Synthesis and in Vitro Screening of Phenylbipyridinylpyrazole Derivatives as Potential Antiproliferative Agents
Abstract
:1. Introduction
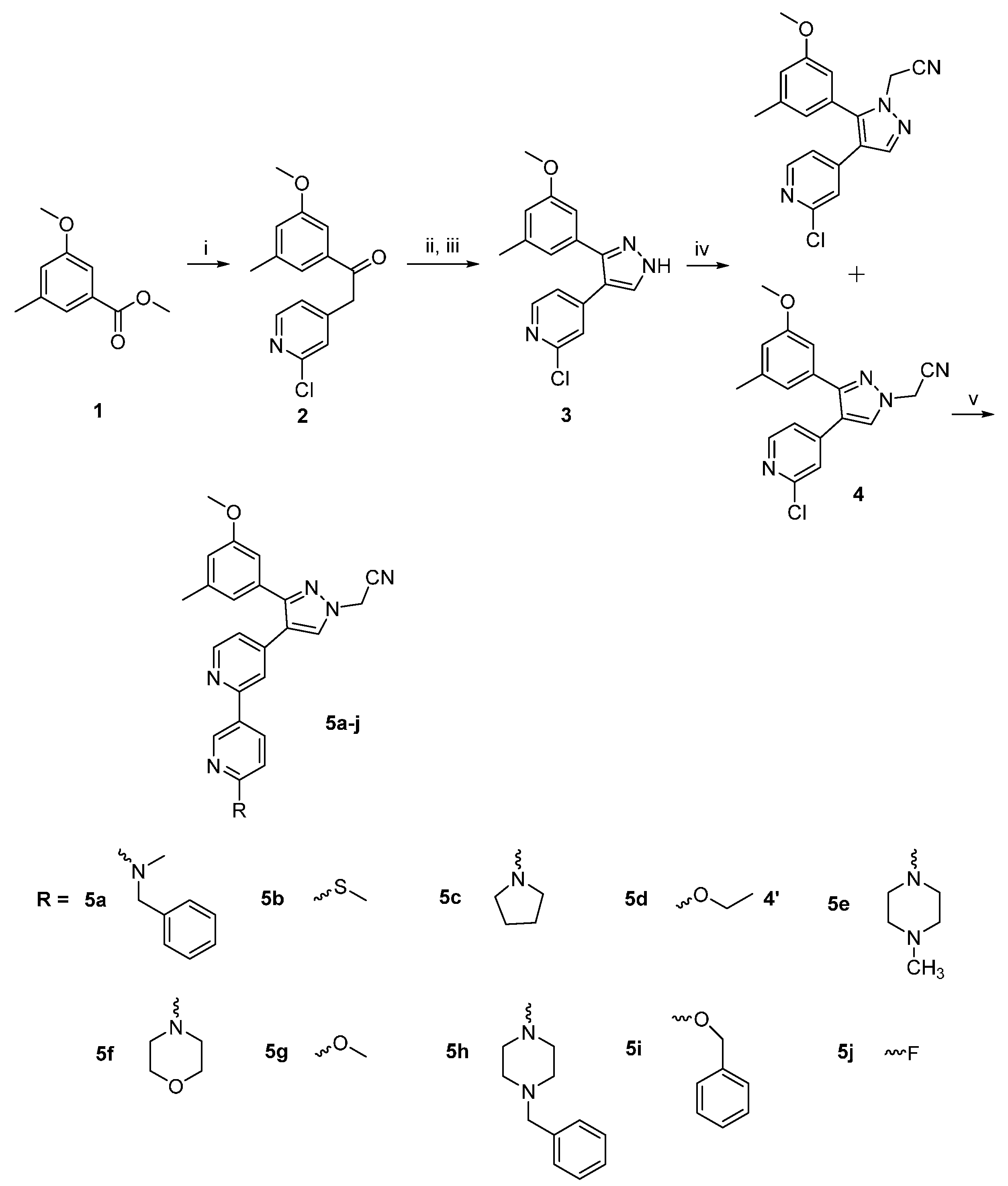
2. Results and Discussion
2.1. Chemistry
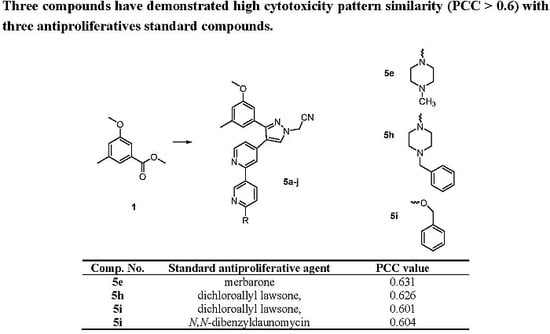
2.2. Biological Evaluation
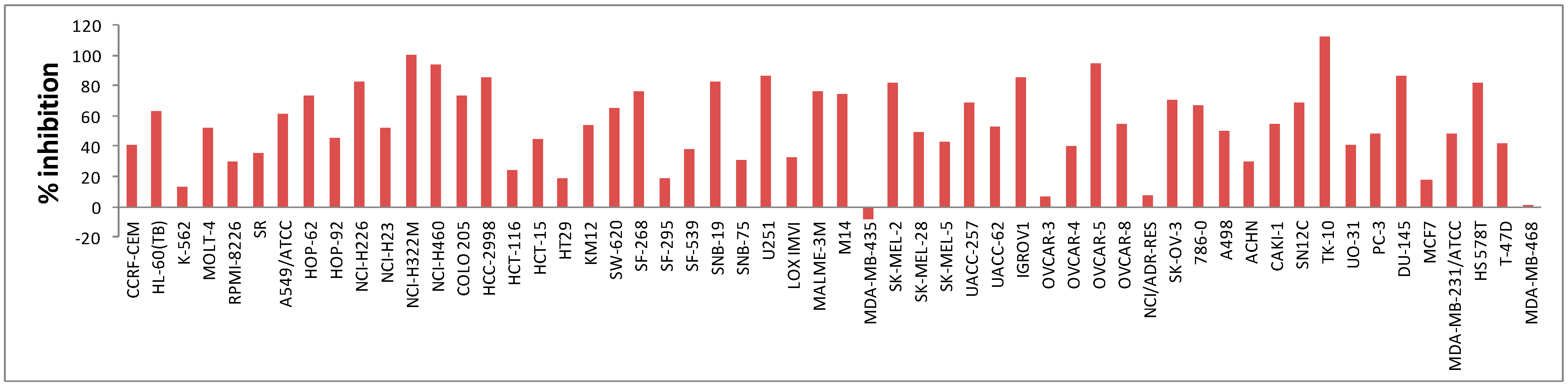
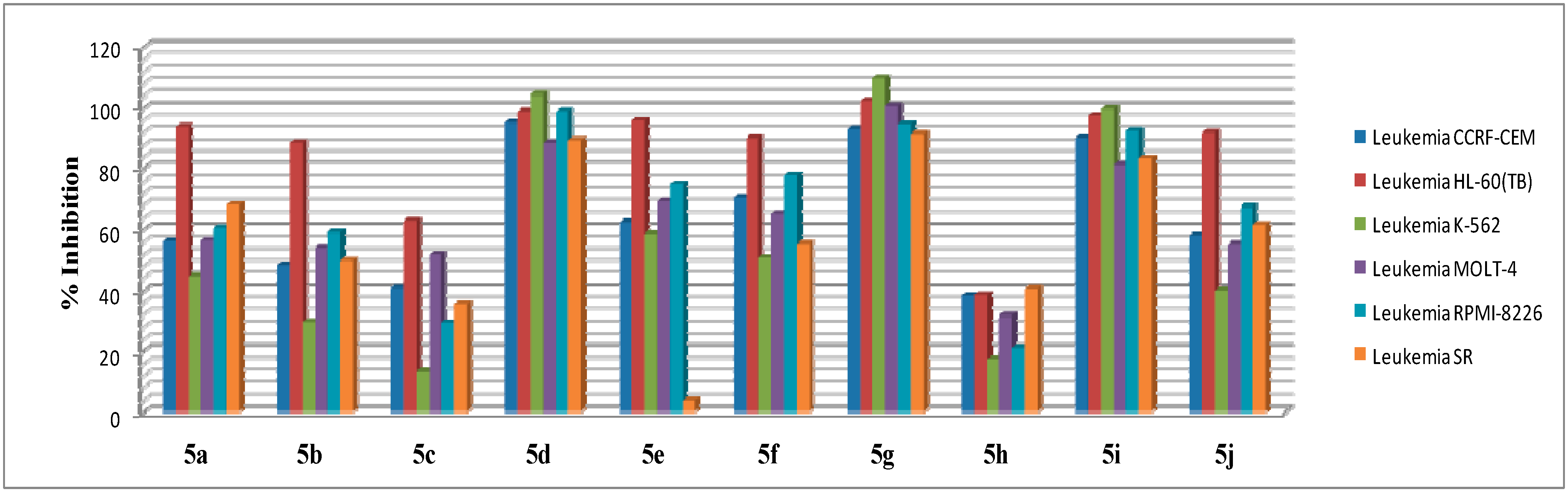
2.2.1. In Vitro Anticancer Screening
| Cell Lines | Percentage of Cell Growth a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | 5i | 5j | ||
| Leukemia | CCRF-CEM | 56.06 | 48.23 | 40.94 | 95.08 | 62.47 | 70.45 | 92.95 | 38.31 | 90.4 | 57.97 |
| HL-60(TB) | 93.71 | 88.74 | 63 | 98.85 | 95.75 | 90.3 | 102.6 | 38.58 | 97.47 | 91.84 | |
| K-562 | 44.4 | 29.76 | 13.4 | 104.4 | 58.46 | 50.75 | 109.7 | 17.62 | 99.8 | 40.04 | |
| MOLT-4 | 56.34 | 54.13 | 51.91 | 88.68 | 69.09 | 65.33 | 100.7 | 31.55 | 81.16 | 55.14 | |
| RPMI-8226 | 60.48 | 58.94 | 29.52 | 98.94 | 75.11 | 77.93 | 94.57 | 20.91 | 92.35 | 67.43 | |
| SR | 67.87 | 49.8 | 35.47 | 89.52 | 4.04 | 55.17 | 91.36 | 40.73 | 83.31 | 61.59 | |
| NSCLC | A549/ATCC | 87.9 | 91.94 | 61.47 | 85.83 | 102.7 | 87.01 | 86.18 | 60.74 | 88.37 | 74.32 |
| HOP-62 | 107.0 | 95.83 | 73.86 | 85.9 | 104.2 | 88.22 | 99.97 | 110.8 | 87.28 | 110.1 | |
| HOP-92 | 69.97 | 43.6 | 45.34 | 49.16 | 44.52 | 46.04 | 52.65 | 52.61 | 60.87 | 84.65 | |
| NCI-H226 | 88.11 | 74.6 | 83.09 | 87.54 | 92.64 | 88.58 | 86.04 | 74.88 | 92.03 | 81.26 | |
| NCI-H23 | 85.56 | 83.56 | 51.85 | 99.59 | 94.98 | 92.09 | 96.39 | 72.15 | 92.21 | 86.9 | |
| NCI-H322M | 91.42 | 77.33 | 100.2 | 113.6 | 89.82 | 85.69 | 101.0 | 95.42 | 93.24 | 97.23 | |
| NCI-H460 | 94.42 | 98.84 | 94.09 | 102.9 | 102.4 | 101.6 | 96.78 | 57.17 | 98.8 | 94.88 | |
| Colon Cancer | COLO 205 | 82.6 | 76.8 | 73.72 | 96.46 | 79.75 | 100.9 | 100.4 | 41.02 | 102.3 | 82.76 |
| HCC-2998 | 88.15 | 84.24 | 85.57 | 98.48 | 100.0 | 99.21 | 106.2 | 67.09 | 99.95 | 92.82 | |
| HCT-116 | 67.45 | 60.72 | 24.5 | 91.03 | 61.7 | 86.09 | 103.9 | 43.2 | 85.8 | 69.84 | |
| HCT-15 | 57.32 | 52.74 | 44.88 | 94.81 | 70.42 | 68.16 | 93.73 | 24.6 | 88.06 | 53.49 | |
| HT29 | 37.1 | 48.01 | 19.08 | 104.3 | 51.26 | 77.25 | 94.72 | 11.94 | 92.24 | 37.66 | |
| KM12 | 86.66 | 54.28 | 54.32 | 104.7 | 95.36 | 79.26 | 102.7 | 43.97 | 100.5 | 87.17 | |
| SW-620 | 92.45 | 76.97 | 65.02 | 102.5 | 82.84 | 94.36 | 92.91 | 60.55 | 97.48 | 77.97 | |
| CNS Cancer | SF-268 | 100.2 | 98.85 | 76.61 | 100.6 | 104.1 | 94.35 | 96.35 | 76.17 | 90.2 | 96.09 |
| SF-295 | 89.92 | 84.01 | 18.24 | 88.62 | 95.43 | 56.53 | 95.6 | 63.38 | 97.89 | 90.11 | |
| SF-539 | 95.97 | 78.11 | 37.87 | 95.06 | 88.77 | 79.27 | 96.35 | 30.21 | 92.94 | 88.07 | |
| SNB-19 | 92.1 | 85.25 | 82.27 | 91.29 | 89.56 | 90.84 | 92.17 | 79.89 | 94.61 | 88.35 | |
| SNB-75 | 95.38 | 44.53 | 30.49 | 66.83 | 55.01 | 63.06 | 76.9 | 65.89 | 73.17 | 85.68 | |
| U251 | 94.47 | 91.11 | 86.66 | 93.01 | 95.17 | 96.88 | 93.53 | 68.64 | 93.18 | 88.99 | |
| Melanoma | LOX IMVI | 69.37 | 61.68 | 32.53 | 97.91 | 89.63 | 65.72 | 97.2 | 32.95 | 91.89 | 67.44 |
| MALME-3M | 107.5 | 77.19 | 76.41 | 102.9 | 93.51 | 92.92 | 104.1 | 79.55 | 97.85 | 104.28 | |
| M14 | 90.13 | 92 | 74.58 | 93.6 | 107.8 | 98.58 | 104.3 | 64.79 | 120.8 | 100.25 | |
| MDA-MB-435 | 96.21 | 46.59 | −8.52 | 96.15 | 96.69 | 59.14 | 102.0 | 68.22 | 106.4 | 95.83 | |
| SK-MEL-2 | 105.3 | 117.9 | 82.05 | 121.0 | 123.4 | 96.91 | 101.4 | 83.3 | 120.9 | 112.4 | |
| SK-MEL-28 | 101.5 | 90.11 | 48.94 | 94.86 | 97.45 | 86.64 | 108.0 | 69.36 | 102.6 | 107.17 | |
| SK-MEL-5 | 93.55 | 77.97 | 42.91 | 97.01 | 81.82 | 73.07 | 96.02 | 70.71 | 100.4 | 93.82 | |
| UACC-257 | 107.7 | 114.0 | 69.05 | 97.1 | 114.1 | 95.97 | 105.4 | 83.45 | 102.2 | 105.51 | |
| UACC-62 | 73.42 | 72.26 | 52.73 | 87.66 | 94.86 | 80.73 | 87.72 | 65.51 | 85.59 | 87.91 | |
| IGROV1 | 109.7 | 69.24 | 85.16 | 110.2 | 105.7 | 93.48 | 95.63 | 87.36 | 101.5 | 96.37 | |
| Ovarian Cancer | OVCAR-3 | 98.29 | 77.61 | 6.29 | 104.3 | 97.5 | 92.24 | 105.0 | 55.41 | 94.35 | 91.6 |
| OVCAR-4 | 87.46 | 63.79 | 39.58 | 90.49 | 82.78 | 81.73 | 100.8 | 60.1 | 90.31 | 90.57 | |
| OVCAR-5 | 91.13 | 72.62 | 94.75 | 87.78 | 91.08 | 90.84 | 84.93 | 77.7 | 74.9 | 91.76 | |
| OVCAR-8 | 81.22 | 84.54 | 55.05 | 89.33 | 92.19 | 84.12 | 88.68 | 63.03 | 89.73 | 79.8 | |
| NCI/ADR-RES | 83.72 | 77.68 | 7.25 | 92.72 | 91.54 | 66.54 | 95.56 | 60.92 | 84.09 | 75.16 | |
| SK-OV-3 | 98.39 | 91.79 | 70.56 | 95.22 | 106.3 | 101.1 | 104.1 | 81.49 | 98.24 | 92.76 | |
| Renal Cancer | 786-0 | 91.57 | 76.97 | 67.03 | 91.19 | 81.84 | 86.41 | 98.75 | 67.64 | 92 | 83.91 |
| A498 | 91.0 | 110.2 | 50.46 | 103.4 | 80.4 | 93.82 | 97.71 | 87.81 | 87.49 | 98.66 | |
| ACHN | 82.25 | 63.48 | 29.86 | 87.6 | 81.27 | 55.86 | 91.97 | 51.64 | 75.87 | 95.16 | |
| CAKI-1 | 82.27 | 72.03 | 54.56 | 83.76 | 90.18 | 84.89 | 87.76 | 61.49 | 86.4 | 81.52 | |
| SN12C | 78.84 | 72.33 | 68.88 | 90.66 | 78.88 | 85.35 | 90.12 | 51.19 | 87.72 | 79.58 | |
| TK-10 | 109.8 | 100.5 | 112.1 | 107.4 | 113.3 | 115.5 | 96 | 90.95 | 86.43 | 111.5 | |
| UO-31 | 91.95 | 61.17 | 41.05 | 82.51 | 67.72 | 71.4 | 70.5 | 57.74 | 69.35 | 80.58 | |
| Prostate Cancer | PC-3 | 61.14 | 65.35 | 48.35 | 85.21 | 87.85 | 67.24 | 83.77 | 34.01 | 86.85 | 69.44 |
| DU-145 | 93.7 | 84.57 | 86.53 | 112.5 | 90.3 | 96.15 | 109.2 | 66.92 | 111.1 | 93.49 | |
| Breast Cancer | MCF7 | 76.85 | 26.85 | 18.15 | 98.85 | 76.91 | 43.18 | 102.5 | 28.28 | 89.1 | 63.72 |
| MDA-MB-231/ATCC | 60.44 | 52.64 | 48 | 89.4 | 79.16 | 69.26 | 80.57 | 31.94 | 77.26 | 47.6 | |
| HS 578T | 84.41 | 81.85 | 82.21 | 109.4 | 91.78 | 89.77 | 85.33 | 65.96 | 82.47 | 79.81 | |
| T-47D | 57.58 | 26.9 | 42.13 | 70.07 | 72.63 | 72.37 | 80.58 | 28.42 | 68.23 | 40.82 | |
| MDA-MB-468 | 68.1 | 19.33 | 1.31 | 87.94 | 63.44 | 76.26 | 86.7 | 40.28 | 69.27 | 61.8 | |
| Cell Lines | 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | 5i | 5j |
|---|---|---|---|---|---|---|---|---|---|---|
| K-562 | - a | 29.76 | 13.4 | - | - | - | - | 17.62 | - | - |
| SR | - | - | 35.47 | - | 4.04 | - | - | - | - | - |
| HT29 | 37.1 | - | 19.08 | - | - | - | - | 11.94 | - | 37.66 |
| MDA-MB-435 | - | - | −8.52 | - | - | - | - | - | - | - |
| OVCAR-3 | - | - | 6.29 | - | - | - | - | - | - | - |
| NCI/ADR-RES | - | - | 7.25 | - | - | - | - | - | - | - |
| MCF7 | - | 26.85 | 18.15 | - | - | - | - | 28.28 | - | - |
| MDA-MB-468 | - | 19.33 | 1.31 | - | - | - | - | - | - | - |
| Mean % growth | 84 | 72 | 53 | 94 | 85 | 81 | 94 | 58 | 82 | 91 |
2.2.2. Topoisomerase IIα Inhibitory Activity
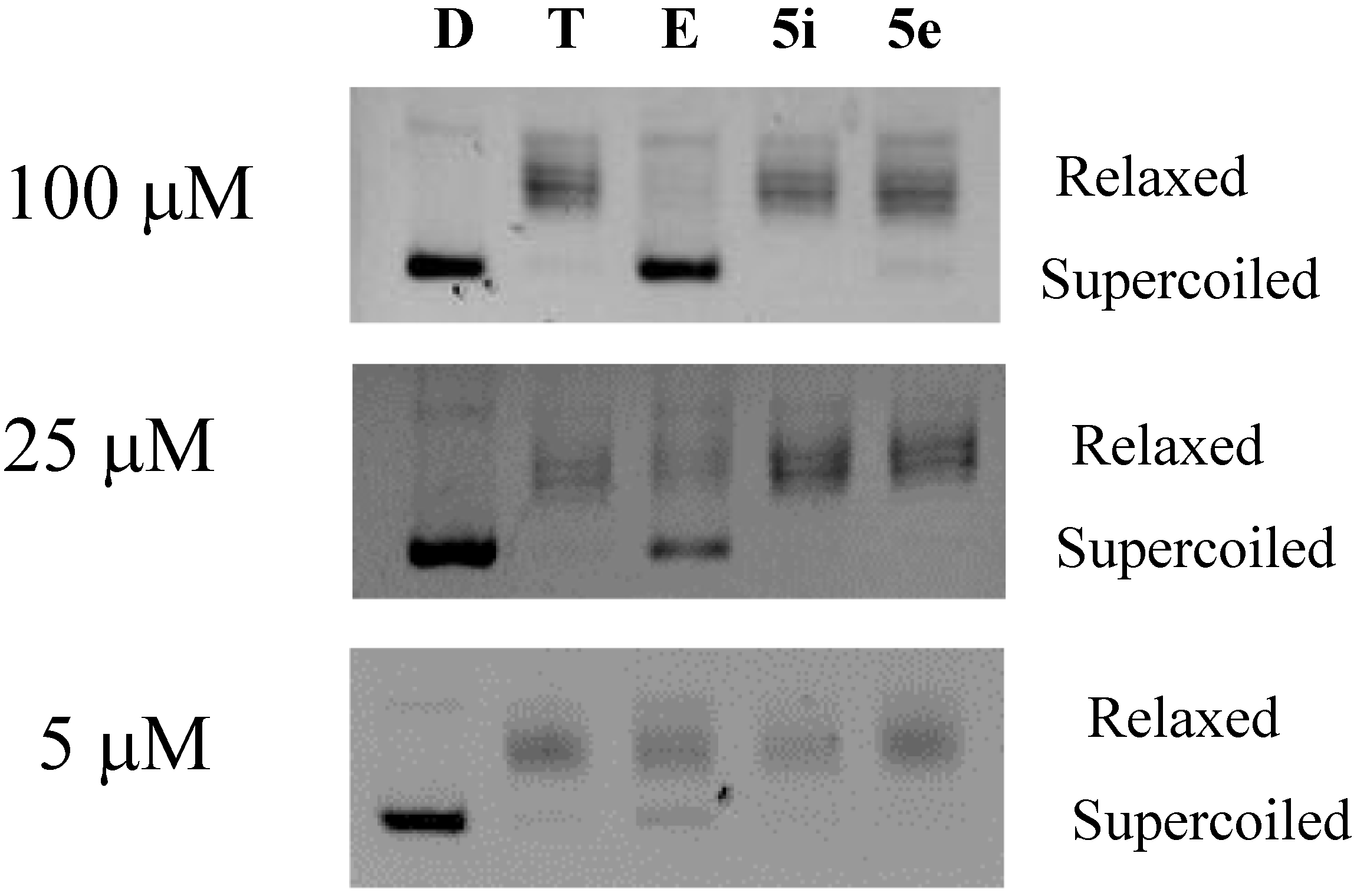
2.3. Assessment of Toxicity Profiles and Lipinski’s Rule of Five
| Comp No. | TPSA a | MW b | nHBA c | nHBD d | V e | RB f | Volume g | Clog P h | Toxicity i |
|---|---|---|---|---|---|---|---|---|---|
| 5a | 85.87 | 425.492 | 7 | 0 | 0 | 7 | 389.283 | 3.34 | -j |
| 5b | 83.112 | 479.588 | 8 | 0 | 0 | 6 | 445.429 | 2.78 | - |
| 5c | 89.108 | 466.545 | 8 | 0 | 0 | 6 | 425.069 | 2.68 | - |
| 5d | 85.87 | 411.465 | 7 | 0 | 0 | 6 | 372.481 | 2.94 | - |
| 5e | 83.112 | 555.686 | 8 | 0 | 2 | 8 | 517.079 | 4.2 | - |
| 5f | 76.636 | 427.533 | 6 | 0 | 0 | 6 | 381.625 | 3.23 | - |
| 5g | 79.874 | 450.546 | 7 | 0 | 0 | 6 | 416.084 | 3.5 | - |
| 5h | 79.874 | 500.606 | 7 | 0 | 2 | 8 | 464.491 | 4.32 | - |
| 5i | 76.636 | 399.429 | 6 | 0 | 0 | 5 | 351.867 | 3.11 | - |
| 5j | 85.87 | 487.563 | 7 | 0 | 1 | 8 | 444.13 | 4.35 | - |
3. Experimental Section
3.1. Chemistry
3.1.1. General
3.1.2. General Procedure for Synthesis of Compounds 5a–j
3.2. In Vitro Anticancer Screening
- [(Ti − Tz)/(C − Tz)] × 100 for concentrations for which Ti ≥ Tz.
- [(Ti − Tz)/Tz] × 100 for concentrations for which Ti < Tz.
3.3. DNA Topoisomerase IIα Inhibition Screening in Vitro
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA:Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.L.; Smithers, D.; Rose, L.M.; Adamson, D.J.; Thomas, H.J. Inhibition of synthesis of pyrimidine nucleotides by 2-hydroxy-3-(3,3-dichloroallyl)-1,4-naphthoquinone. Cancer Res. 1979, 39, 4868–4874. [Google Scholar] [PubMed]
- Wang, L.; Eastmond, D.A. Catalytic inhibitors of topoisomerase ii are DNA-damaging agents: Induction of chromosomal damage by merbarone and icrf-187. Environ. Mol. Mutagen. 2002, 39, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Lange, J.; Kett, A.; Dornhofer, N.; Meinert, R.; Arand, M.; Knapstein, P.G.; Becker, R.; Oesch, F.; Tanner, B. Contribution of c-erbb-2 and topoisomerase ii alpha to chemoresistance in ovarian cancer. Cancer Res. 1999, 59, 3206–3214. [Google Scholar] [PubMed]
- Larsen, A.K.; Eseargueil, A.E.; Skladanowski, A. Catalytic topoisomerase ii inhibitors in cancer therapy. Pharmacol. Ther. 2003, 99, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Skouboe, C.; Bjergbaek, L.; Oestergaard, V.H.; Larsen, M.K.; Knudsen, B.R.; Andersen, A.H. A human topoisomerase ii alpha heterodimer with only one atp binding site can go through successive catalytic cycles. J. Biol. Chem. 2003, 278, 5768–5774. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Diversity of DNA topoisomerases i and inhibitors. Biochimie 1998, 80, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Kellner, U.; Rudolph, P.; Parwaresch, R. Human DNA-topoisomerases—Diagnostic and therapeutic implications for cancer. Onkologie 2000, 23, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Lee, K.; Park, S.-J.; Ahn, B.; Lee, J.-C.; Cho, H.; Lee, K.-I. Identification of antitumor activity of pyrazole oxime ethers. Bioorg. Med. Chem. Lett. 2005, 15, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, P.; Liu, J.; Lin, Y.; Yao, H.; Jiang, J.; Ye, W.; Wu, X.; Xu, J. Synthesis, in vitro and in vivo antitumor activity of pyrazole-fused 23-hydroxybetulinic acid derivatives. Bioorg. Med. Chem. Lett. 2014. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Martorana, A.; Gia, O.; DallaVia, L.; Cirrincione, G. 3,5-Bis(3'-indolyl)pyrazoles, analogues of marine alkaloid nortopsentin: Synthesis and antitumor properties. Bioorg. Med. Chem. Lett. 2007, 17, 6134–6137. [Google Scholar] [CrossRef]
- Barraja, P.; Spanò, V.; Giallombardo, D.; Diana, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of [1,2]oxazolo[5,4-e] indazoles as antitumour agents. Tetrahedron 2013, 69, 6474–6477. [Google Scholar] [CrossRef]
- El-Deeb, I.M.; Lee, S.H. Design and synthesis of new potent anticancer pyrazoles with high flt3 kinase inhibitory selectivity. Bioorg. Med. Chem. 2010, 18, 3961–3973. [Google Scholar] [CrossRef]
- El-Deeb, I.M.; Park, B.S.; Jung, S.J.; Yoo, K.H.; Oh, C.H.; Cho, S.J.; Han, D.K.; Lee, J.Y.; Lee, S.H. Design, synthesis, screening, and molecular modeling study of a new series of ros1 receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 5622–5626. [Google Scholar] [PubMed]
- Park, B.S.; El-Deeb, I.M.; Yoo, K.H.; Oh, C.H.; Cho, S.J.; Han, D.K.; Lee, H.S.; Lee, J.Y.; Lee, S.H. Design, synthesis and biological evaluation of new potent and highly selective ros1-tyrosine kinase inhibitor. Bioorg. Med. Chem. Lett. 2009, 19, 4720–4723. [Google Scholar] [CrossRef] [PubMed]
- Al-Sanea, M.M.; El-Deeb, I.M.; Lee, S.H. Design, synthesis and in vitro screening of new 1H-pyrazole and 1,2-isoxazole derivatives as potential inhibitors for ROS and MAPK14 kinases. Bull. Korean Chem. Soc. 2013, 34, 437–442. [Google Scholar] [CrossRef]
- Zaharevitz, D.W.; Holbeck, S.L.; Bowerman, C.; Svetlik, P.A. Compare: A web accessible tool for investigating mechanisms of cell growth inhibition. J. Mol. Graph. Model. 2002, 20, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Paull, K.D.; Shoemaker, R.H.; Hodes, L.; Monks, A.; Scudiero, D.A.; Rubinstein, L.; Plowman, J.; Boyd, M.R. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: Development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst. 1989, 81, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E.; Pickett, S.D. Computational methods for the prediction of “drug-likeness”. Drug Discov. Today 2000, 5, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Thapa, P.; Yoo, H.Y.; Kadayat, T.M.; Park, P.H.; Na, Y.; Lee, E.; Jeon, K.H.; Cho, W.J.; Choi, H.; et al. Dihydroxylated 2,4,6-triphenyl pyridines: Synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship study. Eur. J. Med. Chem. 2012, 49, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sanea, M.M.; Elkamhawy, A.; Zakaria, A.; Park, B.S.; Kwon, Y.; Lee, S.H.; Lee, S.W.; Kim, I.T. Synthesis and in Vitro Screening of Phenylbipyridinylpyrazole Derivatives as Potential Antiproliferative Agents. Molecules 2015, 20, 1031-1045. https://doi.org/10.3390/molecules20011031
Al-Sanea MM, Elkamhawy A, Zakaria A, Park BS, Kwon Y, Lee SH, Lee SW, Kim IT. Synthesis and in Vitro Screening of Phenylbipyridinylpyrazole Derivatives as Potential Antiproliferative Agents. Molecules. 2015; 20(1):1031-1045. https://doi.org/10.3390/molecules20011031
Chicago/Turabian StyleAl-Sanea, Mohammad M., Ahmed Elkamhawy, Ahmed Zakaria, Byung Sun Park, Youngjoo Kwon, So Ha Lee, Sang Woo Lee, and In Tae Kim. 2015. "Synthesis and in Vitro Screening of Phenylbipyridinylpyrazole Derivatives as Potential Antiproliferative Agents" Molecules 20, no. 1: 1031-1045. https://doi.org/10.3390/molecules20011031
APA StyleAl-Sanea, M. M., Elkamhawy, A., Zakaria, A., Park, B. S., Kwon, Y., Lee, S. H., Lee, S. W., & Kim, I. T. (2015). Synthesis and in Vitro Screening of Phenylbipyridinylpyrazole Derivatives as Potential Antiproliferative Agents. Molecules, 20(1), 1031-1045. https://doi.org/10.3390/molecules20011031