Improved Method for the Synthesis of New 1,5-Benzothiazepine Derivatives as Analogues of Anticancer Drugs
Abstract
:Introduction
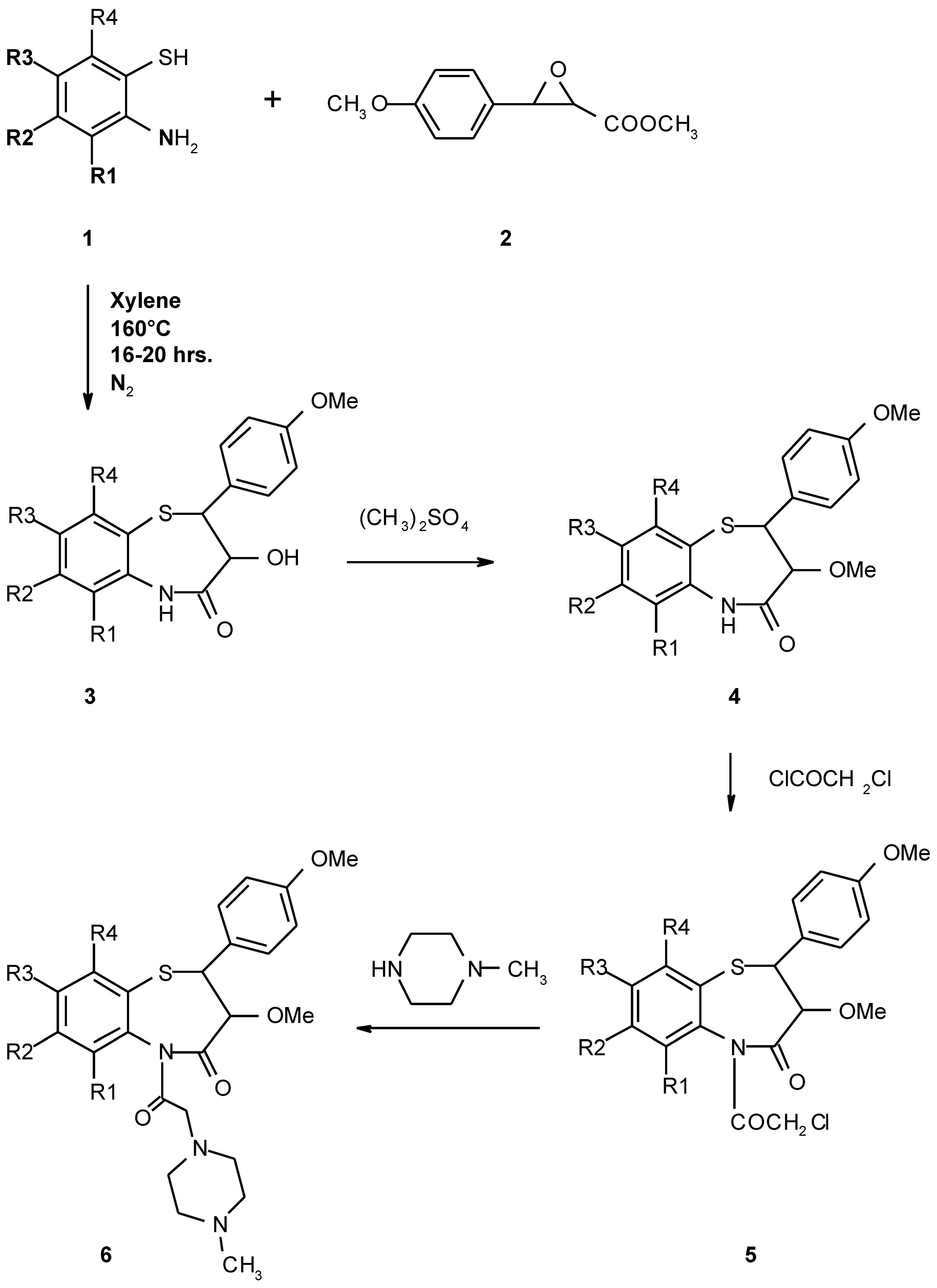
Results and Discussion
Synthesis
Antimicrobial activity
Experimental
General
(±)-cis-2-(4-Methoxyphenyl)-3-hydroxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one (3)
(±)-cis-2-(4-Methoxyphenyl)-3-methoxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one (4)
(±)-cis-2-(4-Methoxyphenyl)-3-methoxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4(5-chloroacetyl)-one (5)
(±)-cis-2-(4-Methoxyphenyl)-3-methoxy-8-phenoxy/-7-chloro-8-methyl/-9-chloro-6-methoxy-2,3-dihydro-1,5-benzothiazepin-4[5-(4'-methylpiperazino-1')acetyl]-one (6)
Acknowledgmentt
References
- Salim, H. A.; Abdul, R.; Mohamed, S.; Dahab, G. M. J. Appl. Toxicol. 1993, 13(2), 85, Chem. abstr. 1993, 118, 204993.
- Lochead, A.; Muller, J. C.; Hoornaert, C.; Denys, C. Eur. Pat. Appl. EP320,362; (ClA61 K31/55), 14 Jun 1989. FR Appl 87/17, 044, 08 Dec 1987. Chem Abstr. 1990, 112, 172330.
- Ben, H.; Ruben, S.; Melissa, S.; Kenneth, M. Life Sci. 1992, 51(26), 2049, Chem. Abstr. 1993, 118, 52185.
- Miranda, H. F.; Pelissier, T.; Sierralta, F. Gen. Pharmacol. 1993, 24(1), 201, Chem. Abstr. 1993, 118, 22558. [PubMed]
- Hiroki, Y.; Koichi, F.; Yasuo, S.; Takuro, K.; Hiroyuki, K.; Hiroshi, N. Eur. Pat. Appl. EP 353, 032; (ClCO7D281/10), 31 Jan 1990. JP Appl. 88/185, 097, 25 Jul 1988. Chem. Abstr. 1991, 114, 122434.
- Ahmed, N. K. Can. Pat. Appl. CA 2,030, 159; (Cl.A61k31/55), 23 May 1991. US Appl. 441, 083, 22 Nov. 1989.
- Ahmed, N. K. Eur. Pat. Appl. EP430, 036; (ClA61k31/55), 05 Jun 1991. US Appl. 440, 121, 22 Nov 1989. Chem. Abstr. 1992, 116, 717.
- Bauer, A. W.; Kibby, W. M. M.; Sherris, J. C.; Turk, M. Am. J. Clin. Path. 1966, 45(4), 493. [PubMed]
- Samples Availability: Available from the authors.
| Compd. No. | R1 | R2 | R3 | R4 | Molecular formula | Molecula Wt.r | MP °C | Yield % | Elemental Analysis % Calcd. (found) | ||
| C | H | N | |||||||||
| 3a | H | H | OC6H5 | H | C22H19NO4S | 393 | 268-270 | 68 | 67.18 | 4.83 | 3.56 |
| (67.21) | (4.87) | (3.51) | |||||||||
| 3b | H | Cl | CH3 | H | C17H16ClNO3S | 349.5 | 242 | 65 | 58.37 | 4.58 | 4.01 |
| (58.32) | (4.54) | (3.98) | |||||||||
| 3c | OCH3 | H | H | Cl | C17H16ClNO4S | 365.5 | 280 | 71 | 55.81 | 4.38 | 3.83 |
| (55.82) | (4.41) | (3.79) | |||||||||
| 4a | H | H | OC6H5 | H | C23H21NO4S | 407 | 215-217 | 62 | 67.81 | 5.16 | 3.44 |
| (67.76) | (5.12) | (3.41) | |||||||||
| 4b | H | Cl | CH3 | H | C18H18ClNO3S | 363.5 | 220 | 65 | 59.42 | 4.95 | 3.85 |
| (58.38) | (4.90) | (3.81) | |||||||||
| 4c | OCH3 | H | H | Cl | C18H18ClNO4S | 379.5 | 236 | 63 | 56.92 | 4.74 | 3.69 |
| (56.89) | (4.71) | (3.66) | |||||||||
| 5a | H | H | OC6H5 | H | C25H22ClNO5S | 483.5 | 277-279 | 65 | 62.05 | 4.55 | 2.90 |
| (62.01) | (4.51) | (2.89) | |||||||||
| 5b | H | Cl | CH3 | H | C20H19Cl2NO4S | 440 | 265-267 | 66 | 54.55 | 4.32 | 3.18 |
| (54.59) | (4.34) | (3.13) | |||||||||
| 5c | OCH3 | H | H | Cl | C20H19Cl2NO5S | 456 | 253-55 | 60 | 52.63 | 4.17 | 3.07 |
| (52.67) | (4.20) | (3.02) | |||||||||
| 6a | H | H | O C6H5 | H | C30H33N3O5S | 547 | 291-93 | 65 | 65.81 | 6.03 | 7.68 |
| (65.79) | (6.00) | (7.64) | |||||||||
| 6b | H | Cl | CH3 | H | C25H30ClN3O4S | 503.5 | 295 | 62 | 59.58 | 5.96 | 8.34 |
| (59.52) | (5.93) | (8.32) | |||||||||
| 6c | OCH3 | H | H | Cl | C25H30ClN3O5S | 519.5 | 301 | 71 | 59.75 | 5.78 | 8.08 |
| (59.71) | (5.75) | (8.03) | |||||||||
| Comopd. | NH/OH | C-H str. | C-H str. | C-H | C-Cl | |||
| No. | (Aliphatic) | (Aromatic) | C=C | C=O | C-O-C | (Cyclic) | ||
| 3a | 3600-3150(b) | 2880(w) | 3090(w) | 1580(ms) | 1620(s) | 1345(s) | 1450(m) | - |
| 1020(m) | ||||||||
| 3b | 3580-3110(b) | 2850(w) | 3100(w) | 1600(ms) | 1600(s) | 1350(s) | 1420(m) | 780(m) |
| 1050(m) | ||||||||
| 3c | 3550-3200(b) | 2900(w) | 3050(w) | 1560(ms) | 1610(s) | 1360(s) | 1445(m) | 740(m) |
| 1070(m) | ||||||||
| 4a | 3150(s) | 2920(m) | 3000(w) | 1580(ms) | 1635(s) | 1355(s) | 1410(m) | - |
| 1050(m) | ||||||||
| 4b | 3180(s) | 2850(m) | 3080(w) | 1560(ms) | 1640(s) | 1350(s) | 1440(m) | 780(m) |
| 1030(m) | ||||||||
| 4c | 3140(s) | 2885(m) | 3010(w) | 1600(ms) | 1620(s) | 1355(s) | 1450(m) | 760(m) |
| 1065(m) | ||||||||
| 5a | - | 2880(m) | 3010(w) | 1580(ms) | 1650(s) | 1360(s) | 1420(m) | 740(m) |
| 1060(m) | ||||||||
| 5b | - | 2910(m) | 3100(w) | 1585(ms) | 1660(s) | 1345(s) | 1430(m) | 770(m) |
| 1020(m) | ||||||||
| 5c | - | 2875(m) | 3080(w) | 1600(ms) | 1658(s) | 1355(s) | 1450(m) | 780(m) |
| 1035(m) | ||||||||
| 6a | - | 2850(m) | 3090(w) | 1550(ms) | 1650(s) | 1340(s) | 1440(m) | - |
| 1045(m) | ||||||||
| 6b | - | 2900(m) | 3080(w) | 1540(ms) | 1648(s) | 1360(s) | 1450(m) | 780(m) |
| 1025(m) | ||||||||
| 6c | - | 2845(m) | 3010(w) | 1510(ms) | 1655(s) | 1345(s) | 1420(m) | 750(m) |
| 1035(m) |
| Compound | Ar-H | N-H | C2-H | C3-H | CH2 | OCH3 | CH3 |
| No. | (m) | (d,J=8Hz,lH) | (d,J=8Hz,lH) | (s,2H) | (s,3H) | ||
| 3a | 6.20-7.90 | 8.10 | 2.80 | 3.36 | - | 3.54 (s,3H) | - |
| 3b | 6.10-7.50 | 8.25 | 2.92 | 3.44 | - | 3.50 (s,3H) | 1.96 |
| 3c | 6.00-7.60 | 8.15 | 2.84 | 3.20 | - | 3.52 (s,3H) | - |
| 4a | 6.10-7.90 | 8.10 | 2.82 | 3.24 | - | 3.48, 3.32(d,6H) | - |
| 4b | 6.20-7.50 | 8.20 | 2.74 | 3.10 | - | 3.58, 3.45(d,6H) | 1.76 |
| 4c | 6.05-7.85 | 8.25 | 2.76 | 3.05 | - | 3.44, 3.30(d,9H) | - |
| 5a | 6.00-7.45 | - | 2.66 | 3.15 | 4.40 | 3.50, 3.44(d6H) | - |
| 5b | 6.20-7.90 | - | 2.78 | 3.25 | 4.35 | 3.48, 3.36(d,6H) | 1.65 |
| 5c | 6.10-7.60 | - | 2.68 | 3.30 | 4.50 | 3.55, 3.48(d,9H) | - |
| 6a | 6.00-7.38 | - | 2.45 | 3.18 | 4.30 | 3.58, 3.40(d,6H) | 2.30 |
| 6b | 6.05-7.50 | - | 2.35 | 3.30 | 4.25 | 3.56, 3.46(d,6H) | 1.82 |
| 6c | 6.10-7.40 | - | 2.45 | 3.15 | 4.15 | 3.54, 3.26(d,9H) | 2.10 |
| Compound No. | E. coli | S. aureus | A. flavus | A. niger | C. lunata | F. moniliformae |
| 3a | 10.9 | 10.1 | 9.8 | 10.0 | 9.3 | 10.5 |
| (0.99) | (1.01) | (0.96) | (0.95) | (1.03) | (0.94) | |
| 3b | 10 | 7.0 | 8.2 | 8.9 | 8.2 | 10.0 |
| (0.90) | (0.70) | (0.80) | (0.85) | (0.91) | (0.89) | |
| 3c | 11 | 8.9 | 8.9 | 10.2 | 9.1 | 9.8 |
| (1.00) | (0.89) | (0.87) | (0.97) | (1.07) | (0.88) | |
| 4a | 10.5 | 10.2 | 10.1 | 10.1 | 8.5 | 8.8 |
| (0.95) | (1.02) | (0.99) | (0.96) | (0.94) | (0.79) | |
| 4b | 8.8 | 7.9 | 10.0 | 9.9 | 7.8 | 9.2 |
| (0.80) | (0.79) | (0.98) | (0.94) | (0.87) | (0.82) | |
| 4c | 10.8 | 9.8 | 10.1 | 10.1 | 7.8 | 10.0 |
| (0.98) | (0.98) | (0.99) | (0.96) | (0.87) | (0.89) | |
| 5a | 11.2 | 10.4 | 10.2 | 10.7 | 9.0 | 10.5 |
| (1.02) | (1.04) | (1.00) | (1.02) | (1.00) | (0.94) | |
| 5b | 10.0 | 10.4 | 10.2 | 10.0 | 8.7 | 9.8 |
| (0.91) | (1.04) | (1.00) | (0.95) | (0.99) | (0.88) | |
| 5c | 10.9 | 10.6 | 10.4 | 10.5 | 9.1 | 11.0 |
| (0.99) | (1.06) | (1.02) | (1.00) | (1.01) | (0.98) | |
| 6a | 11.0 | 10.5 | 10.9 | 10.9 | 9.5 | 11.0 |
| (1.00) | (1.05) | (1.07) | (1.04) | (1.06) | (0.98) | |
| 6b | 9.7 | 7.9 | 9.8 | 10.2 | 8.8 | 11.2 |
| (0.88) | (0.79) | (0.96) | (0.97) | (0.98) | (1.00) | |
| 6c | 10.8 | 10.4 | 10.2 | 10.4 | 9.0 | 11.8 |
| (0.98) | (1.04) | (1.00) | (0.99) | (1.80) | (1.05) |
© 1997 MDPI. All rights reserved
Share and Cite
Sharma, A.K.; Singh, G.; Yadav, A.K.; Prakash, L. Improved Method for the Synthesis of New 1,5-Benzothiazepine Derivatives as Analogues of Anticancer Drugs. Molecules 1997, 2, 129-134. https://doi.org/10.3390/20900129
Sharma AK, Singh G, Yadav AK, Prakash L. Improved Method for the Synthesis of New 1,5-Benzothiazepine Derivatives as Analogues of Anticancer Drugs. Molecules. 1997; 2(9):129-134. https://doi.org/10.3390/20900129
Chicago/Turabian StyleSharma, Anoop K., Gajendra Singh, Ashok K. Yadav, and L. Prakash. 1997. "Improved Method for the Synthesis of New 1,5-Benzothiazepine Derivatives as Analogues of Anticancer Drugs" Molecules 2, no. 9: 129-134. https://doi.org/10.3390/20900129
APA StyleSharma, A. K., Singh, G., Yadav, A. K., & Prakash, L. (1997). Improved Method for the Synthesis of New 1,5-Benzothiazepine Derivatives as Analogues of Anticancer Drugs. Molecules, 2(9), 129-134. https://doi.org/10.3390/20900129



