Synthesis and Cytotoxicity Evaluation of Some Novel Thiazoles, Thiadiazoles, and Pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones Incorporating Triazole Moiety
Abstract
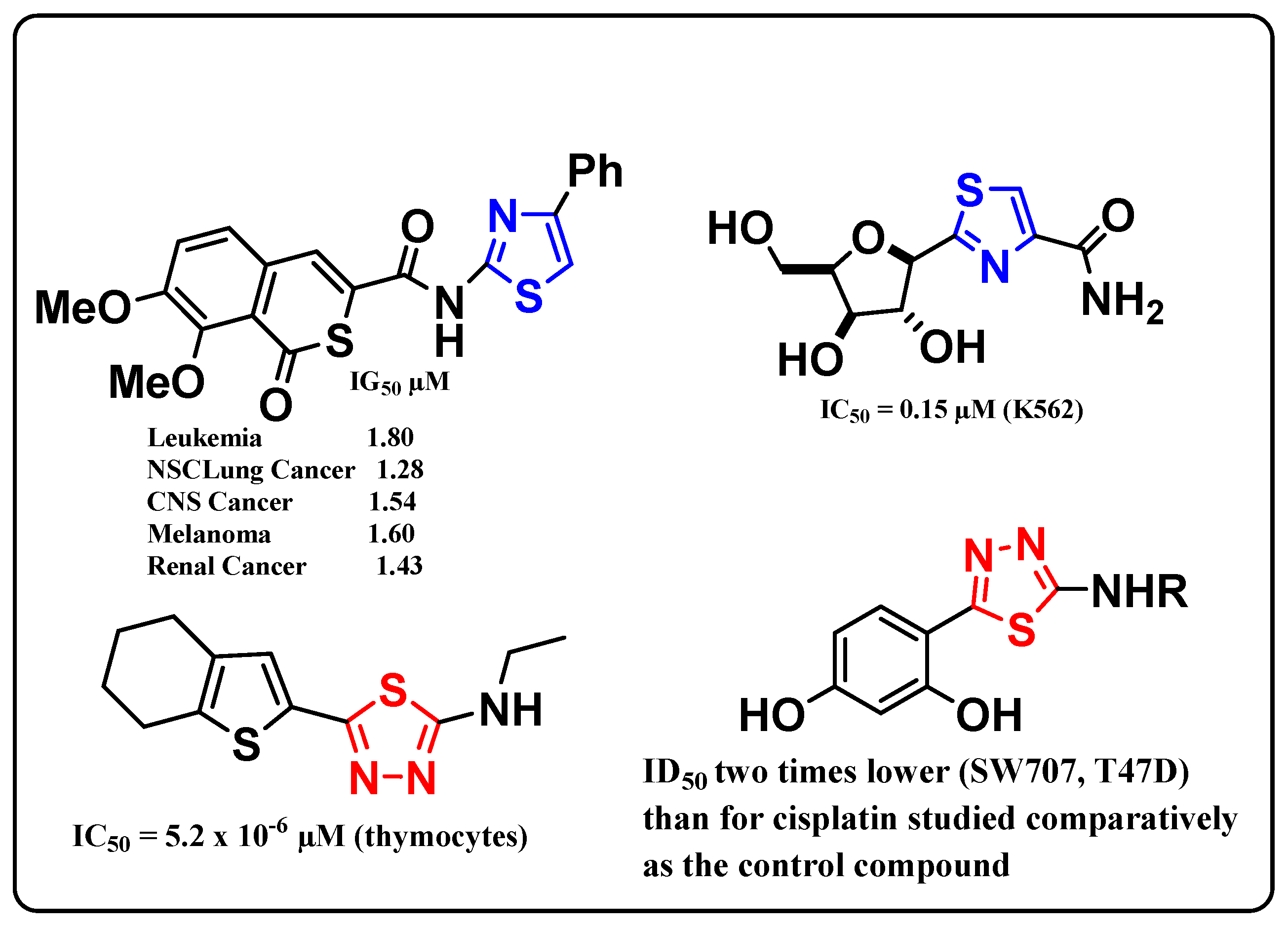
:1. Introduction
2. Results and Discussion
2.1. Chemistry
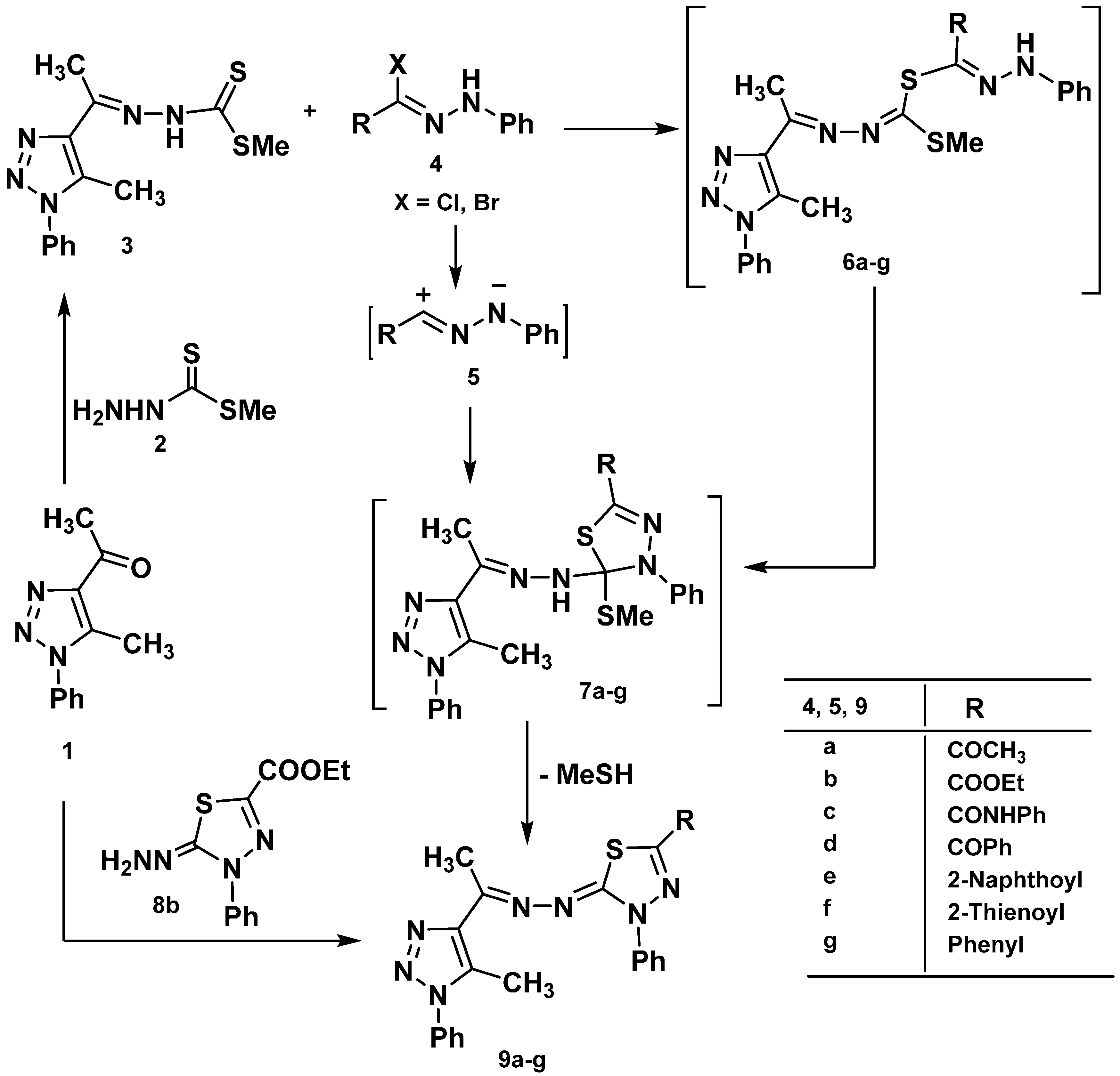
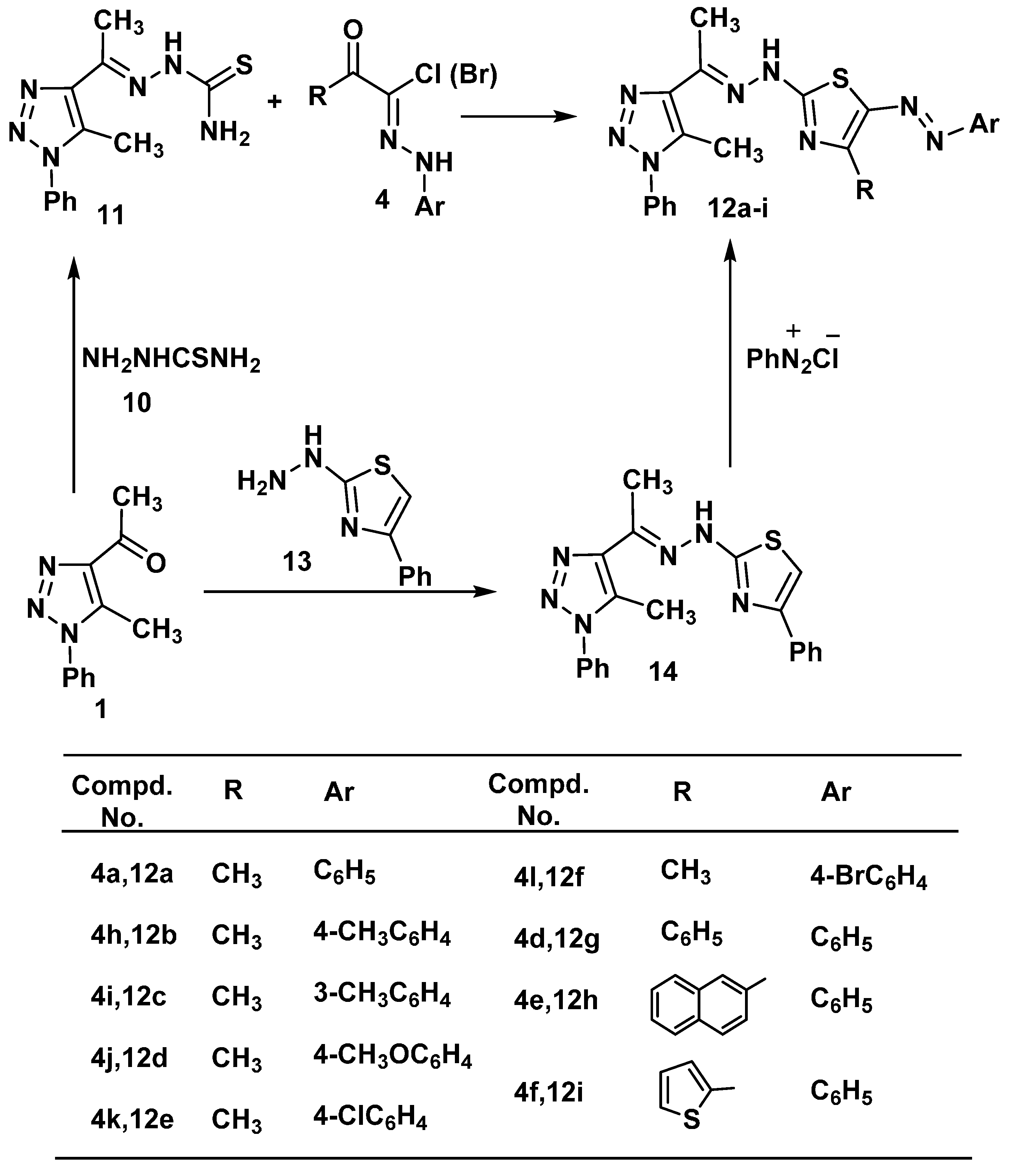
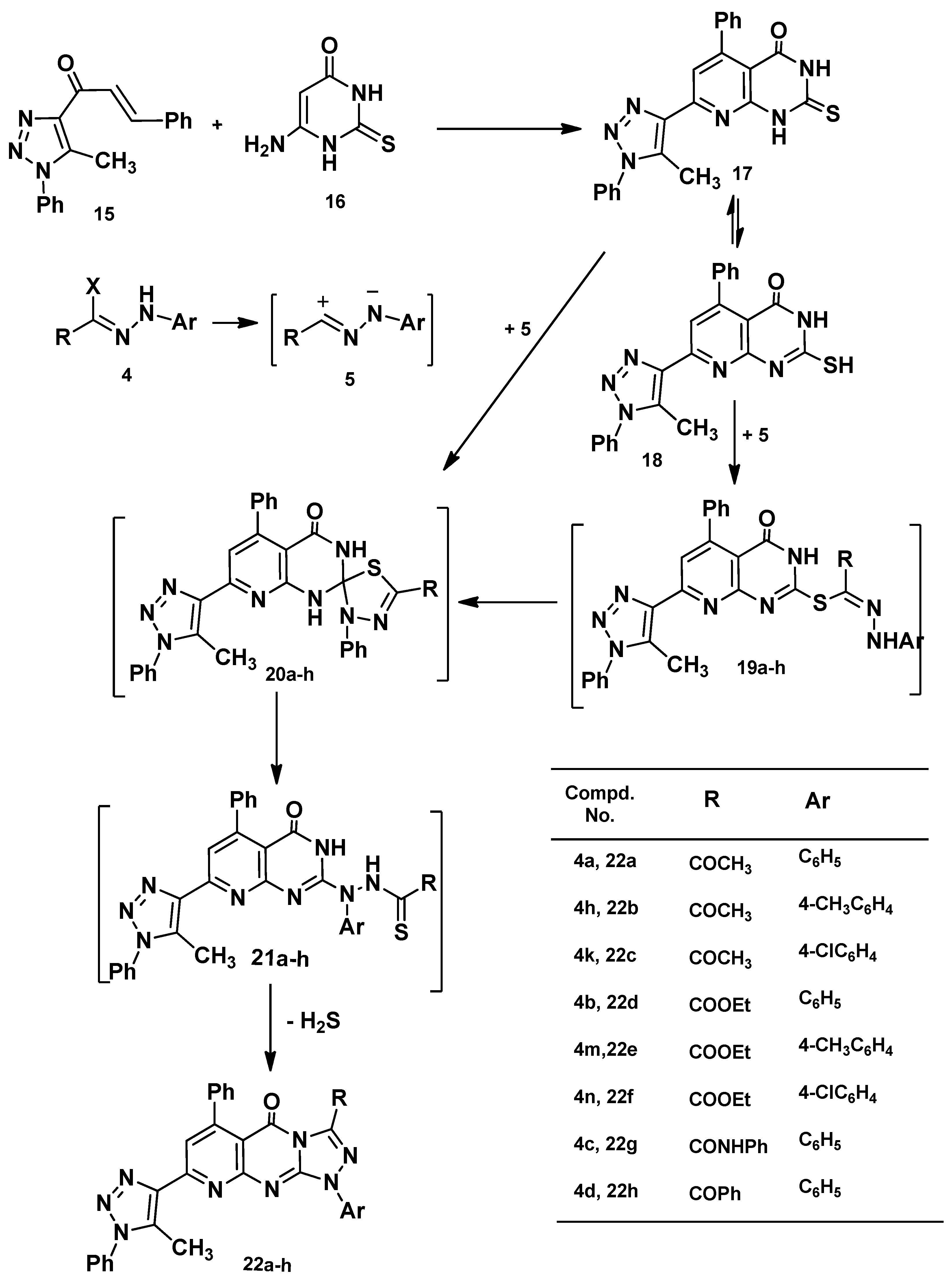
2.2. Cytotoxic Activity
| Tested Compounds | Tumor Cell Lines | |
|---|---|---|
| MCF-7 | HepG2 | |
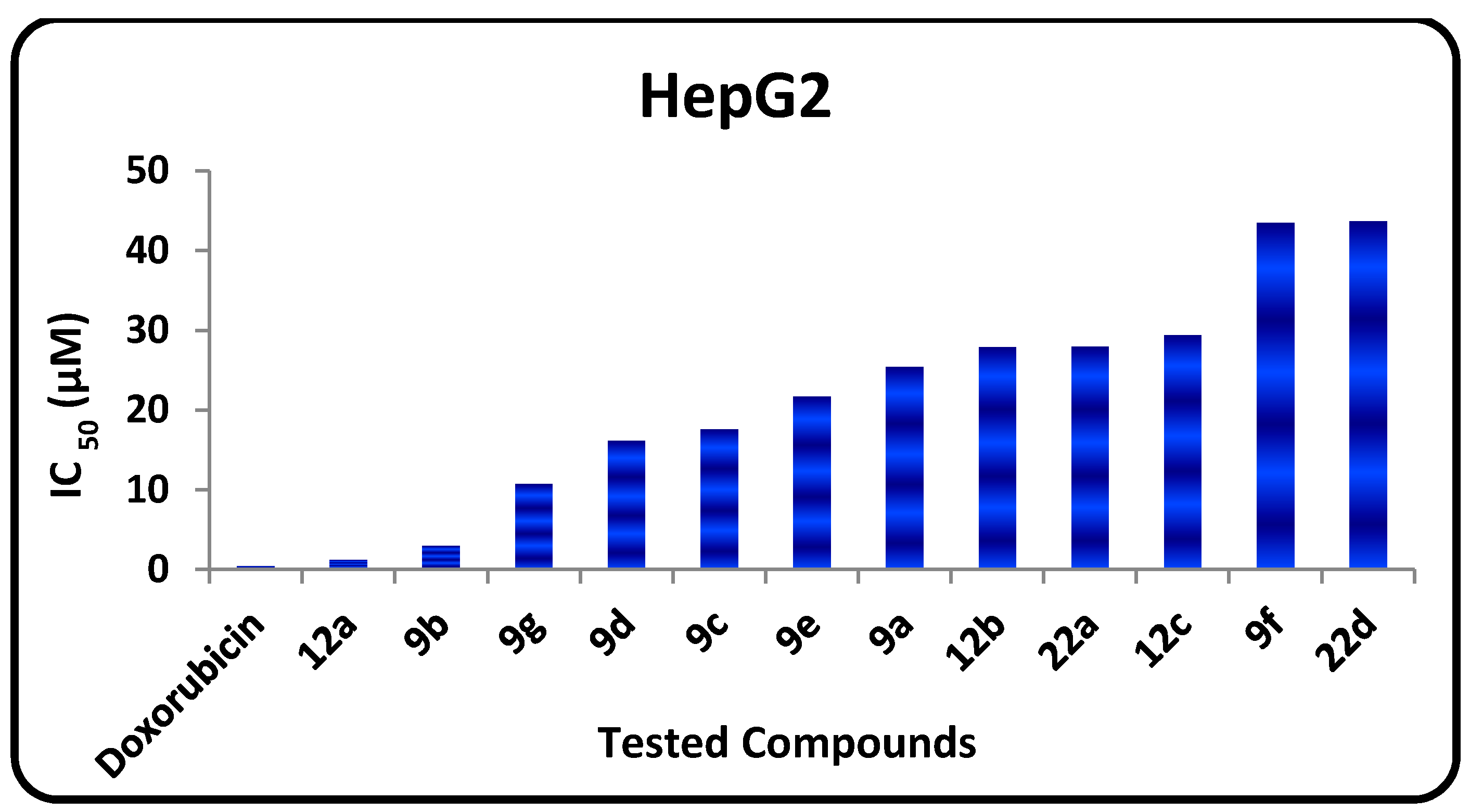
| 9a | 29.11 ± 0.21 | 25.42 ± 0.21 |
| 9b | 8.67 ± 0.30 | 2.94 ± 0.12 |
| 9c | 7.72 ± 0.18 | 17.60 ± 0.23 |
| 9d | 22.40 ± 0.20 | 16.13 ± 0.21 |
| 9e | 22.94 ± 0.18 | 21.72 ± 0.14 |
| 9f | 38.21 ± 0.16 | 43.43 ± 0.19 |
| 9g | 19.72 ± 0.20 | 10.71 ± 0.27 |
| 12a | 3.4 ± 0.23 | 1.19 ± 0.07 |
| 12b | 22.5 ± 0.24 | 27.90 ± 0.24 |
| 12c | 20.1 ± 0.12 | 29.41± 0.07 |
| 22a | 37.7 ± 0.11 | 27.94 ± 0.13 |
| 22d | 39.9 ± 0.07 | 43.62 ± 0.14 |
| Doxorubicin | 0.46 ± 0.21 | 0.42 ± 0.22 |
- -
- The in vitro inhibitory activities of tested compounds against the human breast carcinoma (MCF-7) have the following descending order: 12a > 9c > 9b > 9g > 12e > 9d > 12b > 9e > 9a > 22d > 9f > 22a.
- -
- The in vitro inhibitory activities of tested compounds against the human hepatocellular carcinoma (HepG2) cell line have the following descending order: 12a > 9b > 9g > 9d > 9c > 9e > 9a > 12e > 22d > 12b > 9f > 22a.

- -
- The 1,3,4-thiadiazole 9b (IC50 = 2.94 µM) has promising antitumor activity against the human hepatocellular carcinoma cell line while the other 1,3,4-thiadiazole derivatives 9a, 9c–f have moderate activities (IC50 = 7.72‒43.43 µM).
- -
- Thiazole 12a has promising inhibitory activity against both the human hepatocellular carcinoma cell line and the breast carcinoma cell line (IC50 = 1.19, and 3.4 µM, respectively) while the other thiazole derivatives 12b and 12e have moderate activity.
- -
- Pyridotriazolopyrimidinone derivatives 22a,d have moderate activity.
- -
- For substituents at position 2 of the 1,3,4-thiadiazole ring, the in vitro inhibitory activity of tested compounds against the human breast carcinoma cell line have the following descending order: CONHC6H5 > COOC2H5 > C6H5 > C6H5CO > C10H7CO > CH3CO > C4H3SCO group.
- -
- For substituents at position 2 of the 1,3,4-thiadiazole ring, the in vitro inhibitory activity of tested compounds against the human hepatocellular carcinoma cell line have the following descending order: COOC2H5 > C6H5 > C6H5CO > CONHC6H5 > C10H7CO > CH3CO > C4H3SCO group.
3. Experimental Section
3.1. Chemistry
3.1.1. General
3.1.2. Synthesis of Methyl 2-(1-(5-methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazine-1-carbodithioate (3)
3.1.3. General Procedure for Synthesis of 2-((1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)-hydrazono)-3-phenyl-5-subsitituted-2,3-dihydro-1,3,4-thiadiazoles 9a–g
3.1.4. Alternate synthesis of 9b
3.1.5. Synthesis of 2-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinecarbothioamide (11)
3.1.6. Synthesis of 2-(2-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-5-(aryl-diazenyl)-4-substitutedthiazoles 12a–i
3.1.7. Synthesis of 2-(2-(1-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)ethylidene)hydrazinyl)-4-phenylthiazole (14)
3.1.8. Alternate Synthesis of 12g
3.1.9. Synthesis of 7-(5-Methyl-1-phenyl-1H-1,2,3-triazol-4-yl)-5-phenyl-2-thioxo-2,3-dihydropyrido-[2,3-d]pyrimidin-4(1H)-one (17)
3.1.10. General Procedure for Synthesis of Pyrido[2,3-d][1,2,4]triazolo-[4,3-a] pyrimidin-5(1H)-ones 22a–h
3.2. Evaluation of the Antitumor Activity Using Viability Assay
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Su, N.N.; Li, Y.; Yu, S.J.; Zhang, X.; Liu, X.H.; Zhao, W.G. Microwave-assisted synthesis of some novel 1,2,3-triazoles by click chemistry, and their biological activity. Res. Chem. Intermed. 2013, 39, 759–766. [Google Scholar] [CrossRef]
- Su, N.N.; Xiong, L.X.; Yu, S.J.; Zhang, X.; Cui, C.; Li, Z.M.; Zhao, W.G. Larvicidal activity and click synthesis of 2-alkoxyl-2-(1,2,3-triazole-1-yl)acetamide library. Comb. Chem. High Throughput Screen. 2013, 16, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.-Q.; Katritzky, A.R. 1,2,3-Triazoles. In Comprehensive Heterocycle Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: New York, NY, USA, 1996; Volume 4, pp. 1–126. [Google Scholar]
- Katritzky, A.R.; Zhang, Y.; Singh, S.K. 1,2,3-Triazole formation under mild conditions via 1,3-dipolar cycloaddition of acetylenes with azides. Heterocycles 2003, 60, 1225–1239. [Google Scholar] [CrossRef]
- Christian, W.T.; Caspar, C.; Morten, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper (i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Biorn, A.C.; Cocklin, S.; Madani, N.; Si, Z.; Ivanovic, T.; Samanen, J.; Ryk, D.I.V.; Pantophlet, R.; Burton, D.R.; Freire, E.; et al. Mode of action for linear peptide inhibitors of HIV-1 gp120 interactions. Biochemistry 2004, 43, 1928–1938. [Google Scholar] [CrossRef] [PubMed]
- Whiting, M.; Muldoon, J.; Lin, Y.C.; Silverman, S.M.; Lindstrom, W.; Olson, A.J.; Kolb, H.C.; Finn, M.G.; Sharpless, B.K.; Elder, J.H.; et al. Inhibitors of HIV-1 protease by using in situ click chemistry. Angew. Chem. Int. Ed. 2006, 45, 1435–1439. [Google Scholar] [CrossRef]
- Brik, A.; Muldoon, J.; Lin, Y.C.; Elder, J.C.; Goodsell, D.S.; Olson, A.J.; Fokin, V.V.; Sharpless, B.K.; Wong, C.H. Rapid diversity-oriented synthesis in microtiter plates for in situ screening of HIV protease inhibitors. ChemBioChem. 2003, 4, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Gao, Y.; Hou, Y.L.; Zhang, C.; Yu, S.J.; Bian, Q.; Li, Z.M.; Zhao, W.G. Design, synthesis, and fungicidal evaluation of a series of novel 5-methyl-1H-1,2,3-trizole-4-carboxyl amide and ester analogues. Eur. J. Med. Chem. 2014, 86, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Brockunier, L.L.; Parmee, E.R.; Ok, H.O.; Candelore, M.R.; Cascieri, M.A.; Colwell, L.F.; Eng, L.; Feeney, W.P.; Forrest, M.J.; Hom, G.J.; et al. Human beta3-adrenergic receptor agonists containing 1,2,3-triazole substituted benzenesulfonamides. Bioorg. Med. Chem. Lett. 2000, 10, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Pande, V.; Ramos, M.J. Structural basis for the GSK-3beta binding affinity and selectivity against CDK-2 of 1-(4-aminofurazan-3yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 5129–5135. [Google Scholar] [CrossRef] [PubMed]
- Olesen, P.H.; Sørensen, A.R.; Ursö, B.; Kurtzhals, P.; Bowler, A.N.; Ehrbar, U.; Hansen, B.F. Synthesis and in vitro characterization of 1-(4-Aminofurazan-3-yl)-5-dialkylaminomethyl-1H-[1,2,3]triazole-4-carboxylic acid derivatives. A new class of selective GSK-3 inhibitors. J. Med. Chem. 2003, 46, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Krasinski, A.; Radic, Z.; Manetsch, R.; Raushel, J.; Taylor, P.; Sharpless, B.K.; Kolb, H.C. In situ selection of lead compounds by click chemistry: Target-guided optimization of aceylcholinesterase inhibitors. J. Am. Chem. Soc. 2005, 127, 6686–6692. [Google Scholar] [CrossRef] [PubMed]
- Mocharla, V.P.; Colasson, B.; Lee, L.V.; Roeper, S.; Sharpless, B.K.; Wong, C.H.; Kolb, H.C. In situ click chemistry: Enzyme-generated inhibitors of carbonic anhydrase II. Angew. Chem. Int. Ed. 2005, 44, 116–120. [Google Scholar] [CrossRef]
- Caballé, C.; Urdaneta, E.; Marzo, F.; Larralde, J.; Santidrián, S. Inhibition of in vitro intestinal absorption of d-galactose by cefroxadine, cefatrizine and cefaloglycin. Indian J. Pharm. 2003, 35, 163–167. [Google Scholar]
- Syed, M.A.; Ramappa, A.K.; Alegaon, S. Synthesis and evaluation of antitubercular and anti fungal activity of some novel 6-(4-substituted aryl)-2-(3,5-dimethyl-1H-pyrazol-1-yl)imidazo[2,1-b][1,3,4] thiadiazole derivatives. Asian J. Pharm. Clin. Res. 2013, 6, 47–51. [Google Scholar]
- Zhang, L.J.; Yang, M.Y.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 2014, 11, 1107–1111. [Google Scholar] [CrossRef]
- Yan, S.L.; Yang, M.Y.; Sun, Z.H.; Min, L.J.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole moiety. Lett. Drug Des. Discov. 2014, 11, 940–943. [Google Scholar] [CrossRef]
- Tong, J.Y.; Sun, N.B.; Wu, H.K.; Liu, X.H. Synthesis, crystal structure and biological activity of N-(5-(O-tolyl)-1,3,4-thiadiazol-2-yl)cyclopropanecarboxamide. J. Chem. Soc. Pak. 2013, 35, 1349–1353. [Google Scholar]
- Yang, M.Y.; Zhao, W.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Liu, X.H. Synthesis and biological activity of acylthiourea derivatives contain 1,2,3-thiadiazole and 1,3,4-thiadiazole. Lett. Drug Des. Discov. 2015. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Da, Y. Synthesis of 2-(5-(2-chlorophenyl)-2-furoylamino)-5-aryloxymethyl-1,3,4-thiadiazoles under microwave irradiation. Synth. Commun. 2001, 31, 1829–1836. [Google Scholar] [CrossRef]
- Liu, X.; Shi, Y.; Ma, Y.; Zhang, C.; Dong, W.; Pan, L.; Wang, B.; Li, Z. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropane carboxamides. Eur. J. Med. Chem. 2009, 44, 2782–2786. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Singh, A.K.; Jaiswal, N.; Singh, D. Synthesis and pharmacological activity of 1,3,4-thiadiazole derivatives: A review. Int. Res. J. Pharm. 2012, 3, 70–82. [Google Scholar]
- Gomha, S.M.; Khalil, K.D.; El-Zanate, A.M.; Riyadh, S.M. A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo[5,1-c][1,2,4]triazine, and 1,3,4-thiadiazoline. Heterocycles 2013, 87, 1109–1120. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M. Synthesis under microwave irradiation of [1,2,4]triazolo[3,4-b] [1,3,4]thiadiazoles and other diazoles bearing indole moieties and their antimicrobial evaluation. Molecules 2011, 16, 8244–8256. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdel-Aziz, H.A. Synthesis of new heterocycles derived from 3-(3-methyl-1H-indol-2-yl)-3-oxopropanenitrile as potent antifungal agents. Bull. Korean Chem. Soc. 2012, 33, 2985–2990. [Google Scholar] [CrossRef]
- Hargrave, K.D.; Hess, F.K.; Oliver, J.T. N-(4-Substituted-thiazolyl)oxamic acid derivatives, new series of potent, orally active antiallergy agents. J. Med. Chem. 1983, 26, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Patt, W.C.; Hamilton, H.W.; Taylor, M.D.; Ryan, M.J.; Taylor, D.G., Jr.; Connolly, C.J.C.; Doherty, A.M.; Klutchko, S.R.; Sircar, I.; Steinbaugh, B.A.; et al. Structure-activity relationships of a series of 2-amino-4-thiazole containing renin inhibitors. J. Med. Chem. 1992, 35, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.N.; Xavier, F.P.; Vasu, K.K.; Chaturvedi, S.C.; Pancholi, S.S. Synthesis of 4-benzyl-1,3-thiazole derivatives as potential anti-inflammatory agents: An analogue-based drug design approach. J. Enzym. Inhib. Med. Chem. 2009, 24, 890–897. [Google Scholar] [CrossRef]
- Jaen, J.C.; Wise, L.D.; Caprathe, B.W.; Tecle, H.; Bergmeier, S.; Humblet, C.C.; Heffner, T.G.; Meltzner, L.T.; Pugsley, T.A. 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist properties. J. Med. Chem. 1990, 33, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Ishikawa, H. Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg. Med. Chem. Lett. 1994, 4, 1601–1606. [Google Scholar] [CrossRef]
- Bell, F.W.; Cantrell, A.S.; Hogberg, M.; Jaskunas, S.R.; Johansson, N.G.; Jordon, C.L.; Kinnick, M.D.; Lind, P.; Morin, J.M., Jr.; Noreen, R.; et al. Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J. Med. Chem. 1995, 38, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Ergenc, N.; Capan, G.; Gunay, N.S.; Ozkirimli, S.; Gungor, M.; Ozbey, S.; Kendi, E. Synthesis and hypnotic activity of new 4-thiazolidinone and 2-thioxo-4,5-imidazolidinedione derivatives. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 343–347. [Google Scholar] [CrossRef]
- Carter, J.S.; Kramer, S.; Talley, J.J.; Penning, T.; Collins, P.; Graneto, M.J.; Seibert, K.; Koboldt, C.; Masferrer, J.; Zweifel, B. Synthesis and activity of sulfonamide-substituted 4,5-diaryl thiazoles as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Badorc, A.; Bordes, M.F.; de Cointet, P.; Savi, P.; Bernat, A.; Lale, A.; Petitou, M.; Maffrand, J.P.; Herbert, J.M. New orally active non-peptide fibrinogen receptor (GpIIb-IIIa) antagonists: Identification of ethyl 3-[N-[4-[4-amino[(ethoxycarbonyl)imino]methyl]phenyl]-1,3-thiazol-2-yl]-N-[1-(ethoxycarbonyl)methyl]piperid-4-yl]amino]propionate (SR 121787) as a potent and long-acting antithrombotic agent. J. Med. Chem. 1997, 40, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Theis, H.; Hanke, R.; Endermann, R.; Johannsen, L.; Geschke, F.U. seco-Cyclothialidines: New concise synthesis, inhibitory activity toward bacterial and human DNA topoisomerases, and antibacterial properties. J. Med. Chem. 2001, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Abbas, S.E.S.; Youssef, M.M.; Eladwy, R.A. Synthesis and antitumor activity of pyrido [2,3-d]pyrimidine and pyrido[2,3-d] [1,2,4]triazolo[4,3-a]pyrimidine derivatives that induce apoptosis through G(1) cell-cycle arrest. Eur. J. Med. Chem. 2014, 83, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Astakhov, A.V.; Chernyshev, V.M. Molecular structure of 3-amino[1,2,4]triazolo-[4,3-a] pyrimidin-5-one in various tautomeric forms: Investigation by DFT and QTAIM methods. Chem. Heterocycl. Compd. 2014, 50, 319–326. [Google Scholar] [CrossRef]
- Liu, X.H.; Sun, Z.H.; Yang, M.Y.; Tan, C.X.; Weng, J.Q.; Zhang, Y.G.; Ma, Y. Microwave assistant one pot synthesis, crystal structure, antifungal activities and 3D-QSAR of novel 1,2,4-triazolo[4,3-a]pyridines. Chem. Biol. Drug Des. 2014, 84, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, T.A.; Hassaneen, H.M.E. Synthesis of pyrido[2,3-d][1,2,4] triazolo[4,3-a]pyrimidin-5-ones as potential antimicrobial agents. Arch. Pharm. Res. 2013, 36, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M. A facile one-pot synthesis of 6,7,8,9-tetrahydrobenzo[4,5]thieno[2,3-d]-1,2,4-triazolo[4,5-a]pyrimidin-5-ones. Monatsh. Chem. 2009, 140, 213–220. [Google Scholar] [CrossRef]
- Gomha, S.M. Badrey, M.G. Ecofriendly regioselective one-pot synthesis of chromeno[4,3-d] [1,2,4]triazolo[4,3-a]pyrimidine. Eur. J. Chem. 2013, 4, 180–184. [Google Scholar] [CrossRef]
- Mohmed, A.M.; Abdelall, E.K.A.; Zaki, Y.H.; Abdelhamid, A.O. Synthesis of some new of thieno[2,3-b]pyridines, pyrazolo[1,5-a]pyrimidine, [1,2,4]triazolo[1,5-a]pyrimidine and pyrimido[1,2-a]benzimidazole derivatives containing pyridine moiety. Eur. J. Chem. 2011, 2, 509–513. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Shokry, A.S.; Tawfiek, S.M. A new approachforthe synthesis of some pyrazolo[5,1-c]triazines and pyrazolo[1,5-a]pyrimidines containing naphtofuran moiety. J. Heterocycl. Chem. 2012, 49, 116–124. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; Fahmi, A.A.; Halim, K.N.M. Design and synthesis of some new pyrazolo[1,5-a]pyrimidines, pyrazolo[5,1-c]triazines, pyrazolo[3,4-d]pyridazines, oxazolo[3,4-d]pyridazines containing pyrazole moiety. Synth. Commun. 2013, 43, 1101–1126. [Google Scholar] [CrossRef]
- Abdel-Aziem, A.; Abdelhamid, A.O. One pot synthesis of pyridine, thiazolidine, pyrazole and 2,3-dihydro-1,3,4-thiadiazole derivatives under solvent-free condition. Int. J. Adv. Res. 2013, 1, 717–728. [Google Scholar]
- Abdelhamid, A.O.; Gomha, S.M. Synthesis of new pyrazolo[1,5-a]pyrimidine, triazolo[4,3-a] pyrimidine derivatives and thieno[2,3-b]pyridine derivatives from sodium 3-(5-methyl-1-phenyl-1H-pyrazol-4-yl)-3-oxoprop-1-en-1-olate. J. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Gomha, S.M.; Shawali, S.A.; Abdelhamid, A.O. Convenient methods for synthesis of various fused heterocycles via utility of 4-acetyl-5-methyl-1-phenyl-pyrazole as precursor. Turk. J. Chem. 2014, 38, 865–879. [Google Scholar] [CrossRef]
- Pokhodylo, N.T.; Savka, R.D.; Matiichuk, V.S.; Obushak, N.D. Synthesis and selected transformations of 1-(5-methyl-1-aryl-1H-1,2,3-triazol-4-yl)ethanones and 1-[4-(4-R-5-methyl-1Н-1,2,3-triazol-1-yl)phenyl]ethanones. Zh. Obshch. Khim. 2009, 79, 320–325. [Google Scholar]
- Abdelhamid, A.O.; Zohdi, H.F.; Rateb, N.M. Reactions with hydrazonoyl halides XXI: Reinvestigation of the reactions of hydrazonoyl bromides with 1,1-dicyanothioacetanilide. J. Chem. Res. 1999, 184, 920. [Google Scholar]
- Butler, R.N. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: New York, NY, USA, 1996; Volume 4, pp. 621–678. [Google Scholar]
- Huisgen, R.; Grashey, R.; Seidel, M.; Knupfer, H.; Schmidt, R. 1.3-Dipolare additionen, III. Umsetzungen des diphenylnitrilimins mit carbonyl und thiocarbonyl-verbindungen. Justus Liebigs Annalen der Chemie 1962, 658, 169–180. [Google Scholar] [CrossRef]
- Yadav, R.C.; Sharma, P.K.; Singh, J. Synthesis and biological activity of 4''-substituted-2-(4'-formyl-3'-phenylpyrazole)-4-phenyl thiazole. J. Chem. Pharm. Res. 2013, 5, 78–84. [Google Scholar]
- Abdelhamid, A.O.; Abdel‐Riheem, N.A.; El‐Idreesy, T.T.; Rashdan, H.R.M. Synthesis of 5‐arylazothiazoles, pyridines and thieno[2,3‐b]pyridines derivatives containing 1,2,3‐triazole moiety. Eur. J. Chem. 2012, 3, 322–331. [Google Scholar] [CrossRef]
- Popsavin, M.; Spaić, S.; Svirčev, M.; Kojić, V.; Bogdanović, G.; Popsavin, V. Synthesis and in vitro antitumour screening of 2-(β-d-xylofuranosyl)thiazole-4-carboxamide and two novel tiazofurin analogues with substituted tetrahydrofurodioxol moiety as a sugar mimic. Bioorg. Med. Chem. Lett. 2012, 22, 6700–6704. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Kryshchyshyn, A.; Nektegayev, I.; Vasylenko, O.; Grellier, P.; Lesyk, R. Isothiocoumarin-3-carboxylic acid derivatives: Synthesis, anticancer and antitrypanosomal activity evaluation. Eur. J. Med. Chem. 2014, 75, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Opolski, A. Synthesis and antiproliferative activity of N-substituted 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Bioorg. Med. Chem. 2006, 14, 4483–4489. [Google Scholar] [CrossRef] [PubMed]
- Mavrova, T.; Wesselinova, D.; Tsenov, Y.A.; Denkova, P. Synthesis, cytotoxicity and effects of some 1,2,4-triazole and 1,3,4-thiadiazole derivatives on immunocompetent cells. Eur. J. Med. Chem. 2009, 44, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.M.; Zayed, M.E.M.; Ng, S.W. Ethyl (Z)-2-chloro-2-(2-phenylhydrazin-1-ylidene)acetate. Acta Cryst. 2011, 67, o1962. [Google Scholar]
- Eweiss, N.F.; Osman, A. Synthesis of heterocycles-2. New routes to acetylthiadiazolines and arylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Shawali, A.S.; Osman, A. Reaction of dimethylphenacylsulfonium bromide with N-nitrosoacetarylamides and reactions of the products with nucleophiles. Bull. Chem. Soc. Jpn. 1976, 49, 321–324. [Google Scholar] [CrossRef]
- Shawali, A.S.; Osman, A. Synthesis and reactions of phenyl carbamoyl-aryl hydrazidic chlorides. Tetrahedron 1971, 27, 2517–2528. [Google Scholar]
- Abdelhamid, A.O.; El-Shiatey, F.H.H. Reactions with hydrazonoyl halides II. Synthesis and reactions of 2-bromothienyl-2-phenylhydrazone. Phosphorus Sulfur Silicon Relat. Elem. 1988, 39, 45–49. [Google Scholar] [CrossRef]
- Hassaneen, H.M.; Shawali, A.S.; Elwan, N.M.; Abounada, N.M. Reaction of 1-(2-naphthoyl) methyl-2-dimethylsulfonium bromide with N-nitroso-N-arylacetamides and reactions of the products with some nucleophiles. Sulfur Lett. 1992, 13, 273–285. [Google Scholar]
- Wolkoff, P. A new method of preparing hydrazonyl halides. Can. J. Chem. 1975, 53, 1333–1335. [Google Scholar] [CrossRef]
- Gangadevi, V.; Muthumary, J. Preliminary studies on cytotoxic effect of fungal taxol on cancer cell lines. Afr. J. Biotechnol. 2007, 6, 1382–1386. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the synthesized compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomha, S.M.; Ahmed, S.A.; Abdelhamid, A.O. Synthesis and Cytotoxicity Evaluation of Some Novel Thiazoles, Thiadiazoles, and Pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones Incorporating Triazole Moiety. Molecules 2015, 20, 1357-1376. https://doi.org/10.3390/molecules20011357
Gomha SM, Ahmed SA, Abdelhamid AO. Synthesis and Cytotoxicity Evaluation of Some Novel Thiazoles, Thiadiazoles, and Pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones Incorporating Triazole Moiety. Molecules. 2015; 20(1):1357-1376. https://doi.org/10.3390/molecules20011357
Chicago/Turabian StyleGomha, Sobhi M., Sayed A. Ahmed, and Abdou O. Abdelhamid. 2015. "Synthesis and Cytotoxicity Evaluation of Some Novel Thiazoles, Thiadiazoles, and Pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones Incorporating Triazole Moiety" Molecules 20, no. 1: 1357-1376. https://doi.org/10.3390/molecules20011357
APA StyleGomha, S. M., Ahmed, S. A., & Abdelhamid, A. O. (2015). Synthesis and Cytotoxicity Evaluation of Some Novel Thiazoles, Thiadiazoles, and Pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-ones Incorporating Triazole Moiety. Molecules, 20(1), 1357-1376. https://doi.org/10.3390/molecules20011357







