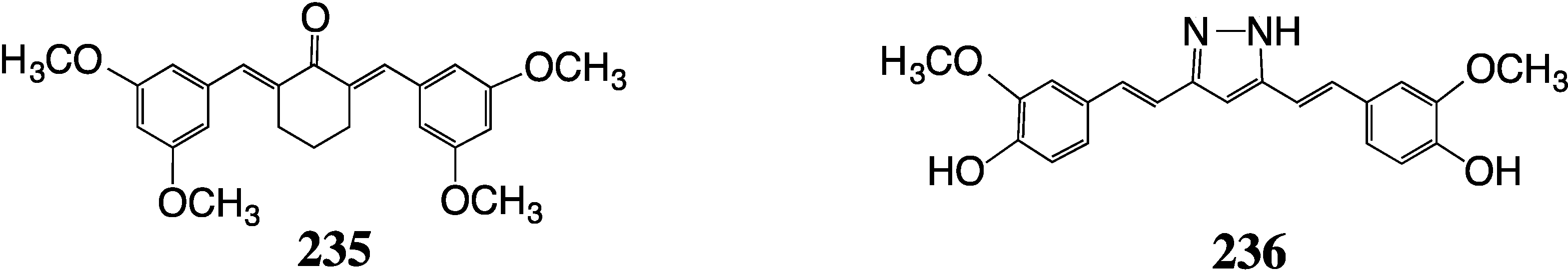
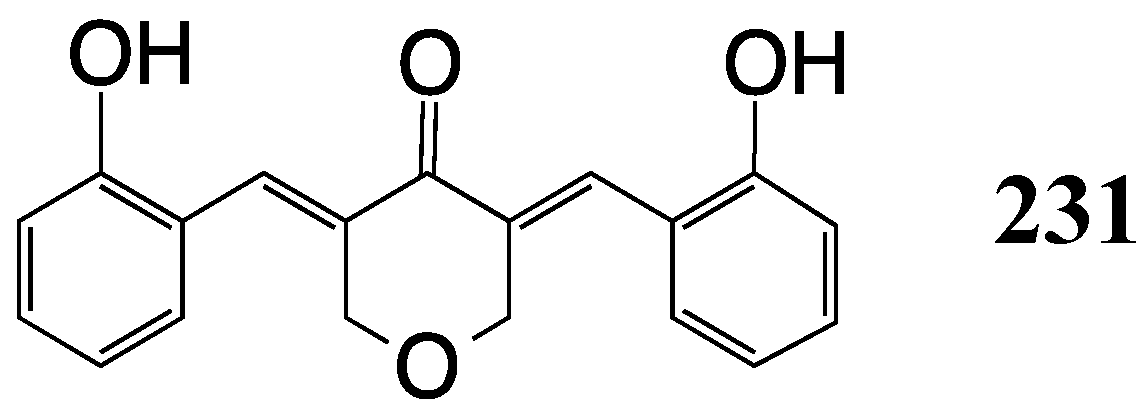
8. Malaria
9. Summary
Acknowledgments
Abbreviation
| MACs | monocarbonyl analogs of curcumin |
| Aβ | amyloid β |
| AD | Alzheimer’s Disease |
| AKT | protein kinase B |
| ALR | aldose reductase |
| AP-1 | the activator protein 1 |
| ATP | adenosine triphosphate |
| COX-2 | cyclooxygenase |
| DNMT | DNA methyltransferase |
| ERK | extracellular-signal regulated kinase |
| HIF | hypoxia inducible factor |
| HSP | heat shock protein |
| HUVEC | human umbilical vein endothelial cells |
| IL | interleukin |
| i.p. | intraperitoneal |
| i.v. | intravenous |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MID | minimum inhibitory concentration |
| MTD | maximum tolerance dose |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| LOX | lipoxygenase |
| LPS | lipopolysaccharide |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NCI | National Cancer Institute |
| NF-κB | nuclear factor κB |
| NO | nitric oxide |
| NSAID | nonsteroidal anti-inflammatory drug |
| ODC | ornithine decarboxylase |
| p38 | stress-activated protein kinase |
| PGE | prostaglandine E |
| p.o. | per os (by mouth) |
| RAW264.7 | mouse macrophage-like cell line |
| ROS | reactive oxygen species |
| SAR | structure-activity relationship |
| STAT | signal transducer and activator of transcription |
| TB | tuberculosis |
| TF | tissue factor |
| TNF | tumor necrosis factor |
| TPA | 12-O-tetradecanoyl-13-acetate |
| VEGF | vascular endothelial growth factor |
| VHL | von Hippel-Lindau |
Conflicts of Interest
References and Notes
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P.; Aggarwal, B.B. Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.). Pharm. Crops 2011, 2, 28–54. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 27 October 2014).
- Mishra, S.B.; Mukerjee, A.; Singh, S. Turmeric: A time tested folk medicine with Ayurvedic perspective. J. Pharm. Biomed. Res. 2011, 1, 67–70. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.N.; Schneider, C. Vanillin and ferulic acid: not the major degradation products of curcumin. Trends Mol. Med. 2012, 18, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.-F. Low stability remedies the low bioavailability of curcumin. Trends Mol. Med. 2012, 18, 363–364. [Google Scholar] [CrossRef]
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg. Med. Chem. 2009, 17, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Fossey, S.L.; Bear, M.D.; Lin, J.; Li, C.; Schwartz, E.B.; Li, P.-K.; Fuchs, J.R.; Fenger, J.; Kisseberth, W.C.; London, C.A. The novel curcumin analog FLLL32 decreases STAT3 DNA binding activity and expression, and induces apoptosis in osteosarcoma cell lines. BMC Cancer 2011, 11, e112. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.; Liang, G. Promising curcumin-based drug design: Mono-carbonyl analogues of curcumin (MACs). Curr. Pharm. Des. 2013, 19, 2114–2135. [Google Scholar] [PubMed]
- Yu, W.; Xiao, H.; Lin, J.; Li, C. Discovery of novel STAT3 small molecule inhibitors via in silico site-directed fragment-based drug design. J. Med. Chem. 2013, 56, 4402–4412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Moore, T.W.; Lin, X.; Morii, N.; Mancini, A.; Howard, R.B.; Culver, D.; Arrendale, R.F.; Reddy, P.; Evers, T.J.; et al. Synthetic curcumin analog EF31 inhibits the growth of head and neck squamous cell carcinoma xenografts. Integr. Biol. (Camb.) 2012, 4, 633–640. [Google Scholar] [CrossRef]
- Zhu, S.; Moore, T.W.; Morii, N.; Howard, R.B.; Culver, D.; Arrendale, R.F.; Reddy, P.; Evers, T.J.; Zhang, H.; Sica, G.; et al. Synthetic curcumin analog UBS109 inhibits the growth of head and neck squamous cell carcinoma xenografts. Curr. Cancer Drug Targets 2014, 14, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Zhu, S.; Wen, J.; Farris, A.; Adsay, V.; Diaz, R.; Snyder, J.P.; Shoji, M.; El-Rayes, B. Novel Synthetic Curcumin Analogs EF31 and UBS109 are Potent DNA Hypomethyating Agents in Pancreatic Cancer. Cancer Lett. 2013, 341, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Yamakoshi, H.; Sato, A.; Ohori, H.; Kakudo, Y.; Kudo, C.; Takahashi, Y.; Watanabe, M.; Takano, H.; Ishioka, C.; et al. Newly synthesized curcumin analog has improved potential to prevent colorectal carcinogenesis in vivo. Cancer Sci. 2009, 100, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Kudo, C.; Yamakoshi, H.; Sato, A.; Nanjo, H.; Ohori, H.; Ishioka, C.; Iwabuchi, Y.; Shibata, H. Synthesis of 86 species of 1,5-diaryl-3-oxo-1,4-pentadienes analogs of curcumin can yield a good lead in vivo. BMC Pharmacol. 2011, 11. [Google Scholar] [CrossRef]
- Sato, A.; Kudo, C.; Yamakoshi, H.; Uehara, Y.; Ohori, H.; Ishioka, C.; Iwabuchi, Y.; Shibata, H. Curcumin analog GO-Y030 is a novel inhibitor of IKKb that suppresses NF-κB signaling and induces apoptosis. Cancer Sci. 2011, 102, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.M.; Buhrow, S.A.; Gilbert, J.A.; Jia, L.; Shoiji, M.; Snyder, J.P.; Ames, M.M. Mouse pharmacokinetics and metabolism of the curcumin analog, 4-Piperidione,3,5-bis[(2-fluorophenyl)methylene]-acetate(3E,5E) (EF-24; NSC 716993). Cancer Chemother. Pharmacol. 2014, 73, 1137–1146. [Google Scholar] [CrossRef]
- Moore, T.W.; Zhu, S.; Shoji, M.; Snyder, J.P. Liver S9 fraction-derived metabolites of curcumin analog UBS109. ACS Med. Chem. Lett. 2014, 5, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.; Orr, S.; Jones, D.J.L.; Verschoyle, R.; Lim, C.-L.; Luo, J.-L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar] [PubMed]
- Hoehle, S.I.; Pfeiffer, E.; Sólyom, A.; Metzler, M. Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver. J. Agric. Food Chem. 2006, 54, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A.; Jantan, I.B.; Jasamai, M.; Ahmad, W. Synthesis and Biological Evaluation of Curcumin Analogues. J. Med. Sci. 2013, 13, 501–513. [Google Scholar] [CrossRef]
- Adams, B.K.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg. Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Koh, S.B.; Ee, P.L.R.; Khan, M.; Go, M.L. Curcumin analogs with potent and selective anti-proliferative activity on acute promyelocytic leukemia: Involvement of accumulated misfolded nuclear receptor co-repressor (N-CoR) protein as a basis of selective activity. ChemMedChem 2012, 7, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Khairia, M.; Youssef, K.M.; El-Sherbeny, M.A. Synthesis and antitumor activity of some curcumin analogs. Arch. Pharm. Chem. 2005, 338, 181–189. [Google Scholar] [CrossRef]
- Brown, A.; Shi, Q.; Moore, T.W.; Younghyoun, Y.; Prussia, A.; Maddox, C.; Liotta, D.D.; Shim, H.; Snyder, J.P. Monocarbonyl Curcumin Analogs: Heterocyclic Pleiotropic Kinase Inhibitors that Mediate Anti-Cancer Properties. J. Med. Chem. 2013, 56, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, K.; Grover, J.; Kania, M.; Jachak, S.M. Recent developments in chemistry and biology of curcumin analogues. RSC Adv. 2014, 4, 13946–13978. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; He, W.; Wang, Z.; Fang, Q.; Xiao, B.; Liu, Z.; Liang, G.; Yang, S. Discovery and evaluation of asymmetrical monocarbonyl analogs of curcumin as anti-inflammatory agents. Drug Des. Dev. Ther. 2014, 8, 373–382. [Google Scholar]
- Padhye, S.; Chavan, D.; Paandey, S.; Deshpande, J.; Swamy, K.V.; Sarkar, F.H. Perspectives on Chemopreventive and Therapeutic Potential of Curcumin Analogs in Medicinal Chemistry. Mini Rev. Med. Chem. 2010, 10, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K.; Mishra, P.K. Curcumin and Its Analogues: Potential Anticancer Agents. Med. Res. Rev. 2010, 30, 818–860. [Google Scholar] [PubMed]
- Vyas, A.; Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr. Pharm. Des. 2013, 19, 2047–2069. [Google Scholar] [PubMed]
- Cridge, B.K.; Larsen, L.; Rosengren, R.J. Curcumin and its derivatives in breast cancer: Current developments and potential for the treatment of drug-resistant cancers. Oncol. Discov. 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Shao, W.Y.; Cao, Y.N.; Yu, Z.W.; Pan, W.J.; Qiu, X.; Bu, X.Z.; An, L.K.; Huang, Z.S.; Guand, L.Q.; Chan, A.S.C. Facile preparation of new unsymmetrical curcumin derivatives by solid-phase synthesis strategy. Tetrahedron Lett. 2006, 47, 4085–4089. [Google Scholar] [CrossRef]
- Huang, J.-D.; Tang, Q.-Q.; Chen, X.-Y.; Ye, Y.; Wang, Y. (1E,4E)-1,5-Bis(2,6-difluorophenyl)penta-1,4-dien-3-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o758. [Google Scholar] [CrossRef] [PubMed]
- Kaftory, M.; Tanaka, K.; Toda, F. Reactions in the solid state. 2. The crystal structures of the inclusion complexes of 1,1,6,6-tetraphenylhexa-2,4-diyne-1,6-diol with benzylideneacetophenone and 2,5-diphenylhydroquinone with dibenzylideneacetone. J. Org. Chem. 1985, 50, 2154–2158. [Google Scholar] [CrossRef]
- Biradha, K.; Nangia, A.; Desiraju, G.R.; Carrell, C.J.; Carrell, H.J. C-HLO Hydrogen bonded multi-point recognition in molecular assemblies of dibenzylidene ketones and 1,3,5-trinitrobenzenes. J. Mater. Chem. 1997, 7, 1111–1122. [Google Scholar] [CrossRef]
- Biradha, K.; Sharma, C.V.K.; Paneerselvam, K.; Shimoni, L.; Carrrell, H.L.; Zacharias, D.E.; Desiraju, G.R. Solid State Supramolecular Assembly via C-H--O Hydrogen Bonds: Crystal Structures of the Complexes of 1,3,5-Trinitrobenzene with Dibenzylideneacetone and 2,5-Dibenzylidene-cyclopentanone. Chem. Commun. 1993, 1473–1475. [Google Scholar]
- Turowska-Tyrk, I. Monitoring initial structural changes in a crystal during photo-induced disapperance of its diffracting propertites. Chem. Phys. 2003, 288, 241–247. [Google Scholar] [CrossRef]
- Shan, Y.; Zhou, H.; Huang, S.D. 1,5-bis(4-methoxyphenyl)penta-1,4-dien-3-one. Z. Kristallogr. New Cryst. Struct. 1999, 214, 381–382. [Google Scholar]
- Butcher, R.J.; Yathirajan, H.S.; Sarojini, B.K.; Narayana, B.; Raj, K.K.V. (1E,4E)-1-(3-Nitrophenyl)-5-phenylpenta-1,4-dien-3-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, o1973. [Google Scholar] [CrossRef]
- Samshuddin, S.; Butcher, R.J.; Akkurt, M.; Narayana, B.; Sarojini, B.K.; Yathirajan, H.S. (1E,4E)-1-(3-Nitrophenyl)-5-phenylpenta-1,4-dien-3-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-G.; Chen, X.-Y.; Hu, J.; Zhang, L.; Zhao, Y.-J.; Yang, S.-L.; Liang, G. Synthesis and Crystal Structure of Monocarbonyl Analogs of Curcumin. Chin. J. Struct. Chem. 2012, 31, 491–498. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, J.; Liang, D.; Liang, D.; Tang, Q.; Zhang, Y.; Liang, G.; Li, X. Synthesis, Crystal Structure and Anti-tumor Properties of Fluorine-Containing Curcumin Analogues. Chin. J. Org. Chem. 2010, 30, 289–294. [Google Scholar]
- Jia, Z.; Quail, J.W.; Arora, V.K.; Dimmock, J.R. Structures of 3,5-bis(benzylidene)-4-piperidone hydrochloride (I) and its N-methyl analog (II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1988, 44, 2114–2117. [Google Scholar] [CrossRef]
- Lagisetty, P.; Powell, D.R.; Awasthi, V. Synthesis and structural determination of 3,5-bis(2-fluorobenzylidene)-4-piperidone analogs of curcumin. J. Mol. Struct. 2009, 936, 23–28. [Google Scholar] [CrossRef]
- Leonova, E.S.; Makarov, M.V.; Rybalkina, E.Y.; Nayani, S.L.; Tongwa, P.; Fonari, A.; Timofeeva, T.V.; Odinets, I.L. Structure-cytotoxicity relationship in a series of N-phosphorus substituted E,E-3,5-bis(3-pyridinylmethylene)- and E,E-3,5-bis(4-pyridinylmethylene)piperid-4-ones. Eur. J. Med. Chem. 2010, 45, 5926–5934. [Google Scholar] [CrossRef] [PubMed]
- Vatsadze, S.Z.; Kovalkina, M.A.; Svirdenkova, N.V.; Zyk, N.V.; Chuarakov, A.V.; Kuz’mina, L.G.; Howard, J.A.K. Novel dienone-based ligands for the synthesis of coordination polymers. Cryst. Eng. Commun. 2004, 6, 112–115. [Google Scholar] [CrossRef]
- Abaee, M.S.; Mojtahedi, M.M.; Zahedi, M.M.; Mesbah, A.W.; Ghandchi, N.M.; Massa, W. Synthesis of Bis (arylmethylidene) thiopyranones and Crystal Structure of the Phenyl Derivative. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 2891–2895. [Google Scholar] [CrossRef]
- Abaee, M.S.; Mojtahedi, M.M.; Sharifi, R.; Zahedi, M.M.; Mesbah, A.W.; Massa, W. (3E,5E)-3,5-Bis(4-hydroxybenzylidene)oxan-4-one. J. Chem. Res. 2008, 388–389. [Google Scholar]
- Du, Z.-Y.; Huang, H.-R.; Lu, Y.-J.; Zhang, K.; Fang, Y.-X. (3E,5E)-3,5-Bis(4-hydroxy-3,5-dimethoxybenzylidene)oxan-4-one monohydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o3334. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, N.S.; Sathiyanarayanan, K.I.; Aravindan, P.G.; Giridharan, P. Synthesis, crystal structure, and anticancer properties of cyclic monocarbonyl analogs of curcumin. Med. Chem. Res. 2011, 20, 81–87. [Google Scholar] [CrossRef]
- Jia, Z.; Quail, J.W.; Arora, V.K.; Dimmock, J.R. Structure of 3,5-bis(benzylidene)-1-methyl-4-piperidone methobromide hemiethanol solvate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1989, 45, 1117–1118. [Google Scholar] [CrossRef]
- This result was achieved by searching the Cambridge Structural Database (CSD) for compound 15 (R = H).
- Theocharis, C.R.; Jones, W.; Thomas, J.M.; Motevalli, M.; Hursthouse, M.B. The solid-state photodimerisation of 2,5-dibenzylidenecyclopentanone (DBCP); a topochemical reaction that yields an amorphous product. J. Chem. Soc. Perkin Trans 2 1984, 71–76. [Google Scholar]
- Arshad, I.; Ashraf, S.; Abbas, A.; Hameed, S.; Lo, K.M.; Naseer, M.M. Conformational isomerism in a conformational polymorph of 2,5-dibenzylidenecyclopentanone: Crystallographic and quantum chemical structures. Eur. Chem. Bull. 2014, 3, 587–592. [Google Scholar]
- Kawamata, J.; Inoue, K.; Inabe, T. Molecular and crystal structures of novel second-order nonlinear optical crystals: a,a'-Dibenzylidenecycloalkanones. Bull. Chem. Soc. Jpn. 1998, 71, 2777. [Google Scholar] [CrossRef]
- Ganesh, T.; Guza, R.C.; Bane, S.; Ravindra, R.; Shanker, N.; Lakdawala, A.S.; Snyder, J.P.; Kingston, D.G.I. The bioactive Taxol conformation on β-tubulin: Experimental evidence from highly active constrained analogs. Proc. Nat. Acad. Sci. USA 2004, 101, 10006–10011, The favored structures: Tubulin-bound Taxol conformations in CDCl3 (4%) and D2O/DMSO-d6 (2%). [Google Scholar] [CrossRef] [PubMed]
- Thepchatri, P.; Eliseo, T.; Cicero, D.O.; Myles, D.; Snyder, J.P. Relationship among ligand conformations in solution, in the solid state, and at the Hsp90 binding site: Geldanamycin and radicicol. J. Am. Chem. Soc. 2007, 129, 3127–3134, The favored structures: Hsp90- bound conformers of geldanamycin (4%) and radicicol (21%). [Google Scholar] [CrossRef] [PubMed]
- Cox, B.D.; Prosser, A.R.; Katzman, B.M.; Alcaraz, A.A.; Liotta, D.C.; Wilson, L.J.; Snyder, J.P. Anti-HIV Small Molecule Binding in the Peptide Sub-Pocket of the CXCR4:CVX15 Crystal Structure. ChemBioChem 2014, 15, 1614–1620, The favored structure: Proposed CXCR4-bound AMD11070 (14%). [Google Scholar] [CrossRef] [PubMed]
- Kasinski, A.L.; Du, Y.; Thomas, S.L.; Zhao, J.; Sun, S.-Y.; Khuri, F.R.; Wang, C.-Y.; Shoji, M.; Sun, A.; Snyder, J.P.; et al. Inhibition of IkB kinase nuclear factor-κB signaling pathway by 3,5-bis(2-flurobenzylidene) piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol. Pharmacol. 2008, 74, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-Bis-(2-pyridinylmethylidene)-4-piperidone (EF31): Anti-inflammatoryand anti-cancer properties. Int. Immunol. Pharmacol. 2012, 12, 368–377. [Google Scholar]
- Thomas, S.L.; Zhao, J.; Li, Z.; Lou, B.; Du, Y.; Purcell, J.; Snyder, J.P.; Khuri, F.R.; Liotta, D.C.; Fu, H. Activation of the p38 pathway by a novel monoketone curcumin analog, EF24, suggests a potential combination strategy. Biochem. Pharmacol. 2010, 80, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.K.; Cai, J.; Armstrong, J. EF24, a novel synthetic curcumin analog induces apoptosis in cancer cells via a redox dependent mechanism. Anticancer Drugs 2005, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Vilekar, P.; Awasthi, S.; Natarajan, A.; Anant, S.; Awasthi, V. EF24 suppresses maturation and inflammatory response in dendritic cells. Int. Immunol. 2012, 24, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Li, X.; Chen, L.; Yang, S.; Wu, X.; Studer, E.; Gurley, E.; Hylemon, P.B.; Ye, F.; Li, Y.; et al. Synthesis and anti-inflammatory activities of mono-carbonyl analogues of curcumin. Bioorg. Med. Chem. Lett. 2008, 18, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, J.; Wang, Y.; Liang, D.; Yang, X.; Li, X.; Wu, J.; Wu, X.; Yang, S.; Li, X.; et al. Synthesis of mono-carbonyl analogues of curcumin and their effects on inhibition of cytokine release in LPS-stimulated RAW 264.7 macrophages. Bioorg. Med. Chem. 2010, 18, 2388–2393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, C.; Pan, Y.; Li, J.; Zhang, Y.; Yang, S.; Zhang, H.; Li, X.; Liang, G. A novel compound C12 inhibits inflammatory cytokine production and protects from inflammatory injury in vivo. PLoS One 2011, 6, e24377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, C.; Pan, Y.; Yang, X.; Huang, Y.; Feng, Z.; Li, X.; Yang, S.; Liang, G. A novel synthetic mono-carbonyl analogue of curcumin, A13, exhibits anti-inflammatory effects in vivo by inhibition of inflammatory mediators. Inflammation 2011, 35, 594–604. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Cai, L.; Cai, Y.; Hu, J.; Yu, C.; Li, J.; Feng, Z.; Yang, S.; Li, X.; et al. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br. J. Pharmacol. 2012, 166, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhu, G.; Wang, Y.; Cai, L.; Cai, Y.; Hu, J.; Li, Y.; Yan, Y.; Wang, Z.; Li, X.; et al. Attenuation of high-glucose-induced inflammatory response by a novel curcumin derivative B06 contributes to its protection from diabetic pathogenic changes in rat kidney and heart. J. Nutr. Biochem. 2013, 24, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Cai, Y.; Pan, Y.; Ye, F.; Zhang, Y.; Zhao, Y.; Yang, S.; Li, X.; Liang, G. Evaluation and Discovery of novel synthetic chalcone derivatives as anti-inflammatory agents. J. Med. Chem. 2011, 54, 8110–8123. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.M.; Hunsaker, L.A.; Roybal, C.N.; Bobrovnikova-Marjon, E.V.; Abcouwer, S.F.; Royer, R.E.; Deck, L.M.; Vander Jagt, D.L. Activation of NF-κB is inhibited by curcumin and related enones. Bioorg. Med. Chem. 2006, 14, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Taurin, S.; Rosengren, R.J.; Schumacher, M.; Diederich, M.; Somers-Edgar, T.J.; Larsen, L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogues of curcumin. Bioorg. Med. Chem. 2010, 18, 6701–6707. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Ding, K.; Neamati, N.; Long, Y.Q. Synthesis of the pyridinyl analogues of dibenzylideneacetone (pyr-dba) via an improved Claisen-Schmidt condensation, displaying diverse biological activities as curcumin analogues. Org. Biomol. Chem. 2012, 10, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Katsori, A.M.; Chatzopoulou, M.; Dimas, K.; Kontogiorgis, C.; Patsilinakos, A.; Trangas, T.; Hadjipavlou-Litina, D. Curcumin analogues as possible anti-proliferative & anti-inflammatory agents. Eur. J. Med. Chem. 2011, 46, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.H. Reversible michael additions: Covalent inhibitors and prodrugs. Mini Rev. Med. Chem. 2012, 12, 1330–1344. [Google Scholar] [PubMed]
- Serafimova, I.M.; Pufall, M.A.; Krishnan, S.; Duda, K.; Cohen, M.S.; Maglathlin, R.L.; McFarland, J.M.; Miller, R.M.; Frödin, M.; Taunton, J. Reversible targeting of noncatalytic cysteines with chemically tuned electrophiles. Nat. Chem. Biol. 2012, 8, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Schlesier, K.; Harwat, M.; Bohm, V.; Bitsch, R. Assessment of anti-oxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, L.; Zou, P.; Zhang, Y.; Wang, Z.; Fang, Q.; Jianag, L.; Chen, G.; Xu, Z.; Zhang, H.; et al. Synthesis and biological evaluation of allylated and prenylated mono-carbonyl analogs of curcumin as anti-inflammatory agents. Eur. J. Med. Chem. 2014, 74, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Nurrochmad, A.; Supardjan, A.M.; Sardjiman. Inhibition of cyclooxygenase by cyclovalone and its three analogue compounds. Majalah Farmasi Indonesia 1998, 9, 180–185. [Google Scholar]
- Gafner, S.; Lee, S.; Cuendet, M.; Barthelemy, S.; Vergnes, L.; Labidalle, S.; Mehta, R.G.; Boone, C.W.; Pezzuto, J.M. Biologic evaluation of curcumin and structural derivatives in cancer chemoprevention model systems. Phytochemistry 2004, 65, 2849–2859. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.M.; Hunsaker, L.A.; Abcouwer, S.F.; Deck, L.M.; Vander Jagt, D.L. Anti-oxidant activities of curcumin and related enones. Bioorg. Med. Chem. 2005, 13, 3811–3820. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.M.; Hunsaker, L.A.; Gonzales, A.M.; Heynekamp, J.J.; Orlando, R.A.; Deck, L.M.; Vander Jagt, D.L. TPA-induced upregulation of activator protein-1 can be inhibited or enhanced by analogs of the natural product curcumin. Biochem. Pharmacol. 2006, 72, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yang, S.; Zhou, H.; Shao, L.; Huang, K.; Xiao, J.; Huang, Z.; Li, X. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur. J. Med. Chem. 2009, 44, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Spartan Student Edition. Available online: http://www.scientificsoftware-solutions.com/product.php?productid=17747 (accessed on 3 December 2014).
- Dimmock, J.R.; Padmanilayam, M.P.; Puthucode, R.N.; Nazarali, A.J.; Motaganahalli, N.L.; Zello, G.A.; Quail, J.W.; Oloo, E.O.; Kraatz, H.B.; Prisciak, J.S.; et al. A conformational and structure activity relationship study of cytotoxic 3,5-bis(arylidene)-4-piperidones and related N-acryloyl analogues. J. Med. Chem. 2001, 44, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Jha, A.; Zello, G.A.; Quail, J.W.; Oloo, E.O.; Nienaber, K.H.; Kowalczyk, E.S.; Allen, T.M.; Santos, C.L.; Clercq, E.D.; et al. Cytotoxic N-[4-(3-aryl-3-oxo-1-propenyl) phenylcarbonyl]-3, 5-bis (phenylmethylene)-4-piperidones and related compounds. Eur. J. Med. Chem. 2002, 37, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.L.; Zhong, D.; Zhou, W.; Malik, S.; Liotta, D.; Snyder, J.P.; Hamel, E.; Giannakakou, P. EF24, a novel curcumin analog, disrupts the microtubule cytoskeleton and inhibits HIF-1. Cell Cycle 2008, 7, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Ohori, H.; Yamakoshi, H.; Tomizawa, M.; Shibuva, M.; Kakudo, Y.; Takahashi, A.; Takahashi, S.; Kato, S.; Suzuki, T.; Ishioka, C.; et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol. Cancer Ther. 2006, 5, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Chandru, H.; Sharada, A.C.; Bettadaiah, B.K.; Kumar, C.S.A.; Rangappa, K.S.; Jayashree, K. In vivo growth inhibitory and anti-angiogenic effects of synthetic novel dienone cyclopropoxy curcumin analogs on mouse Ehrlich ascites tumor. Bioorg. Med. Chem. 2007, 15, 7696–7703. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.P.; Ehlers, T.; Hubbard, R.B., IV; Bai, X.; Arbiser, J.L.; Goldsmith, D.J.; Bowen, J.P. Design, synthesis and biological evaluation of angiogenesis inhibitors: Aromatic enones and dienone analogues of curcumin. Bioorg. Med. Chem. 2003, 13, 115–117. [Google Scholar] [CrossRef]
- Robinson, T.P.; Hubbard, R.B., IV; Ehlers, T.; Arbiser, J.L.; Goldsmith, D.J.; Bowen, J.P. Synthesis and biological evaluation of aromatic enones related to curcumin. Bioorg. Med. Chem. 2005, 13, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.B.; Shin, W.S.; Leed, S.; Ahna, C.M. Synthesis of novel curcumin mimics with asymmetrical units and their anti-angiogenic activity. Bioorg. Med. Chem. Lett. 2005, 15, 3782–3786. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Mukherjee, C.; Prasad, A.K.; Parmar, V.S.; de Clercq, E.; Balzarini, J.; Stables, J.P.; Manavathu, E.K.; Shrivastav, A.; Sharma, R.K.; et al. E,E,E-1-(4-Arylamino-4-oxo-2-butenoyl)-3,5-bis-(arylidene)-4-piperidones: A topographical study of some novel potent cytotoxins. Bioorg. Med. Chem. 2007, 15, 5854–1565. [Google Scholar] [CrossRef] [PubMed]
- Melphalan, a Nitrogen Mustard Alkylating Agent. Available online: http://en.wikipedia.org/wiki/Melphalan (accessed on 26 October 2014).
- Fuchs, J.R.; Pandit, B.; Bhasin, D.; Etter, J.P.; Regan, N.; Abdelhamid, D.; Li, C.; Lin, J.; Li, P.-K. Structure-activity relationship studies of curcumin analogues. Bioorg. Med. Chem. Lett. 2009, 19, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.A.Q.; Rando, D.G.; Santos, R.P.; Gonçalves, C.P.; Ferreira, E.; de Carvalho, J.E.; Kohn, L.; Maria, D.A.; Faião-Flores, F.; Michalik, D.; et al. New antitumoral agents I: In vitro anticancer activity and in vivo acute toxicity of synthetic 1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4- pentadien-3-one and derivatives. Bioorg. Med. Chem. 2010, 18, 6275–6281. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, H.; Ohori, H.; Kudo, C.; Sato, A.; Kanoh, N.; Ishioka, C.; Shibata, H.; Iwabuchi, Y. Structure-activity relationship of C5-curcuminoids and synthesis of their molecular probes thereof. Bioorg. Med. Chem. 2010, 18, 1083–1092. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Y.; Peng, J.; Guo, L.; Hu, J.; Cao, M.; Zhang, X.; Zhang, H.; Wang, Z.; Li, X.; et al. A synthetic compound, 1,5-bis(2-methoxyphenyl)-penta-1,4-dien-3-one (B63), induces apoptosis and activates endoplasmic reticulum stress in non-small cell lung cancer cells. Int. J. Cancer 2012, 131, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, J.; Zhou, H.; Yang, S.; Wu, X.; Jiang, C.; Zhao, Y.; Liang, D.; Li, X.; Liang, G. A novel mono-carbonyl analogue of curcumin,(1E,4E)-1,5-bis(2, 3-dimethoxyphenyl) penta-1,4- dien-3-one, induced cancer cell H460 apoptosis via activation of endoplasmic reticulum stress signaling pathway. J. Med. Chem. 2011, 54, 3768–3778. [Google Scholar] [CrossRef] [PubMed]
- Hutzen, B.; Friedman, L.; Sobo, M.; Lin, L.; Cen, L.; De, A.S.; Yamakoshi, H.; Shibata, H.; Iwabuchi, Y.; Lin, J. Curcumin analogue GO-Y030 inhibits STAT3 activity and cell growth in breast and pancreatic carcinomas. Int. J. Oncol. 2009, 35, 867–872. [Google Scholar] [PubMed]
- Lin, L.; Hutzen, B.; Ball, S.; Foust, E.; Sobo, M.; Deangelis, S.; Pandit, B.; Friedman, L.; Li, C.; Li, P.K.; et al. New curcumin analogues exhibit enhanced growth-suppressive activity and inhibit AKT and signal transducer and activator of transcription 3 phosphorylation in breast and prostate cancer cells. Cancer Sci. 2009, 100, 1719–1727. [Google Scholar] [PubMed]
- Thakur, A.; Manohar, S.; V’elez Gerena, C.E.; Zayas, B.; Kumar, V.; Malhotra, S.V.; Rawat, D.S. Novel 3,5-bis(arylidene)-4-piperidone based monocarbonyl analogs of curcumin: Anticancer activity evaluation and mode of action study. Med. Chem. Commun. 2014, 5, 576. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.; Lin, S.S.; Shi, W.T.; Zhu, J.; Liang, G.; Yuan, S.T. Potent anti-angiogenic activity of B19-α monocarbonyl analogue of curcumin. Chin. J. Nat Med. 2014, 12, 8–14. [Google Scholar] [PubMed]
- Liu, Z.; Sun, Y.; Ren, L.; Huang, Y.; Cai, Y.; Weng, Q.; Shen, X.; Li, X.; Liang, G.; Wang, Y. Evaluation of a curcumin analog as an anti-cancer agent inducing ER stress-mediated apoptosis in non-small cell lung cancer cells. BMC Cancer 2013, 13, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Du, Z.-Y.; Zheng, X.; Cui, X.-X.; Conney, A.H.; Zhang, K. Synthesis and evaluation of curcumin-related compounds for anticancer activity. Eur. J. Med. Chem. 2012, 53, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhou, D.; Wang, H.; Ding, N.; Cui, X.-X.; Wang, H.; Verano, M.; Zhang, K.; Conney, A.H.; Zheng, X.; et al. Effects of Pyridine Analogs of Curcumin on Growth, Apoptosis and NF-κB Activity in Prostate Cancer PC-3 Cells. Anticancer Res. 2013, 33, 1343–1350. [Google Scholar] [PubMed]
- Zhou, D.-Y.; Zhang, K.; Conney, A.H.; Ding, N.; Cui, X.-X.; Wang, H.; Verano, M.; Zhao, S.-Q.; Fan, Y.-X.; Zheng, X.; et al. Synthesis and Evaluation of Curcumin-Related Compounds Containing Benzyl Piperidone for Their Effects on Human Cancer Cells. Chem. Pharm. Bull. 2013, 61, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Samaan, N.; Zhong, Q.; Fernandez, J.; Chen, G.; Hussain, A.; M.; Zheng, S.; Wang, G.; Chen, Q.-H.; Pohl, J.; Alizadeh, A.R.; et al. Design, synthesis, and evaluation of novel heteroaromatic analogs of curcumin as anti-cancer agents. Eur. J. Med. Chem. 2014, 75, 123–131. [Google Scholar]
- Shoji, M.; Sun, A.; Kisiel, W.; Lu, Y.J.; Shim, H.; McCarey, B.E.; Nichols, N.; Parker, E.T.; Pohl, J.; Alizadeh, A.R.; et al. Targeting Tissue Factor-Expressing Tumor Angiogenesis and Tumors with EF24 Conjugated to Factor VIIa. J. Drug Target. 2008, 16, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, Y.; Wang, L.; Tian, L.; Song, R.; Han, T.; Pan, S.; Liu, L. In Vivo and In Vitro Suppression of Hepatocellular Carcinoma by EF24, a Curcumin Analog. PLoS One 2012, 7, e48075. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L.M. The Curcumin Analog EF24 Targets NF-κB and miRNA-21, and Has Potent Anticancer Activity In Vitro and In Vivo. PLoS One 2013, 8, e71130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Dong, L.; Huang, Y.; Yuana, J.; Ben, W.; Yang, Y.; Ning, N.; Lu, M.; Guan, Y. Therapeutic role of EF24 targeting glucose transporter 1-mediated metabolism and metastasis in ovarian cancer cells. Cancer Sci. 2013, 104, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Rath, K.S.; McCann, G.A.; Cohn, D.E.; Rivera, B.K.; Kuppusamy, P.; Selvendiran, K. Safe and targeted anticancer therapy for ovarian cancer using a novel class of curcumin analogs. J. Ovarian Res. 2013, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, D.; May, R.; Sureban, S.M.; Lee, K.B.; George, R.; Kuppusamy, P.; Ramanujam, R.P.; Hideg, K.; Dieckgraefe, B.K.; Houchen, C.W.; et al. Diphenyl difluoroketone: A curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008, 68, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- The HCT-116 human colon tumor xenograft study [111] reported the animals to be treated i.p. with 200 microg/kg (μg/kg) body weight daily for 23 days. This dose is to be compared with that used in almost all other studies, namely 10–200 mg/kg. The robust effects of this diminutive dose remains a paradox.
- Yamaguchi, M.; Moore, T.W.; Sun, A.; Snyder, J.P.; Shoji, M. Novel curcumin analogue UBS 109 potently stimulates osteoblastogenesis and suppresses osteoclastogenesis: Involvement in Smad activation and NF-κB inhibition. Integr. Biol. (Camb.) 2012, 4, 905–913. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Zhu, S.; Zhang, S.; Wu, D.; Moore, T.M.; Snyder, J.P.; Shoji, M. Curcumin analogue UBS109 prevents bone loss in breast cancer bone metastasis mouse model: Involvement in osteoblastogenesis and osteoclastogenesis. Cell Tissue Res. 2014, 357, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Zhu, S.; Ko, J.E.; Ashritha, N.; Kandimalla, R.; Snyder, J.P.; Shoji, M.; El-Rayes, B.F. Antiangiogenic effects of a novel synthetic curcumin analogs in pancreatic cancer. Cancer Lett. 2014. [Google Scholar] [CrossRef]
- Yadav, B.; Taurin, S.; Larsen, L.; Rosengren, R.J. RL66 a second-generation curcumin analog has potent in vivo and in vitro anticancer activity in ER-negative breast cancer models. Int. J. Oncol. 2012, 41, 1723–1732. [Google Scholar] [PubMed]
- Antibiotic/Antimicrobial Resistance. Available online: http://www.cdc.gov/drugresistance/ (accessed on 20 September 2014).
- White House orders plan for antibiotic resistance. Available online: http://apnews.excite.com/article/20140918/us--obama-antibiotics-828e636215.html (accessed on 21 September 2014).
- Mahady, G.B.; Pendland, S.L.; Yun, G.; Lu, Z.Z. Turmeric (curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002, 22, 4179–4181. [Google Scholar] [PubMed]
- Rudrappa, T.; Bais, H.P. Curcumin, a known phenolic from curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J. Agric. Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chem. 2009, 115, 265–271. [Google Scholar] [CrossRef]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin inhibits FtsZ assembly: An attractive mechanism for its antibacterial activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef] [PubMed]
- FtsZ. Available online: http://en.wikipedia.org/wiki/FtsZ (accessed on 4 December 2014).
- Kaur, S.; Modi, N.H.; Panda, D.; Roy, N. Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ—A structural insight to unveil antibacterial activity of curcumin. Eur. J. Med. Chem. 2010, 45, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Beuria, T.K.; Singh, P.; Surolia, A.; Panda, D. Promoting assembly and bundling of FtsZ as a strategy to inhibit bacterial cell division; a new approach for developing novel antibacterial drugs. Biochem. J. 2009, 423, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Keffer, J.L.; Bifulco, G.; Lloyd, J.R.; Bewley, C.A. Chrysophaentins A–H, antibacterial bisdiarylbutene macrocycles that inhibit the bacterial cell division protein FtsZ. J. Am. Chem. Soc. 2010, 132, 9069–9077. [Google Scholar] [CrossRef] [PubMed]
- Chatterji, B.P.; Jindal, B.; Srivastava, S.; Panda, D. Microtubules as antifungal and antiparasitic drug targets. Expert Opin. Ther. Pat. 2011, 21, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yang, S.; Jiang, L.; Zhao, Y.; Shao, L.; Xiao, J.; Ye, F.; Li, Y.; Li, X. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem. Pharm. Bull. 2008, 56, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, B.; Venkataraman, R. Synthesis and Biological Evaluation of Some Curcumin Analogs and Their Derivatives. Rasāyan J. Chem. 2010, 3, 260–265. [Google Scholar]
- Vilekar, P.; Ding, C.; Lagisetty, P.; Awasthi, V.; Awasthi, S. Antibacterial Activity of Synthetic Curcumin Derivatives: 3,5-Bis(benzylidene)-4-Piperidone (EF24) and EF24-Dimer Linked via Diethylenetriaminepentacetic Acid (EF2DTPA). Appl. Biochem. Biotechnol. 2014, 172, 3363–3373. [Google Scholar] [CrossRef] [PubMed]
- Tuberculosis. WHO. Available online: http://www.who.int/mediacentre/factsheets/fs104/en/ (accessed on 26 September 2014).
- Treatment. Centers for Disease Control and Prevention. Available online: http://www.cdc.gov/tb/topic/treatment/default.htm (accessed on 25 September 2014).
- How many TB Cases have been Successfully Treated? WHO. Available online: http://www.who.int/gho/tb/epidemic/treatment/en/ (accessed on 27 September 2014).
- Drug-resistant Tuberculosis Now at Record Levels. WHO. Available online: http://www.who.int/mediacentre/news/releases/2010/drug_resistant_tb_20100318/en/ (accessed on 27 September 2014).
- Schraufstätter, E.; Bernt, H. Antibacterial Action of Curcumin and Related Compounds. Nature 1949, 164, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Pasca, M.R.; Guglierame, P.; Arcesi, F.; Bellinzoni, M.; Rossi, E.D.; Riccardi, G. Rv2686c-Rv2687c-Rv2688c, an ABC Fluoroquinolone Efflux Pump in Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2004, 48, 3175–3178. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.E.A.; Bigi, F.; Santangelo, M.D.L.P.; Romano, M.I.; Martin, C.; Cataldi, A.; Ainsa, J.A. Characterization of P55, a Multidrug Efflux Pump in Mycobacterium Bovis and Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2001, 45, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Balganesh, M.; Dinesh, N.; Sharma, S.; Kuruppath, S.; Nair, A.V.; Sharma, U. Efflux Pumps of Mycobacterium Tuberculosis Play a Significant Role in Antituberculosis Activity of Potential Drug Candidates. Antimicrob. Agents Chemother. 2012, 56, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Dube, D.; Kukshal, V.; Srivastava, S.K.; Tripathi, R.P.; Ramachandran, R. NAD+-dependent DNA Ligase (Rv3014c) From M. tuberculosis: Strategies for Inhibitor Design. Med. Chem. Res. 2008, 17, 189–198. [Google Scholar] [CrossRef]
- Takeuchi, T.; Ishidoh, T.; Iijima, H.; Kuriyama, I.; Shimazaki, N.; Koiwai, O.; Kuramochi, K.; Kobayashi, S.; Sugawara, F.; Sakaguchi, K.; et al. Structural Relationship of Curcumin Derivatives Binding to the BRCT Domain of Human DNA Polymerase Lambda. Genes Cells 2006, 11, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K.; Saikia, D.; Tiwari, R.; Ojha, S.; Shanker, K.; Kumar, J.K.; Gupta, A.K.; Tandon, S.; Negi, A.S.; Khanuja, S.P.S. Demethoxycurcumin and its semisynthetic analogues as antitubercular Agents. Planta Med. 2008, 74, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.A.; Dasgupta, I.; Gnanadhas, D.P.; Chakravortty, D. Multifaceted Roles of Curcumin: Two Sides of a Coin! Expert Opin. Biol. Ther. 2011, 11, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Kandepu, N.M.; Das, U.; Zello, G.A.; Nienaber, K.H. Antimycobacterial arylidenecyclohexanones and related Mannich bases. Pharmazie 2004, 59, 502–505. [Google Scholar] [PubMed]
- Das, S.; Das, U.; Bandy, B.; Gorecki, D.K.J.; Dimmock, J.R. 2-[4-(4-Methoxyphenylcarbonyloxy)benzylidene]-6-dimethylaminomethyl cyclohexanone hydrochloride: A Mannich base which inhibits the growth of some drug-resistant strains of Mycobacterium tuberculosis. Pharmazie 2010, 65, 849–850. [Google Scholar]
- Das, U.; Das, S.; Bandy, B.; Sables, J.P.; Dimmock, J.R. N-Aroyl-3,5-bis(benzylidene)-4-piperidones: A novel class of antimycobacterial agents. Bioorg. Med. Chem. 2008, 16, 3602–3607. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Reeves, A.Z.; Powell, K.R.; Napier, R.J.; Swimm, A.I.; Sun, A.; Giesler, K.; Bommarius, B.; Shinnick, T.M.; Snyder, J.P.; et al. Monocarbonyl analogs of curcumin inhibit growth of antibiotic sensitive and resistant strains of Mycobacterium tuberculosis. Eur. J. Med. Chem. 2015, in press. [Google Scholar]
- Kim, J.-J.; Lee, H.-M.; Shin, D.-M.; Kim, W.; Yuk, J.-M.; Jin, H.S.; Lee, S.-H.; Cha, G.-H.; Kim, J.-M.; Lee, Z.-W.; et al. Host Cell Autophagy Activated by Antibiotics Is Required for Their Effective Antimycobacterial Drug Action. Cell Host Microbe 2012, 11, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Bai, Y.; Sun, A.; Liotta, D.; Snyder, J.P.; Fu, H.; Huang, B. Autophagy and apoptosis in hepatocellular carcinoma induced by EF25-(GSH)2: A novel curcumin analog. PLoS One 2014, 9, e107876. [Google Scholar] [CrossRef] [PubMed]
- Dementia. WHO. Available online: http://www.who.int/mediacentre/factsheets/fs362/en/ (accessed on 27 September 2014).
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological Activities of Curcumin and Its Analogues (Congeners) Made by Man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Yang, F. Curcumin Inhibits Formation of Amyloid Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo. J. Biol. Chem. 2004, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- A Pilot Study of Curcumin and Ginkgo for Treating Alzheimer’s Disease. Available online: http://clinicaltrials.gov/ct2/show/NCT00164749?term=curcumin+and+alzheimers+disease&rank=1 (accessed on 26 September 2012).
- Ringman, J.M.; Frautschy, S.A.; Cole, G.M.; Masterman, D.L.; Cummings, J.L. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr. Alzheimer Res. 2005, 2, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Landau, M.; Sawaya, M.R.; Faull, K.F.; Laganowsky, A.; Jiang, L.; Sievers, S.A.; Liu, J.; Barrio, J.R.; Eisenberg, D. Towards a pharmacophore for Amyloid. PLoS Biol. 2011, 9, e1001080. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, D.; Shirai, N.; Amatsubo, T.; Taguchi, H.; Hirao, K.; Urushitani, M.; Morikawa, S.; Inubushi, T.; Kato, M.; Kato, F.; et al. Relationship between the tautomeric structures of curcumin derivatives and their Aβ-binding activities in the context of therapies for Alzheimer’s disease. Biomaterials 2010, 32, 4179–4185. [Google Scholar] [CrossRef]
- Payton, F.; Peter Sandusky, P.; Alworth, W.L. NMR Study of the solution structure of curcumin. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Orlando, R.A.; Gonzales, A.M.; Royer, R.E.; Deck, L.M.; Vander Jagt, D.L. A chemical analog of curcumin as an improved inhibitor of amyloid Abeta oligomerization. PLoS One 2012, 7, e31869. [Google Scholar] [CrossRef] [PubMed]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Ao, G.; Chu, X.; Ji, Y.; Wang, J. Antioxidant properties and PC12 cell protective effects of a novel curcumin analogue (2E,6E)-2,6-bis(3,5-Dimethoxybenzylidene)cyclohexanone (MCH). Int. J. Mol. Sci. 2014, 15, 3970–3988. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.C.; Vatsala, P.G.; Keshamouni, V.G.; Padmanaban, G.; Rangarajan, P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Karmodiya, K.; Surolia, N.; Surolia, A. Synthesis and exploration of novel curcumin analogues as anti-malarial agents. Bioorg. Med. Chem. 2008, 16, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Khan, S.I.; Kandi, S.K.; Raj, K.; Sun, G.; Yang, X.; Molina, A.D.C.; Ni, N.; Wang, B.; Rawat, D.S. Synthesis, antimalarial activity and cytotoxic potential of new monocarbonyl analogues of curcumin. Bioorg. Med. Chem. Lett. 2013, 23, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Reference 27 (Bairwa et al. 2014) overviews a sampling of recent SAR studies.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shetty, D.; Kim, Y.J.; Shim, H.; Snyder, J.P. Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs). Molecules 2015, 20, 249-292. https://doi.org/10.3390/molecules20010249
Shetty D, Kim YJ, Shim H, Snyder JP. Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs). Molecules. 2015; 20(1):249-292. https://doi.org/10.3390/molecules20010249
Chicago/Turabian StyleShetty, Dinesh, Yong Joon Kim, Hyunsuk Shim, and James P. Snyder. 2015. "Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs)" Molecules 20, no. 1: 249-292. https://doi.org/10.3390/molecules20010249
APA StyleShetty, D., Kim, Y. J., Shim, H., & Snyder, J. P. (2015). Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs). Molecules, 20(1), 249-292. https://doi.org/10.3390/molecules20010249