Glutathione-Garlic Sulfur Conjugates: Slow Hydrogen Sulfide Releasing Agents for Therapeutic Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Production and Characterization of GS-OSCs from Garlic at a Low Temperature
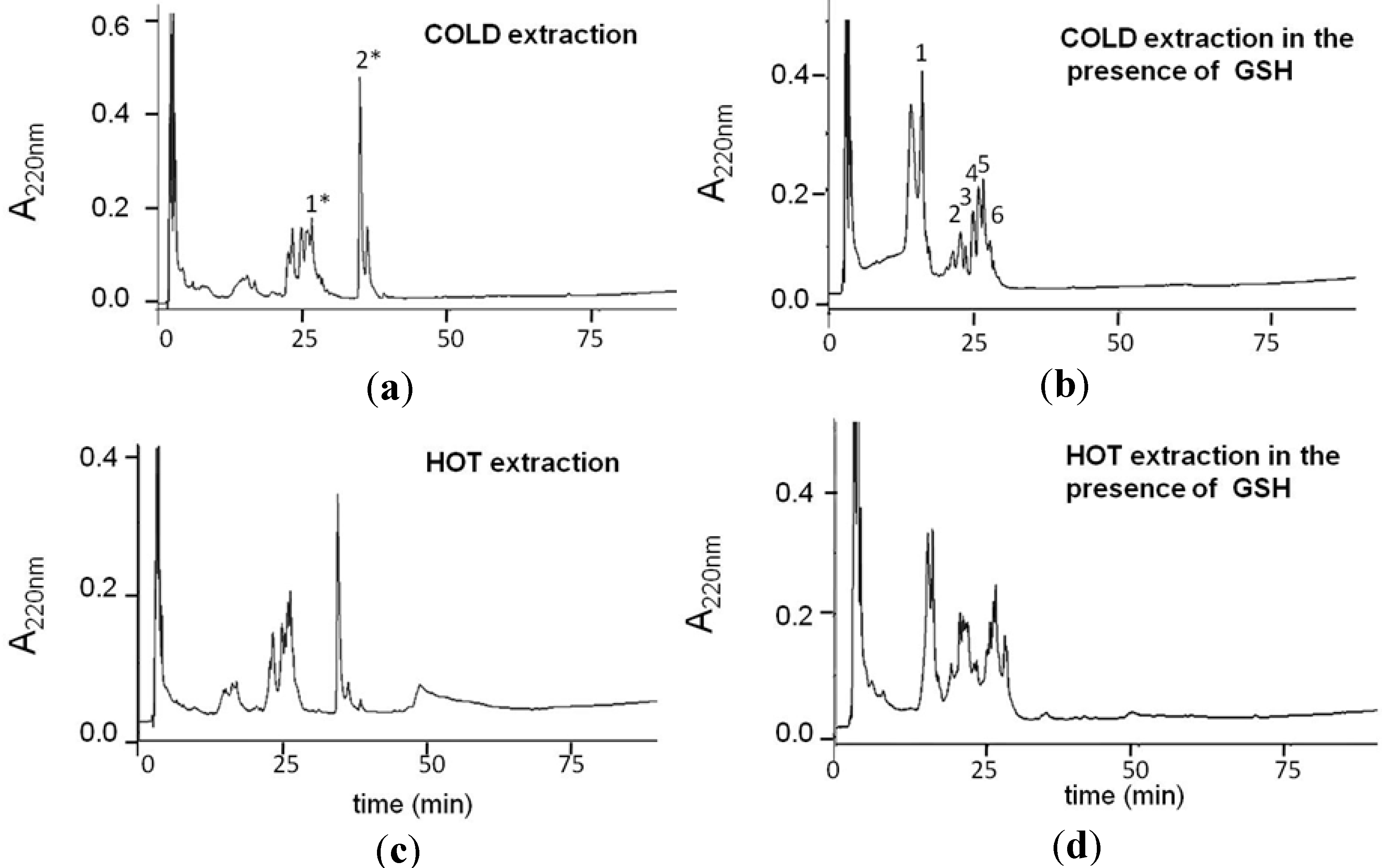
| RP-HPLC Peak | Molecular Mass | Compounds in GSGaWS Cold Extract |
|---|---|---|
| 1 | 614.1 m/z | GSSG |
| 2 | 354.1 m/z |  |
| 3 | 348.1 m/z | 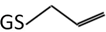 |
| 6 | 380.2 m/z |  |
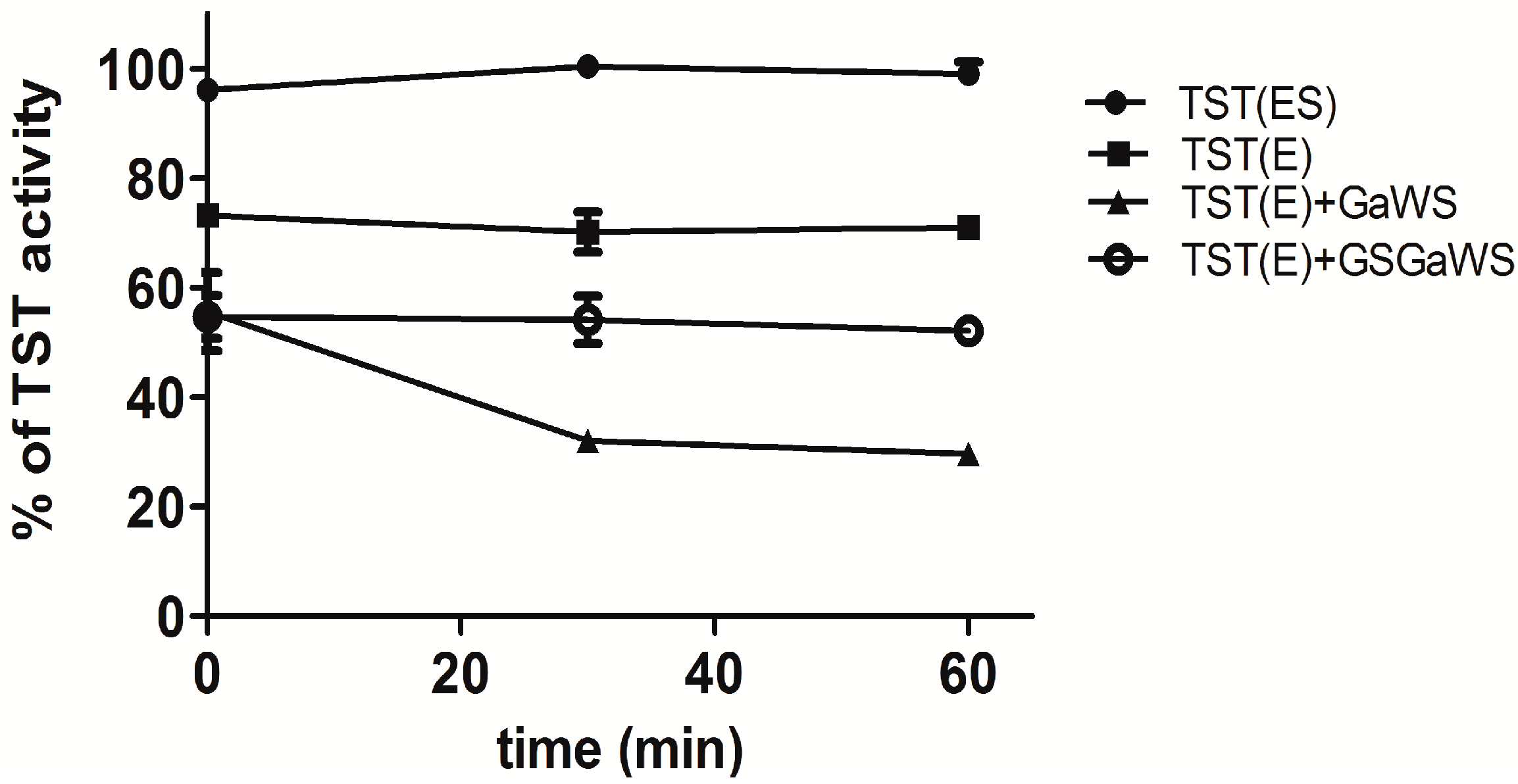
2.2. Effects of the Garlic-WS Obtained at a Low Temperature on the TST Activity
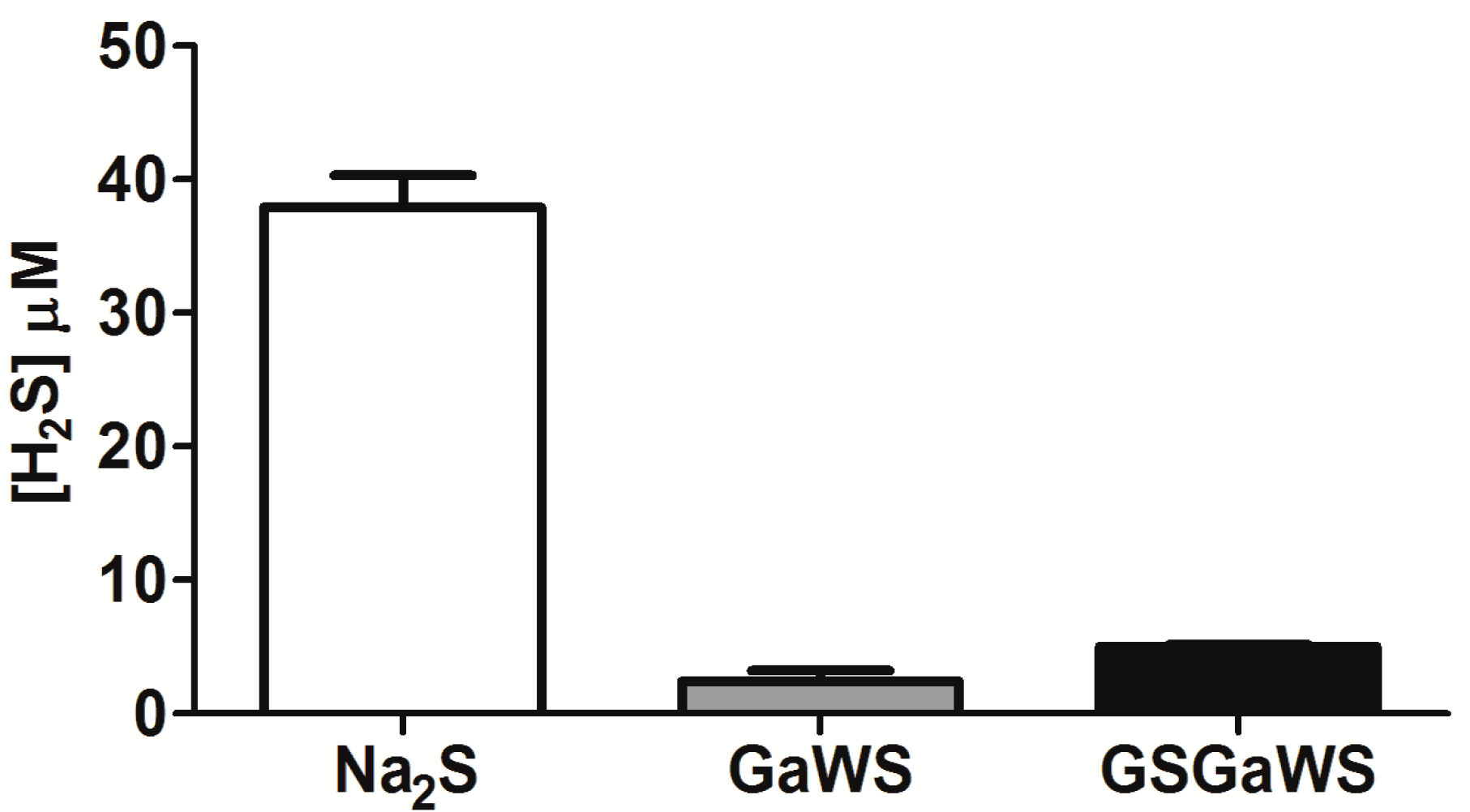
2.3. H2S Production from Garlic Extracts
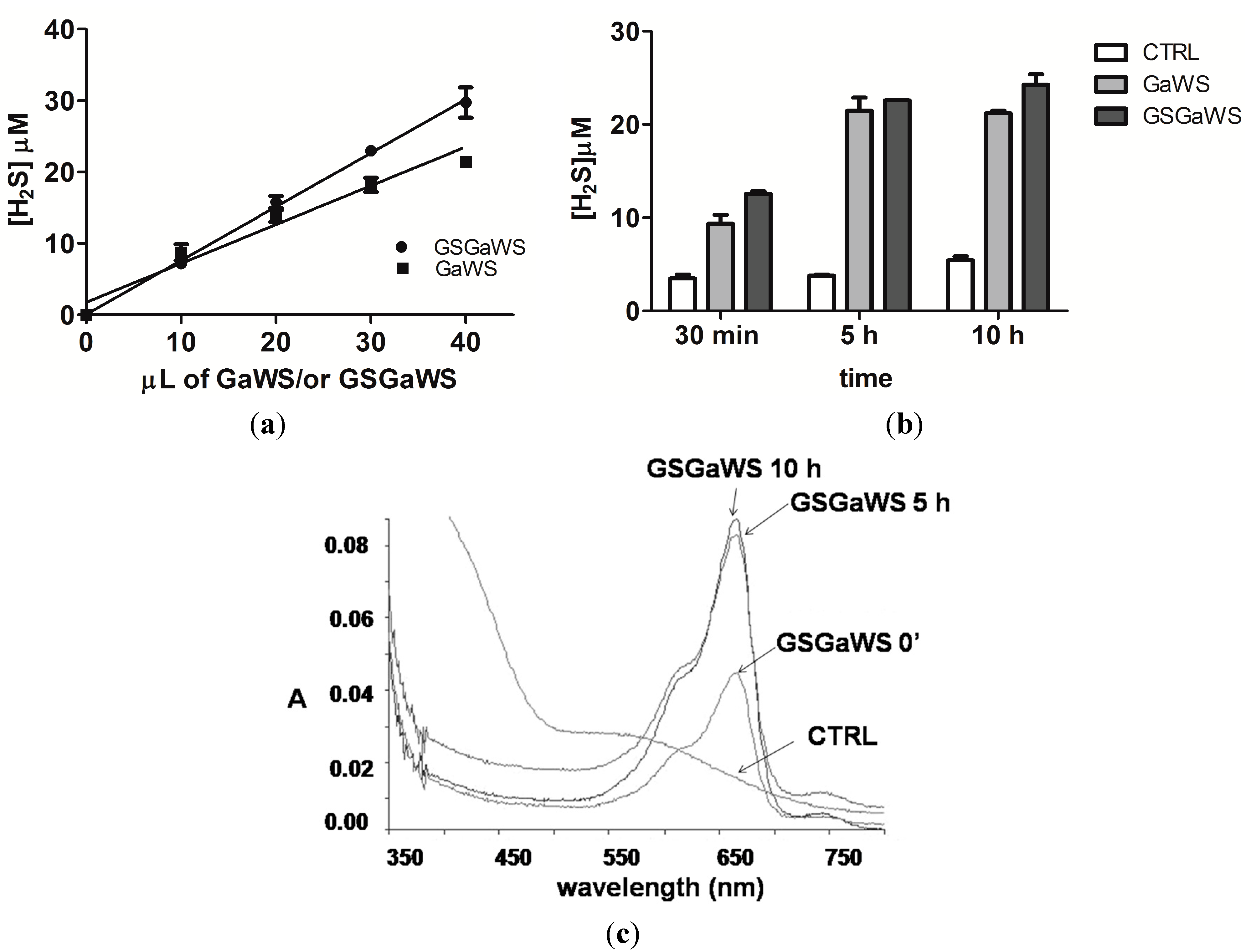
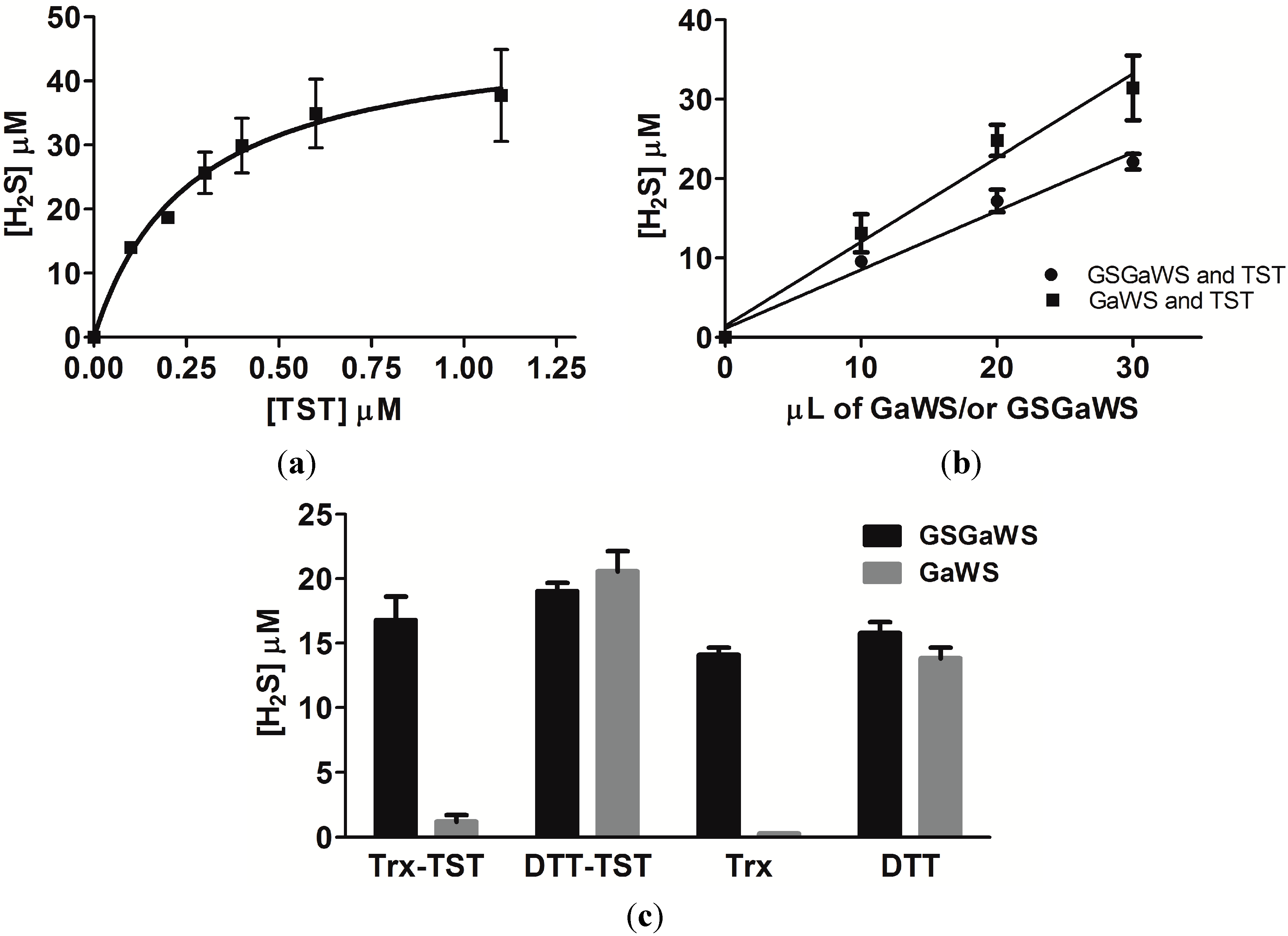
2.4. Thioredoxin as the Reductant in the Endogenous H2S Production from OSCs
2.5. Effects of the Garlic Water-Soluble Extracts on the Proliferation of the Human T-Cell Lymphoma Cell Line, HuT 78
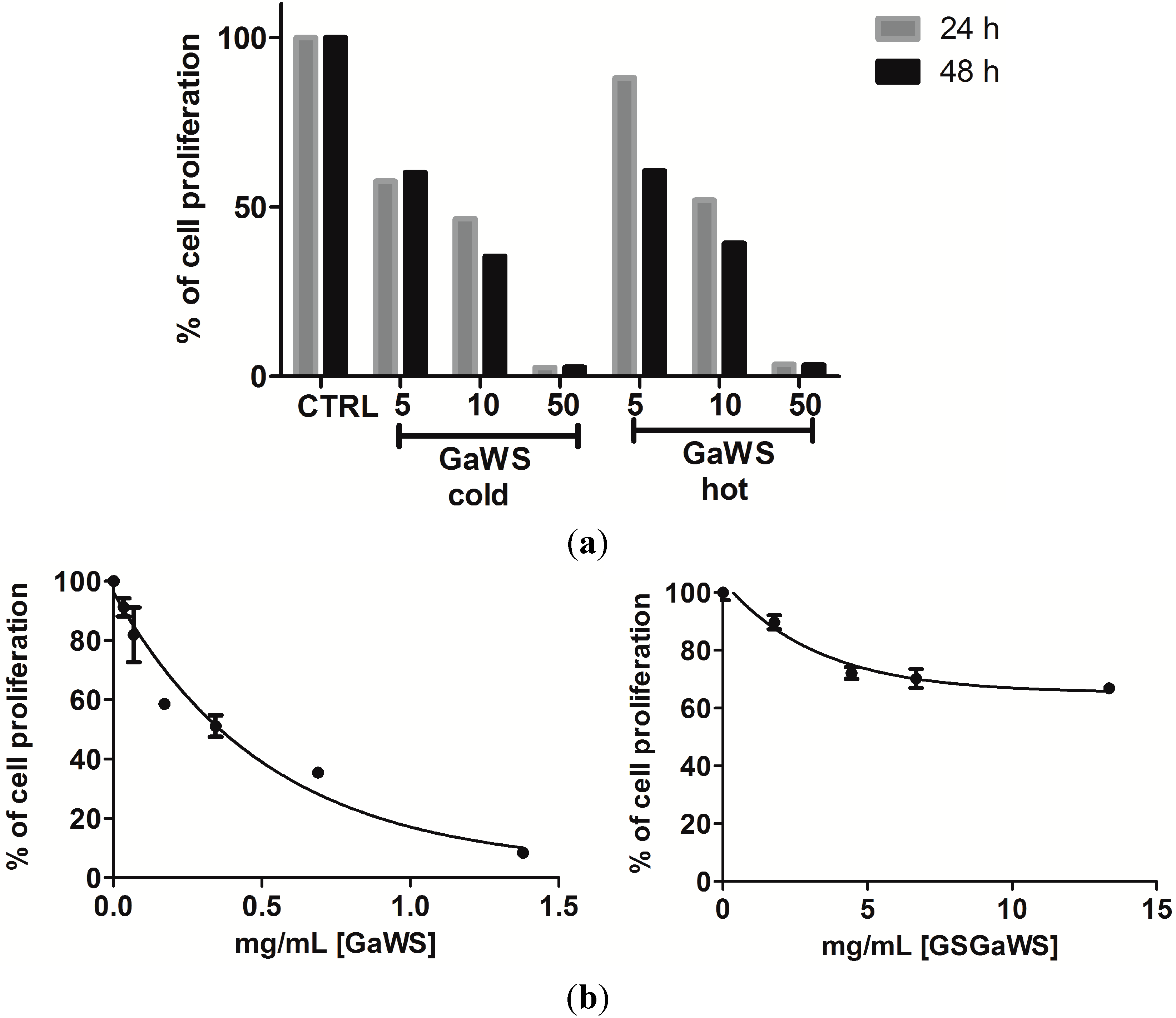
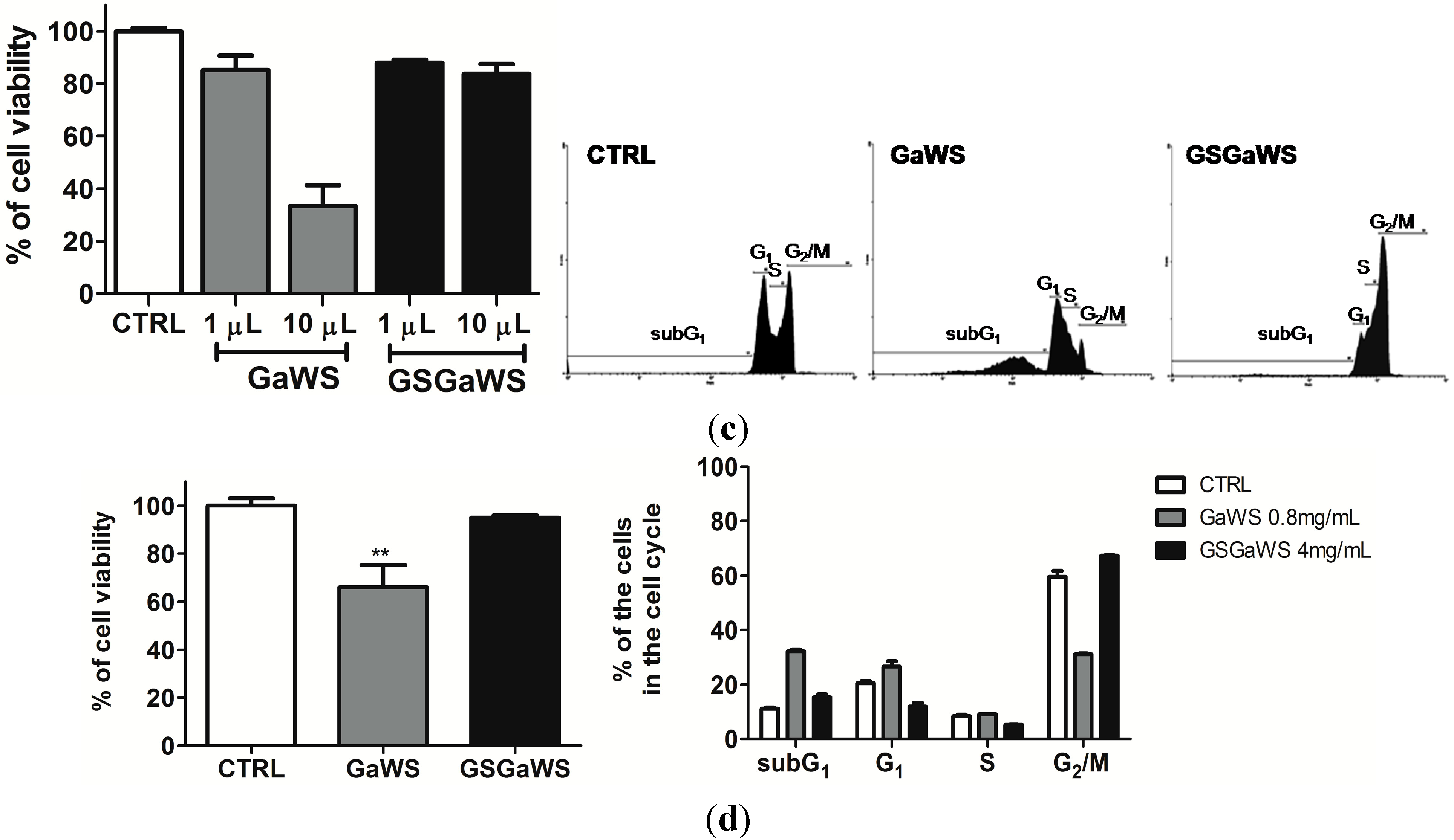
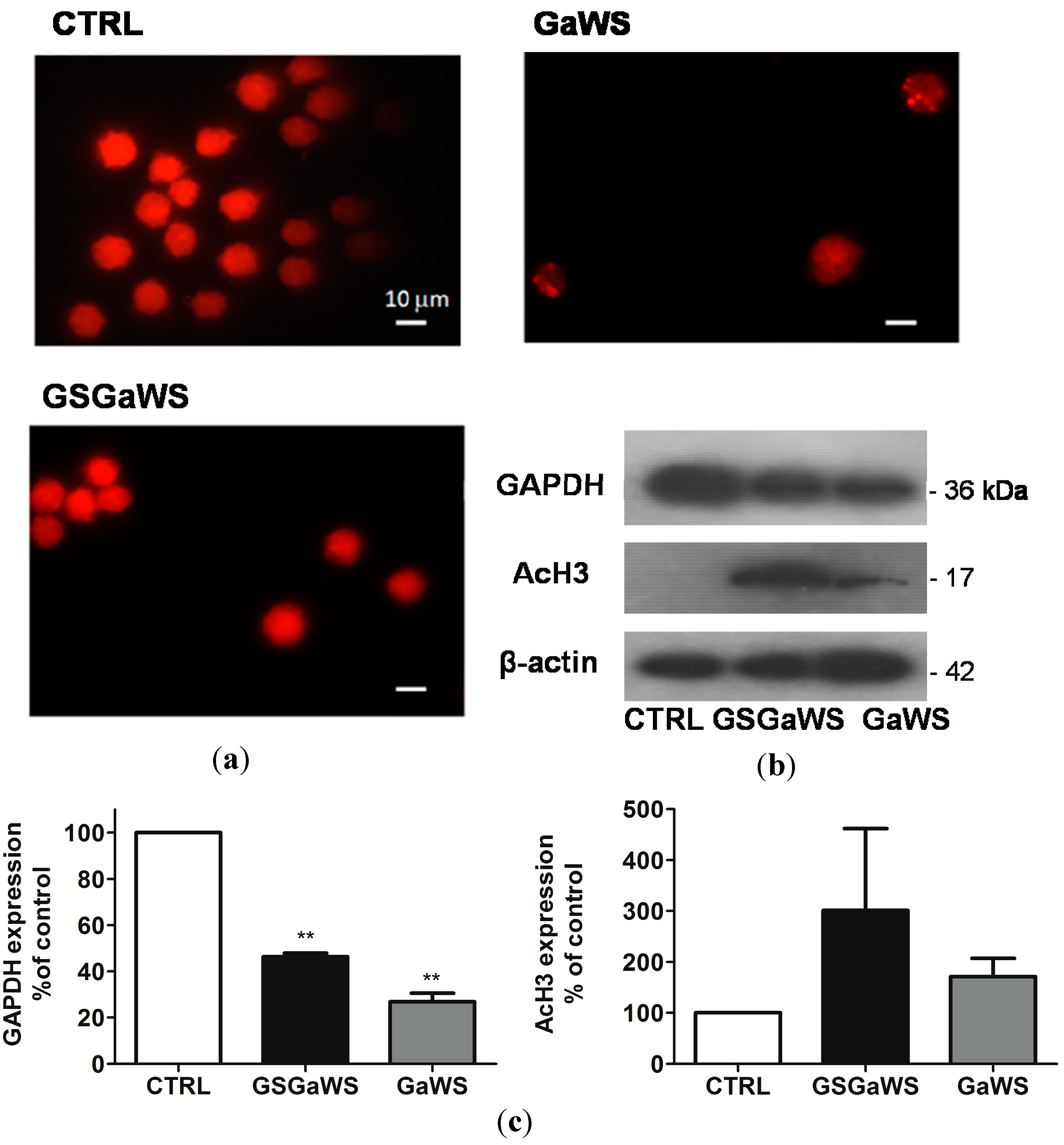
3. Experimental Section
3.1. OSCs Extraction Protocol
3.2. H2S Assay of the Garlic Extracts
3.3. TST Assay
3.4. Cell Proliferation and Vitality Assay
3.5. Cell Cycle Analysis
3.6. Western Blot Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Abbreviations
| 2-PTS | sodium-2-propenyl thiosulfate |
| CBS | cystathionine-β-synthase |
| CSE | cystathionine-γ-lyase |
| E | de-persulfurated form of TST |
| ES | persulfurated form of TST |
| FACS | fluorescence-activated cell sorting |
| GSH | glutathione |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GaWS | water-soluble garlic extract |
| GSSA | S-allyl-mercapto-glutathione |
| GSGaWS | water-soluble glutathione-garlic extract |
| GS-OSCs | glutathione-garlic organosulfur conjugates |
| GST | glutathione-S-transferase |
| RP-HPLC | reversed-phase high-performance liquid chromatography |
| HuT 78 | the human T-cell lymphoma cell line HuT 78 |
| MST | 3-mercaptopyruvate sulfurtransferase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| OSCs | organosulfur compounds |
| PI | propidium iodide |
| SAC | S-allylcysteine |
| Trx | thioredoxin |
| Trd | thioredoxin reductase |
| TST | cyanide:thiosulfate sulfurtransferase |
Conflicts of Interest
References
- Block, E.; Naganathan, S.; Putman, D.; Zhao, S.H. Allium chemistry: HPLC analysis of thiosulfinates from onion, garlic, wild garlic (ramsoms), leek, scallion, shallot, elephant (great-headed) garlic, chive, and Chinese chive. Uniquely high allyl to methyl ratios in some garlic samples. J. Agric. Food Chem. 1992, 40, 2418–2430. [Google Scholar] [CrossRef]
- Harris, J.C.; Cottrell, S.L.; Plummer, S.; Lloyd, D. Antimicrobial properties of Allium sativum (garlic). Appl. Microbiol. Biotechnol. 2001, 57, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Perez-Severiano, F.; Rodriguez-Perez, M.; Pedraza-Chaverri, J.; Maldonado, P.D.; Medina-Campos, O.N.; Ortiz-Plata, A.; Sanchez-Garcia, A.; Villeda-Hernandez, J.; Galvan-Arzate, S.; Aguilera, P.; et al. S-Allylcysteine, a garlic-derived antioxidant, ameliorates quinolinic acid-induced neurotoxicity and oxidative damage in rats. Neurochem. Int. 2004, 45, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Dirsch, V.M.; Gerbes, A.L.; Vollmar, A.M. Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kappaB. Mol. Pharmacol. 1998, 53, 402–407. [Google Scholar] [PubMed]
- Melino, S.; Sabelli, R.; Paci, M. Allyl sulfur compounds and cellular detoxification system: Effects and perspectives in cancer therapy. Amino Acids 2011, 41, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, H.J.; Santamaria, A.B.; Talalay, P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc. Natl. Acad. Sci. USA 1992, 89, 2394–2398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, B.; Lee, C.H.; Parkin, K.L. Cysteine and glutathione mixed-disulfide conjugates of thiosulfinates: Chemical synthesis and biological activities. J. Agric. Food Chem. 2010, 58, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Chen, H.W.; Yang, J.J.; Sheen, L.Y.; Lii, C.K. Diallyl disulfide and diallyl trisulfide up-regulate the expression of the pi class of glutathione S-transferase via an AP-1-dependent pathway. J. Agric. Food Chem. 2007, 55, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Horev-Azaria, L.; Eliav, S.; Izigov, N.; Pri-Chen, S.; Mirelman, D.; Miron, T.; Rabinkov, A.; Wilchek, M.; Jacob-Hirsch, J.; Amariglio, N.; et al. Allicin up-regulates cellular glutathione level in vascular endothelial cells. Eur. J. Nutr. 2009, 48, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, T.; Tian, J.; Yang, K.; Yi, K.; Zhang, P. Garlic in clinical practice: an evidence-based overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Sabelli, R.; Iorio, E.; de Martino, A.; Podo, F.; Ricci, A.; Viticchie, G.; Rotilio, G.; Paci, M.; Melino, S. Rhodanese-thioredoxin system and allyl sulfur compounds. FEBS J. 2008, 275, 3884–3899. [Google Scholar] [CrossRef] [PubMed]
- Nepravishta, R.; Sabelli, R.; Iorio, E.; Micheli, L.; Paci, M.; Melino, S. Oxidative species and S-glutathionyl conjugates in the apoptosis induction by allyl thiosulfate. FEBS J. 2012, 279, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.D.; Wang, Z.J. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: Use in measuring allicin bioavailability. J. Agric. Food Chem. 2005, 53, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Greiner, R.; Palinkas, Z.; Basell, K.; Becher, D.; Antelmann, H.; Nagy, P.; Dick, T.P. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal. 2013, 19, 1749–1765. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I.; Cooper, A.J. Thiosulfoxide (sulfane) sulfur: New chemistry and new regulatory roles in biology. Molecules 2014, 19, 12789–12813. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Signaling Molecules: Hydrogen Sulfide and Polysulfide. Antioxid. Redox Signal. 2014. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Breschi, M.C.; Blandizzi, C.; Virdis, A.; Taddei, S.; Calderone, V. Hydrogen sulphide: Novel opportunity for drug discovery. Med. Res. Rev. 2012, 32, 1093–1130. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Lefer, D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014, 114, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H.; Cerone, R.; Schiaffino, M.C.; Fantasia, A.R.; Minniti, G.; Caruso, U.; Lorini, R.; Watkins, D.; Matiaszuk, N.; Rosenblatt, D.S.; et al. Glycine N-methyltransferase deficiency: A novel inborn error causing persistent isolated hypermethioninaemia. J. Inherit. Metab. Dis. 2001, 24, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Li, Y.; Song, J.N.; Pang, H.G. Role of hydrogen sulfide in secondary neuronal injury. Neurochem. Int. 2014, 64, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Ogasawara, Y.; Kimura, H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011, 439, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Schwab, C.; Yu, S.; McGeer, E.; McGeer, P.L. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol. Aging 2009, 30, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar] [PubMed]
- Amagase, H. Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 136, 716S–725S. [Google Scholar] [PubMed]
- Block, E. The chemistry of garlic and onions. Sci. Am. 1985, 252, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Seki, T.; Ariga, T. Thermostability of allicin determined by chemical and biological assays. Biosci. Biotechnol. Biochem. 2008, 72, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wu, C.; Chen, S. Effects of pH adjustment and heat treatment on the stability and the formation of volatile compounds of garlic. J. Agric. Food Chem. 1989, 37, 730–734. [Google Scholar] [CrossRef]
- Ilić, D.P.; Nikolić, V.D.; Nikolić, L.B.; Stanković, M.Z.; Stanojević, L.P. Thermal degradation, antioxidant and antimicrobial activity of the synthesized allicin and allicin incorporated in gel. Hem. Ind. 2010, 64, 85–91. [Google Scholar] [CrossRef]
- Song, K.; Milner, J.A. The influence of heating on the anticancer properties of garlic. J. Nutr. 2001, 131, 1054S–1057S. [Google Scholar] [PubMed]
- Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Glozman, S.; Yavin, E.; Weiner, L. S-Allylmercaptoglutathione: The reaction product of allicin with glutathione possesses SH-modifying and antioxidant properties. Biochim. Biophys. Acta 2000, 1499, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, G.; Ponmurugan, P.; Senthil Kumar, G.P.; Rajarajan, T. Antidiabetic effect of S-allylcysteine: Effect on plasma and tissue glycoproteins in experimental diabetes. Phytomedicine 2010, 17, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Mundo, M.N.; Silva-Adaya, D.; Maldonado, P.D.; Galvan-Arzate, S.; Andres-Martinez, L.; Perez-De La Cruz, V.; Pedraza-Chaverri, J.; Santamaria, A. S-Allylcysteine prevents the rat from 3-nitropropionic acid-induced hyperactivity, early markers of oxidative stress and mitochondrial dysfunction. Neurosci. Res. 2006, 56, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Lee, D.T.; Tsao, S.W.; Wang, X.; Wong, Y.C. S-allylcysteine, a water-soluble garlic derivative, suppresses the growth of a human androgen-independent prostate cancer xenograft, CWR22R, under in vivo conditions. BJU Int. 2007, 99, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.N.; Williams, D.B.; Cobiac, L.; Head, R.J. Does garlic reduce risk of colorectal cancer? A systematic review. J. Nutr. 2007, 137, 2264–2269. [Google Scholar] [PubMed]
- Moriguchi, T.; Matsuura, H.; Itakura, Y.; Katsuki, H.; Saito, H.; Nishiyama, N. Allixin, a phytoalexin produced by garlic, and its analogues as novel exogenous substances with neurotrophic activity. Life Sci. 1997, 61, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.T.; Krasnikov, B.F.; Cooper, A.J. Redox-sensitive proteins are potential targets of garlic-derived mercaptocysteine derivatives. J. Nutr. 2006, 136, 835S–841S. [Google Scholar] [PubMed]
- Sörbo, B.H. Rhodanese. Acta Chem. Scand. 1953, 7, 1129–1133. [Google Scholar] [CrossRef]
- Westley, J. Rhodanese. Adv. Enzymol. Relat. Areas Mol. Biol. 1973, 39, 327–368. [Google Scholar] [PubMed]
- Wrobel, M.; Jurkowska, H.; Sliwa, L.; Srebro, Z. Sulfurtransferases and cyanide detoxification in mouse liver, kidney, and brain. Toxicol. Mech. Methods 2004, 14, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Aminlari, M.; Gholami, S.; Vaseghi, T.; Azarafrooz, A. Rhodanese (thiosulfate: cyanide sulfurtransferase) in the digestive tract of chicken at different stages of development. Poult. Sci. 1997, 76, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Pagani, S.; Bonomi, F.; Cerletti, P. Enzymic synthesis of the iron-sulfur cluster of spinach ferredoxin. Eur. J. Biochem. 1984, 142, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Lacourciere, G.; Stadtman, T.C. Formation of a selenium-substituted rhodanese by reaction with selenite and glutathione: Possible role of a protein perselenide in a selenium delivery system. Proc. Natl. Acad. Sci. USA 2001, 98, 9494–9498. [Google Scholar] [CrossRef] [PubMed]
- Westley, A.M.; Westley, J. Voltammetric determination of cyanide and thiocyanate in small biological samples. Anal. Biochem. 1989, 181, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Bordo, D.; Bork, P. The rhodanese/Cdc25 phosphatase superfamily. Sequence-structure-function relations. EMBO Rep. 2002, 3, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Powis, G.; Mustacich, D.; Coon, A. The role of the redox protein thioredoxin in cell growth and cancer. Free Radic. Biol. Med. 2000, 29, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H. Thioredoxin as a key molecule in redox signaling. Antioxid. Redox Signal. 2004, 6, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Druesne, N.; Pagniez, A.; Mayeur, C.; Thomas, M.; Cherbuy, C.; Duee, P.H.; Martel, P.; Chaumontet, C. Diallyl disulfide (DADS) increases histone acetylation and p21(waf1/cip1) expression in human colon tumor cell lines. Carcinogenesis 2004, 25, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, A.; Vijayababu, M.R.; Gunadharini, N.; Krishnamoorthy, G.; Arunakaran, J. Induction of apoptosis and histone hyperacetylation by diallyl disulfide in prostate cancer cell line PC-3. Cancer Lett. 2007, 251, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.A. Preclinical perspectives on garlic and cancer. J. Nutr. 2006, 136, 827S–831S. [Google Scholar] [PubMed]
- Wargovich, M.J. Diallylsulfide and allylmethylsulfide are uniquely effective among organosulfur compounds in inhibiting CYP2E1 protein in animal models. J. Nutr. 2006, 136, 832S–834S. [Google Scholar] [PubMed]
- Hara, M.R.; Agrawal, N.; Kim, S.F.; Cascio, M.B.; Fujimuro, M.; Ozeki, Y.; Takahashi, M.; Cheah, J.H.; Tankou, S.K.; Hester, L.D.; et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005, 7, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Stamler, J.S.; Brune, B. Posttranslational modification of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosylation and subsequent NADH attachment. J. Biol. Chem. 1996, 271, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Batthyany, C.; Schopfer, F.J.; Baker, P.R.; Duran, R.; Baker, L.M.; Huang, Y.; Cervenansky, C.; Branchaud, B.P.; Freeman, B.A. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J. Biol. Chem. 2006, 281, 20450–20463. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Hallak, H.; de Boitte, A.; Lapetina, E.G.; Brune, B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 1999, 274, 9427–9430. [Google Scholar] [CrossRef] [PubMed]
- Little, C.; O’Brien, P.J. Mechanism of peroxide-inactivation of the sulphydryl enzyme glyceraldehyde-3-phosphate dehydrogenase. Eur. J. Biochem. 1969, 10, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Radi, R. Glyceraldehyde-3-phosphate dehydrogenase inactivation by peroxynitrite. Arch. Biochem. Biophys. 1998, 360, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Colell, A.; Green, D.R.; Ricci, J.E. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009, 16, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.M.; Arieli, B.; Cohen, Y.; Padan, E.; Strohl, W.R. Sulfur metabolism in Beggiatoa alba. J. Bacteriol. 1987, 169, 5466–5472. [Google Scholar] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.; Chattopadhyay, D.; Mukherjee, H.; Ojha, D.; Mandal, N.; Sarkar, M.C.; Chatterjee, T.; Das, G.; Chakraborti, S. Anti-herpes virus activities of bioactive fraction and isolated pure constituent of Mallotus peltatus: An ethnomedicine from Andaman Islands. Virol. J. 2012, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Wagh, M.P.; Patel, J.S. Biopharmaceutical classification system: Scientific basis for biowaiver extensions. Int. J. Pharm. Pharm. Sci. 2010, 2, 12–19. [Google Scholar]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Sun, X.; Wang, R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004, 18, 1782–1784. [Google Scholar] [PubMed]
- Rose, P.; Whiteman, M.; Moore, P.K.; Zhu, Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005, 22, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhang, L.; Yang, G.; Xu, C.; Wang, R. Butyrate-stimulated H2S production in colon cancer cells. Antioxid. Redox Signal. 2010, 12, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Lu, M.; Wu, Z.Y.; Wong, P.T.; Bian, J.S. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol. Pharmacol. 2009, 75, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.J.; Wang, M.J.; Ju, L.H.; Wang, C.; Zhu, Y.C. Hydrogen sulfide induces human colon cancer cell proliferation: Role of Akt, ERK and p21. Cell Biol. Int. 2010, 34, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.W.; Zhou, J.; Chen, C.S.; Zhao, Y.; Tan, C.H.; Li, L.; Moore, P.K.; Deng, L.W. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One 2011, 6, e21077. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K. Anti-cancer activity of new designer hydrogen sulfide-donating hybrids. Antioxid. Redox Signal. 2013, 20, 831–846. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds GSGaWS, GaWS, TST are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuiyan, A.I.; Papajani, V.T.; Paci, M.; Melino, S. Glutathione-Garlic Sulfur Conjugates: Slow Hydrogen Sulfide Releasing Agents for Therapeutic Applications. Molecules 2015, 20, 1731-1750. https://doi.org/10.3390/molecules20011731
Bhuiyan AI, Papajani VT, Paci M, Melino S. Glutathione-Garlic Sulfur Conjugates: Slow Hydrogen Sulfide Releasing Agents for Therapeutic Applications. Molecules. 2015; 20(1):1731-1750. https://doi.org/10.3390/molecules20011731
Chicago/Turabian StyleBhuiyan, Ashif Iqbal, Vilma Toska Papajani, Maurizio Paci, and Sonia Melino. 2015. "Glutathione-Garlic Sulfur Conjugates: Slow Hydrogen Sulfide Releasing Agents for Therapeutic Applications" Molecules 20, no. 1: 1731-1750. https://doi.org/10.3390/molecules20011731
APA StyleBhuiyan, A. I., Papajani, V. T., Paci, M., & Melino, S. (2015). Glutathione-Garlic Sulfur Conjugates: Slow Hydrogen Sulfide Releasing Agents for Therapeutic Applications. Molecules, 20(1), 1731-1750. https://doi.org/10.3390/molecules20011731









