Abstract
A series of meso-chlorine substituted heptamethine-cyanine dyes and related heptamethine cyanine dyes unsubstituted on the vinyl chain were synthesized by condensation of 2,3,3-trimethyl indolenium salts with reactive intermediates, 2-chloro-formyl-3-hydroxy-methylenecyclohexene or N-[5-(phenylamino)-2,4- phentadienylidene] aniline monohydro-c hloride. The structures of the dyes were characterized by virtue of 1H- NMR, 13C-NMR, IR, MS, etc. The reaction conditions and physicochemical properties of the dyes were extensively explored with classical photo-physical techniques
Introduction
The indotricarbocyanine dyes, due to their relative stability, high molar extinction coefficient, high fluorescent intensity etc., have a wide application in various fields, such as near-infrared laser dyes [1], fluorescent labelling agents for proteins [2,3], fluorescent tags in DNA sequencing [4], immunoassay [5], and flow cytometry [6], as well as sensitizers for various silver halide emulsions [7] and optical recording medium [8]. These applications of indotricarbocyanine dyes take advantage of the property that indocyanine dyes are more stable to light and heat than other cyanine dyes containing the same chromophore and non-indolenine ring, e.g. benzoxazole, benzothiazole and thiazoline. It is known that incorporating more methine groups in the ring can increase the stability of the tricarbocyanine. The physicochemical properties of various indotricarbocyanines containing methine groups in different bridge rings have been studied in several patents [9]. The majority of them are unsubstituted on the indolenine ring, and the systematic investigation of tricarbocyanine dyes carrying different substituents on indolenine ring has not yet been observed. In this paper, we report the synthesis of a series of indotricarbocyanine dyes, investigate both the synthetic conditions and the physicochemical properties and also show the correlation of the structure of the dyes with their properties.
Results and Discussion
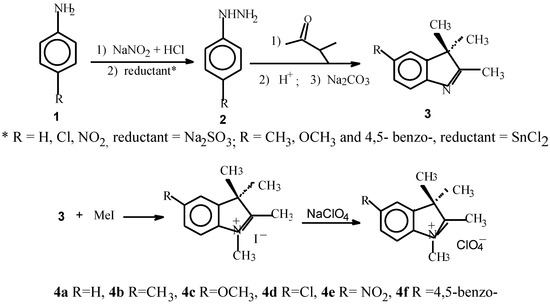
The structures of the dyes studied in this paper are shown in Scheme 1. They were synthesized, as outlined in Scheme 1 and Scheme 2, according to a general route for synthesis of heptamethine cyanine dyes [10].
Synthesis of cyanine dyes
A substituted hydrazine, formed by diazotisation of β- naphthyl-amine or p-substituted anilines with NaNO2 then reduction of the diazonium salt with stannous chloride or sodium sulfate, is condensed with methyl isopropyl ketone in the Fischer indole synthesis [11].
Table 1.
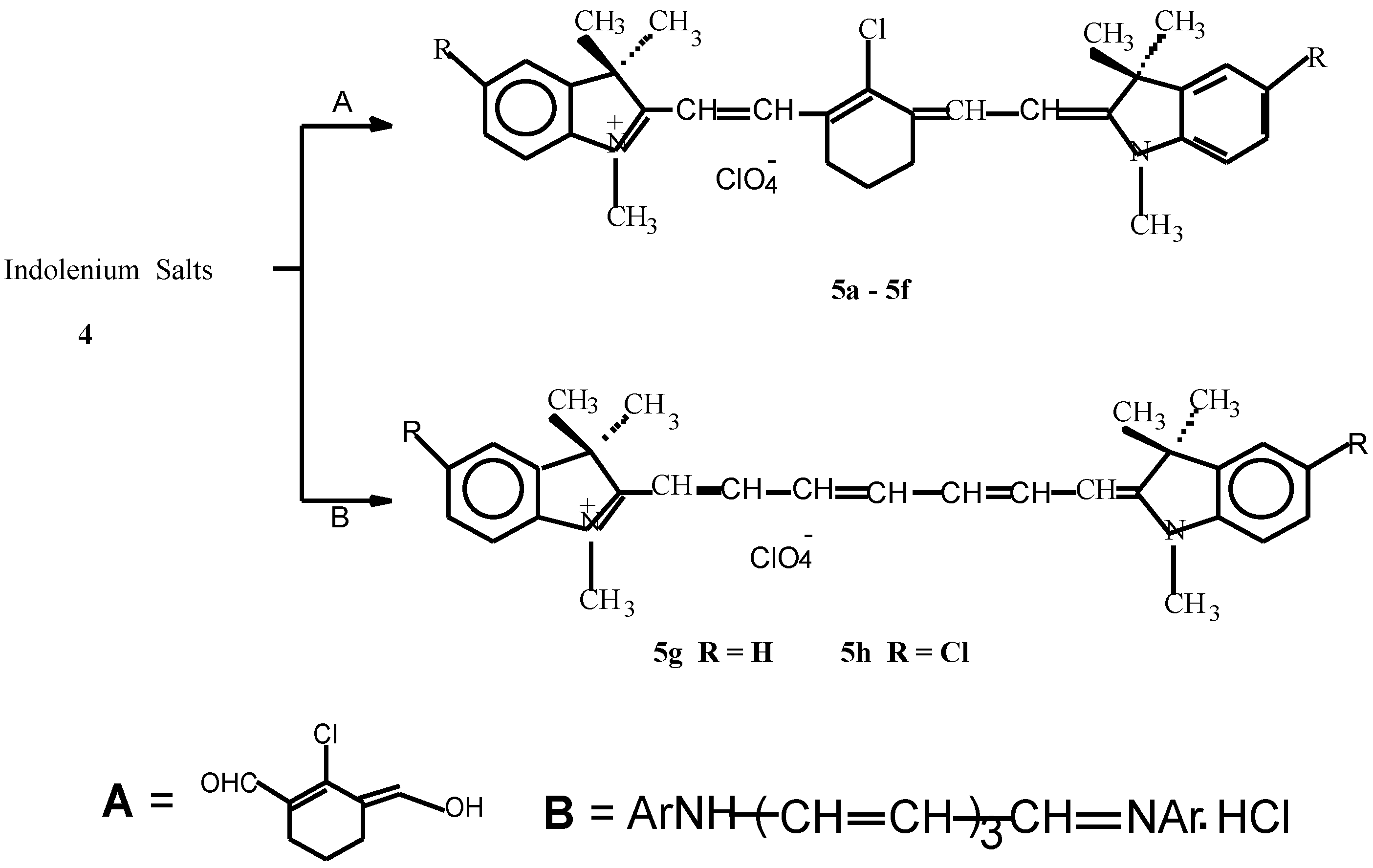
Synthesis of indolenium salts 4a-4f.

| Salts | Indolenine (g) | CH3I (g) | Reflux Time (h.) | Yield (%) | Formula (F.W.) | MS (m/z) |
| 4a | 6 | 12 | 8 | 90 | C12H16NI (301) | 175.159 |
| 4b | 7 | 13.4 | 8 | 75 | C13H18NI (315) | 187.173 |
| 4c | 7.6 | 10 | 10 | 90 | C13H18NI (331) | 203.189 |
| 4d | 13.5 | 10 | 12 | 85 | C12H15NClI (335) | 207.193 |
| 4e | 5.6 | 9.3 | 12 | 42 | C12H16NI (346) | 218.204 |
| 4f | 20.8 | 21 | 6 | 92 | C16H18NI (351) | 223.208 |

| Salts | Analysis Found (Calcd. ) | IR (cm−1) | ||
| C | H | N | ||
| 4a | 47.84 | 5.36 | 4.65 | 1625, 1477, 777 |
| (47.88) | (5.39) | (4.48) | ||
| 4b | 49.21 | 5.43 | 4.20 | 3100, 2967, 1627,1462, 816 |
| (49.68) | (5.41) | (4.46) | ||
| 4c | 47.01 | 5.41 | 4.22 | 1620, 1440, 827 |
| (47.13) | (5.41) | (4.22) | ||
| 4d | 43.00 | 4.54 | 4.21 | 1600, 1520, 960, 840 |
| (42.95) | (4.47) | (4.17) | ||
| 4e | 41.00 | 4.35 | 7.70 | 1625, 1530, 1340, 1250 |
| (41.6) | (4.36) | (8.09) | ||
| 4f | 47.84 | 5.36 | 3.95 | 3200, 2900, 1600, 1550, 1450, |
| (47.88) | (5.39) | (3.99) | 820, 740 |
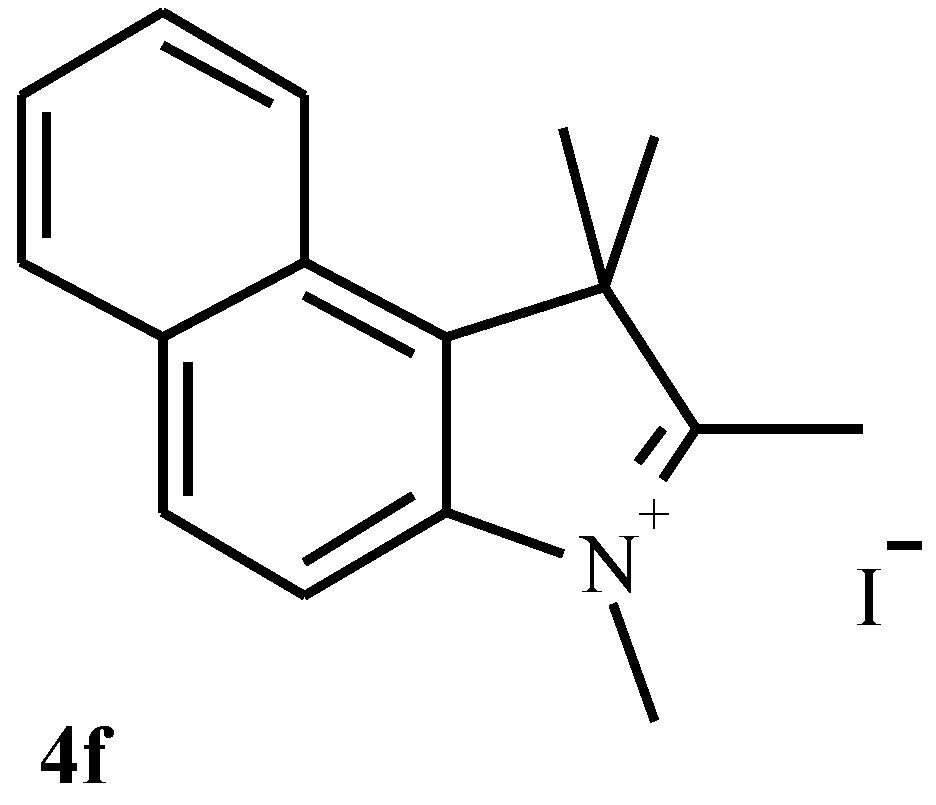
Table 2.
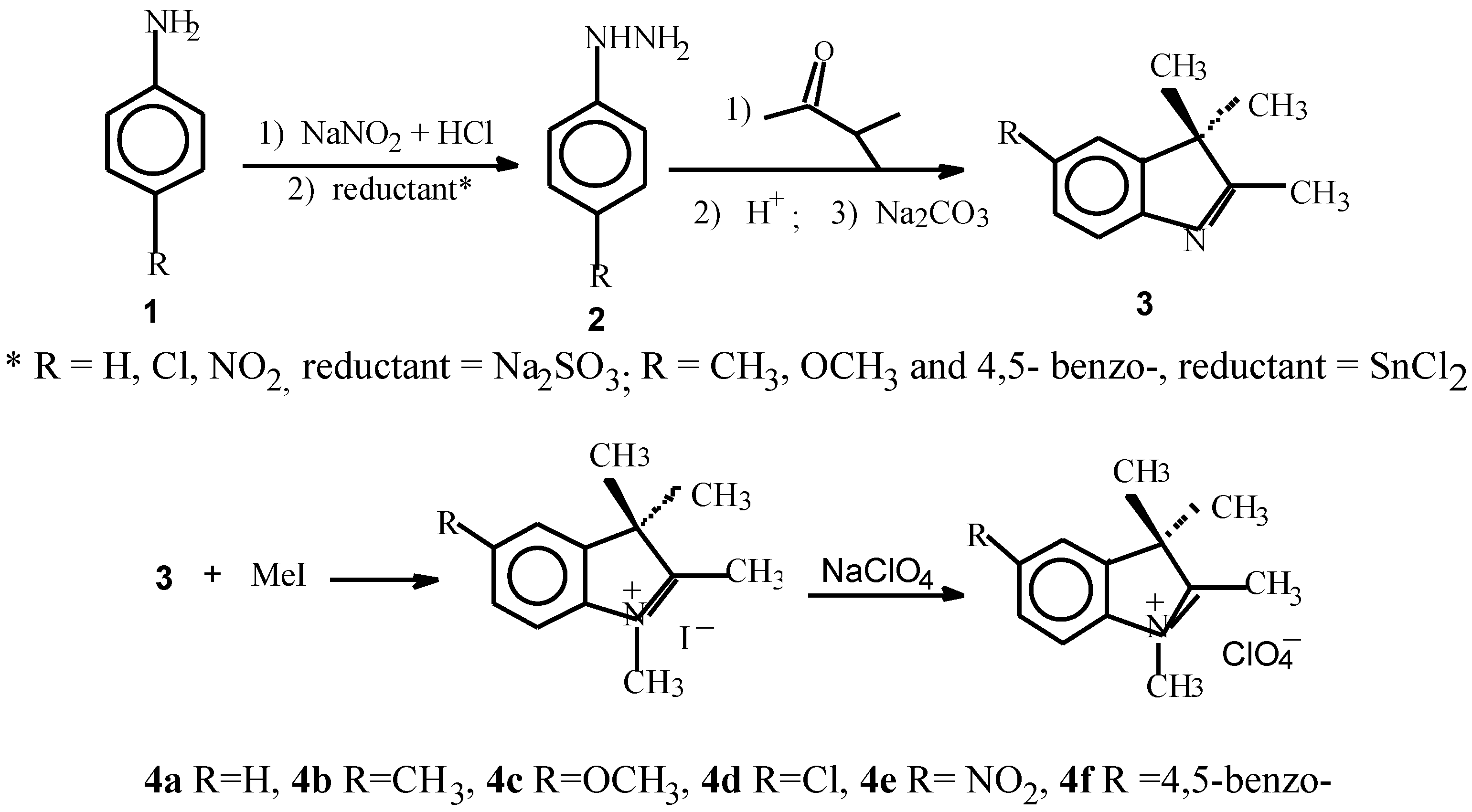
Reaction of indolenium salt (4, ClO4-) with intermediate A or B (Scheme 2) and the products (5).

| Entry | Indolinium Salt + A or B | Products (Dyes) | Temperature (°C) | Reaction Time (min.) | Yield (%) |
| 1 | 4a (A) | 5a | 110 | 15 | 66 |
| 2 | 4b (A) | 5b | 110 | 15 | 61 |
| 3 | 4c (A) | 5c | 100 | 15 | 55 |
| 4 | 4d (A) | 5d | 100 | 20 | 70 |
| 5 | 4e (A) | 5e | 135-140 | 30 | 36 |
| 6 | 4f (A) | 5f | 100(40) | 15(2 hrs) | 53 |
| 7 | 4a (B) | 5g | 85-90 | 55 | 42 |
| 8 | 4b (B) | 5h | 100 | 60 | 45 |

| Products | M.P | Formula | Analysis | Found | (Calcd.) | IR (cm-1) |
| (°C) | (F.W.) | C | H | N | ||
| 5a | 256-258 | C32H36N2Cl2O4 | 62.59 | 6.10 | 4.70 | 1601 1501 1448 1428 1394 |
| (583) | (62.86) | (6.17) | (4.80) | 1365 921 806 771 | ||
| 5b | 266-270 | C34H40N2Cl2O4 | 66.02 | 6.52 | 4.16 | 1546 1507 1434 1387 1356 |
| (611) | (66.70) | (6.55) | (4.58) | 932 738 | ||
| 5c | 279-282 | C34H40N2Cl2O6 | 63.26 | 6.10 | 4.05 | 1545 1504 1431 1386 1358 |
| (643) | (63.45) | (6.22) | (4.35) | 928 806 734 | ||
| 5d | >300 | C32H34N2Cl4O4 | 58.72 | 5.10 | 4.02 | 1623 1546 1433 1387 1360 |
| (652) | (58.89) | (5.21) | (4.29) | 966 860 734 | ||
| 5e | >300 | C32H34N4Cl2O8 1.5H2O | 54.63 | 5.02 | 7.19 | 1600 1544 1477 1429 1381 |
| (700) | (54.85) | (5.14) | (8.00) | 1328 929 860 724 | ||
| 5f | 258-260 | C40H40N2Cl2O4 | 66.35 | 5.43 | 3.33 | 1547 1433 1427 1361 932 |
| (683) | (66.76) | (5.84) | (3.91) | 893 723 | ||
| 5g | 201-203 | C29H33N2ClO4 | 67.97 | 6.27 | 5.08 | 1594 1512 1487 1441 1408 |
| (508.5) | (68.43) | (6.49) | (5.51) | 1391 918 790 751 | ||
| 5h | 206-208 | C29H31N2Cl3O4 | 59.90 | 5.37 | 4.58 | 1513 1439 1405 1389 1346 |
| (577.5) | (60.26) | (5.37) | (4.85) | 1001 921 817 710 |
Scheme 1.
Scheme 1.

Scheme 2.
For 5a–5f, 5a R=H, 5b R=CH3, 5c R=OCH3, 5d R=Cl, 5e R= NO2, 5f R =4,5-benzo-.
Scheme 2.
For 5a–5f, 5a R=H, 5b R=CH3, 5c R=OCH3, 5d R=Cl, 5e R= NO2, 5f R =4,5-benzo-.
Methylation with methyl iodide gives substituted indolinium salts, the purity of the salts recrystallized from ethanol was examined by elemental analysis. The reaction conditions are collated in Table 1. Furthermore, the indolinium salts react with 2-chloro-formyl-3-hydroxy- methylenecyclohexene in a mixture of glacial acetic acid and acetic anhydride to produce the cyanine dyes 5a–5f, in which odd-numbered carbon chain is cyclic. The reaction generally requires 15–60 min for completion. The crude reaction products were purified by washing with cooled acetone and alcohol, recrystallizing from ethyl alcohol or methanol. A high yield was obtained with a ratio of indolinium : intermediate : sodium acetate = 2 : 1 : 2, and a ratio of glacial acetic acid and acetic anhydride = 4:3. It was found (see Table 2) that by using the mixture as solvent and sodium acetate as reaction catalyst the reaction with indolinium iodide (4a, 4b and 4d) afforded the dye 5a, dye 5b and dye 5d in excellent yields (entries 1, 2 and 4), but the dye 5e in low yield (36%). The results clearly indicate that a nitro group on the indolenine ring renders a decreased reactivity of the heterocycle 5e.
The indolenium salts react with N-[5-(phenylamino)- 2,4-phentadienylidene] aniline monohy-drochloride in a mixture of pyridine, acetic anhydride and N,N-dimethyl- formamide to give dye 5g and dye 5h, in which the vinyl chain is unsubstituted.

Table 3.
The 1HNMR sectroscopy data (δ in ppm) of the dyes studied in DMSO-d6.
Table 3.
The 1HNMR sectroscopy data (δ in ppm) of the dyes studied in DMSO-d6.
The synthetic conditions and elemental analysis data of the dyes purified are shown in Table 2 together with melting points.
NMR spectra
Table 3 collates the 1HNMR spectroscopy data of the synthesized dyes. From the 1HNMR spectrum of 5d, the signals of the alkyl hydrogen atom are easily recognized, N-CH3 protons (s, 6H) at δ=3.35 and 3-H (s,12H) at δ=1.7ppm, the signal of 3-H (t,4H)at δ=2.7 as well as the signal of 4-H (m, 2H) at δ=1.85, In the vinyl link of the dyes, the signal of 1-H (d, 2H) is at 6.3 ppm, whereas the signal of 2-H (d, 2H) is at 8.2 ppm. It suggests that the electronic charges are distributed alternately on the conjugation link. For the benzene ring protons, there are two signals apparently ascribed to c-H and d-H. As general concept, we assigned the lower field doublet at 7.50 to d- H, δ=7.40 to c-H. The high field singlet at δ=7.8 was assigned to a-H.
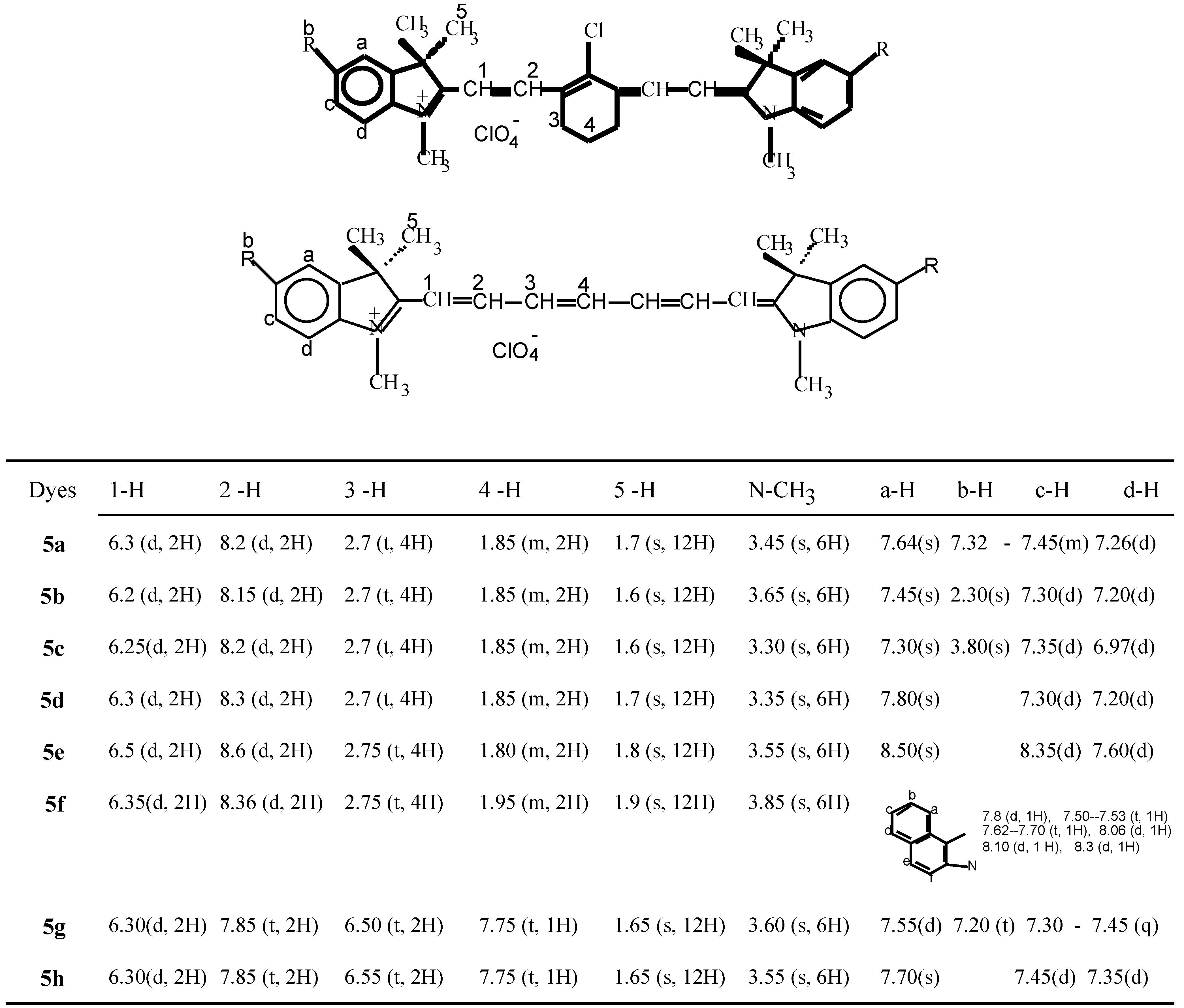
The structures of dye 5f, dye 5g and dye 5h were also characterized by 13CNMR. (300MHz, DMSO-d6). For dye 5f, δ=20.5 (-CH2-CH2-CH2), 25.94 (-CH2-CH2-CH2-), 31.91(N-CH3), 50.56 (H3C-C-CH3). In the vinyl part of the structure 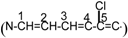 , δ=101.47 (C-2), 131.4 (C-4), 133.4 (C-3), 147.1(C-5), 173.8 (C-1).
, δ=101.47 (C-2), 131.4 (C-4), 133.4 (C-3), 147.1(C-5), 173.8 (C-1).
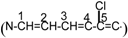 , δ=101.47 (C-2), 131.4 (C-4), 133.4 (C-3), 147.1(C-5), 173.8 (C-1).
, δ=101.47 (C-2), 131.4 (C-4), 133.4 (C-3), 147.1(C-5), 173.8 (C-1).In the naphthalene ring
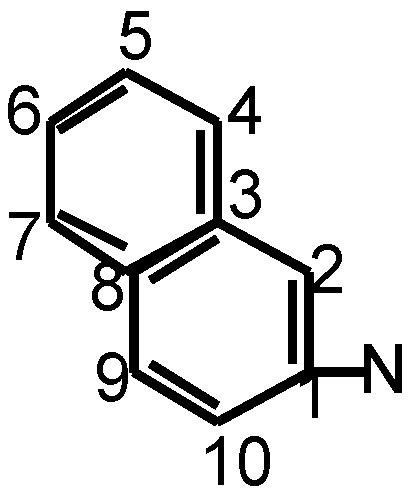 there are 13CNMR peaks 126.08 (C-3), 127.4 (C-8), 141.7(C-1), 140.45 (c-2), 111.7 (C-10), and the peaks 122.26, 124.9, 127.75, 129.9, 130.3.
there are 13CNMR peaks 126.08 (C-3), 127.4 (C-8), 141.7(C-1), 140.45 (c-2), 111.7 (C-10), and the peaks 122.26, 124.9, 127.75, 129.9, 130.3.
 there are 13CNMR peaks 126.08 (C-3), 127.4 (C-8), 141.7(C-1), 140.45 (c-2), 111.7 (C-10), and the peaks 122.26, 124.9, 127.75, 129.9, 130.3.
there are 13CNMR peaks 126.08 (C-3), 127.4 (C-8), 141.7(C-1), 140.45 (c-2), 111.7 (C-10), and the peaks 122.26, 124.9, 127.75, 129.9, 130.3.For dye 5g, there are eleven 13CNMR peaks in the sp2 hybrid range, three peaks in the sp3 hybrid range. δ=27.04 (CH3-C-CH3), δ=31.1 (N-CH3), δ=48.53 (CH3-C-CH3); in the vinyl part of the structure ( 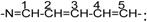 δ=172.1 (C-1), 104 (C-2), 150.55 (C-3), 125.09 (C-4), 156.30 (C-5); on the benzene ring
δ=172.1 (C-1), 104 (C-2), 150.55 (C-3), 125.09 (C-4), 156.30 (C-5); on the benzene ring
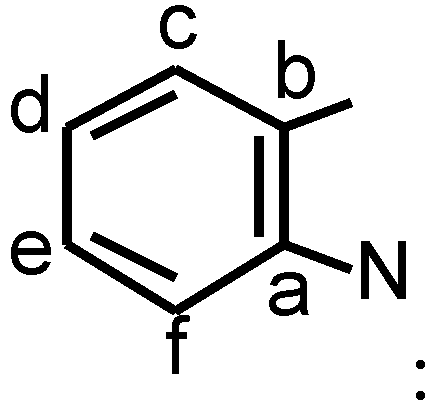 δ=142.87 (C-a), 140.84 (C-b), 122.21 (C-c), 124.51 (C-d), 128.34 (C-e), 110.84 (C-f).
δ=142.87 (C-a), 140.84 (C-b), 122.21 (C-c), 124.51 (C-d), 128.34 (C-e), 110.84 (C-f).
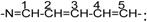 δ=172.1 (C-1), 104 (C-2), 150.55 (C-3), 125.09 (C-4), 156.30 (C-5); on the benzene ring
δ=172.1 (C-1), 104 (C-2), 150.55 (C-3), 125.09 (C-4), 156.30 (C-5); on the benzene ring
 δ=142.87 (C-a), 140.84 (C-b), 122.21 (C-c), 124.51 (C-d), 128.34 (C-e), 110.84 (C-f).
δ=142.87 (C-a), 140.84 (C-b), 122.21 (C-c), 124.51 (C-d), 128.34 (C-e), 110.84 (C-f).For dye 5h, sp3 hybrid range: δ=26.60 (CH3-C-CH3), δ=31.27 (N-CH3), δ=48.72 (CH3-C-CH3); in the vinyl part of the structure ( 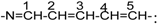 δ=175.1 (C-1), 104.22 (C-2), 150.63 (C-3), 128.75 (C-4), 156.11 (C-5); on the benzene ring
δ=175.1 (C-1), 104.22 (C-2), 150.63 (C-3), 128.75 (C-4), 156.11 (C-5); on the benzene ring
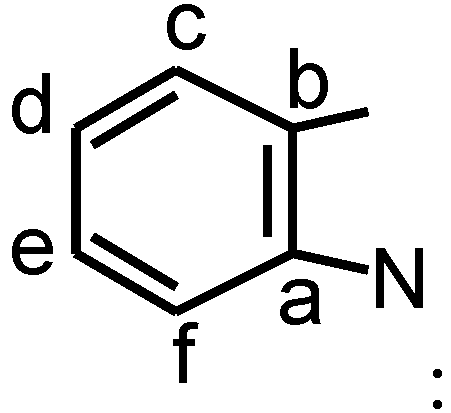 δ=142.65 (C-a), 141.86 (C-b), 122.72 (C-c), 125.71 (C-d), 128.22 (C-e), 112.21 (C-f).
δ=142.65 (C-a), 141.86 (C-b), 122.72 (C-c), 125.71 (C-d), 128.22 (C-e), 112.21 (C-f).
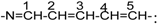 δ=175.1 (C-1), 104.22 (C-2), 150.63 (C-3), 128.75 (C-4), 156.11 (C-5); on the benzene ring
δ=175.1 (C-1), 104.22 (C-2), 150.63 (C-3), 128.75 (C-4), 156.11 (C-5); on the benzene ring
 δ=142.65 (C-a), 141.86 (C-b), 122.72 (C-c), 125.71 (C-d), 128.22 (C-e), 112.21 (C-f).
δ=142.65 (C-a), 141.86 (C-b), 122.72 (C-c), 125.71 (C-d), 128.22 (C-e), 112.21 (C-f).Comparing the δ-values at the same position of hydrogen, it can be seen from Table 3 that the electron- withdrawing substituents on the 5-position of indolenine cycle shifted the δ (ppm) value of the dye to lower field. On the contrary, the electron-donating substituents apparently make the δ (ppm) value of the dyes shift to higher field.
IR spectra
Although the IR spectra of heptamethine cyanine dyes is complex, the typical functional absorption band can be assigned. The aromatic absorption is at 1620-1600, 1550- 1540, (νC=C). and 860-800 (νC=H), 770-730. In addition, the resonance-conjugated unsaturated stretching modes in the chromophore are at 1520≈1480, 1448≈1434 cm-1. Moreover, chromophoric CH out-of-plane bending (ν-CH=CH-) is at 960°920 cm-1. The absorption bands for the appropriate functional groups can also be defined.
UV spectra
Table 4 shows the electric spectra of the dyes (5) in methanol. The polymethine dyes (dye 5a to dye 5f) containing different substituents exhibit different absorption and fluorescence maxima. And the absorption and emission maxima of dye 5b to dye 5f are at longer wavelength range than that of dye 5a; the bathochromic order induced by the substituents (R) is as follows:
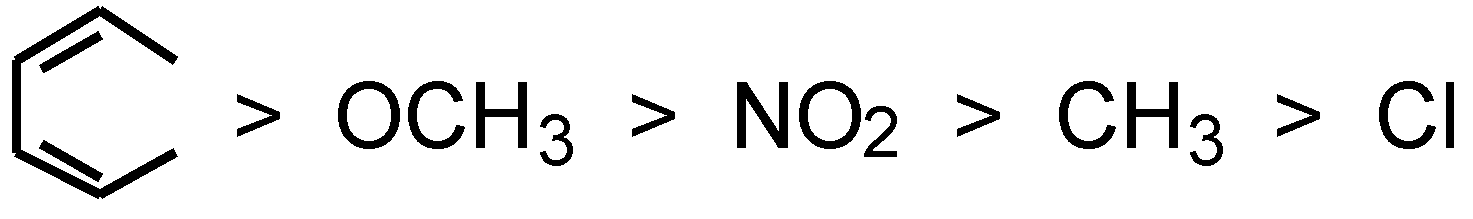
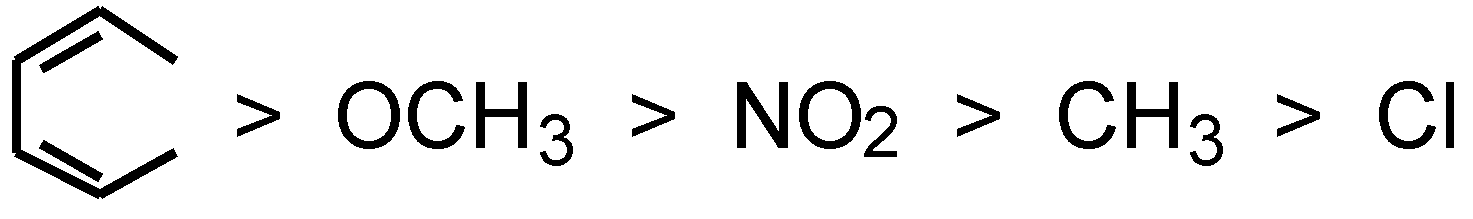
It is understandable that the absorption and emission maxima of dye 5b, dye 5c, dye 5d and dye 5f, carrying chloro, methyl, methoxyl and benzo- respectively, increases as the capacity for electron-donating of the substituents (R) increases. Whereas, dye 5e gives an increase in the number of absorption bands accompanied by a red shift of 20 nm. The main cause is that the introduction of a nitro group increases the delocalizing range of the π-electrons in the chromophore of the dyes and decreases the excitation energy of the dyes. As a result, the absorption and emission maxima of dye 5e shows a bathochromic shift compared with dye 5a (R = H), which was also explained in our earlier work for a series of indocarbocyanine dyes [12].

Table 4.
The electronic spectra data of the dyes.
| Dyes | λmax ab | ε .10-5 | λmax em | λmax ab |
| (nm) | (l cm-1 mol-1) | (nm) | - λmax em (nm) | |
| 5a | 780 | 2.65 | 805 | 25 |
| 5b | 788 | 2.76 | 810 | 22 |
| 5c | 805 | 3.29 | ||
| 5d | 784 | 2.02 | 808 | 24 |
| 5e | 800 | 1.62 | 820 | 20 |
| 5f | 818 | 2.49 | ||
| 5g | 745 | 1.75 | 770 | 25 |
| 5h | 748 | 1.51 | 778 | 30 |
The λmax ab −λmax em value of the dyes is 20-25 (nm) (see Table 4). Furthermore, comparing the absorption and emission maxima of dye 5a and dye 5d with that of dye 5g and dye 5h respectively, we found that the absorption and emission maxima of dye 5a and dye 5d are at 32 −40 nm longer wavelength than those of dye 5g and dye 5h. This indicates that the conjugated chain cyclisized and substituted by chloro can cause the electronic energy of the dyes to decrease, and consequently, increase the stability of the dyes.
The snthesis of 5g and 5h will be reported in detail elsewhere.
Experimental
Absorption spectra of dyes in methanol solution were recorded with a Hitachi−557 spectrophoto-meter and a Hitachi UV−8451A spectrophotometer at room temperature (20°C). The experimental results are compiled in Table 1. Fluorescence spectra of the cyanine dyes in methanol solution were recorded with a Rini-MPF-4 spectrophotofluorimeter. 1H and 13CNMR spectra were taken with a 300 MHz Varian XL200, using TMS as internal standard and DMSO-d6 as solvent. Elemental analysis was performed by the Institute of Chemistry, Analytical Laboratory. Melting points were determined on a micro-melting point apparatus and are uncorrected. IR spectra were run in KBr discs on a Perkin- Elmer 683 spectrometer.
The synthesis of indolenine (3)
The indolenines were made by the method of Fischer indole synthesis from phenylhydrazine and methyl isopropyl ketone, purified by distillation in vacuum. The structure of the indolenines was proved to be correct by 1HNMR data, which was measured on a Varian FT-80 spectrometer with MeSi as an internal reference, CDCl3 as solvent, the spectroscopic data being shown below.
2,3,3-trimethyl-indolenine (3a)
1HNMR: 1.25 (s, 6H, 3-CH3), 2.24 (s, 3H, 2-CH3), 7.10−7.40 (m, 4H, aromatic hydrogen).
2,3,3,5-tetramethyl-indolenine (3b)
1HNMR: 1.25 (s, 6H, 3-CH3), 2.22 (s, 3H, 2-CH3), 2.35 (s, 3H, 5-CH3) 7.10−7.40 (m, 3H, aromatic hydrogen).
2,3,3 trimethyl-5-methoxyl-indolenine (3c)
1HNMR: 1.20 (s, 6H, 3-CH3), 2.20 (s, 3H, 2-CH3), 3.75 (s, 3H), 6.75−7.50 (m, 3H, aromatic hydrogen).
2,3,3-trimethyl-5-chloroindolenine (3d)
1HNMR: 1.25 (s, 6H, 3-CH3), 2.30 (s, 3H, 2-CH3), 6.90−7.45 (m, 3H, aromatic hydrogen).
2,3,3-trimethyl-5-nitroindolenine (3e)
1HNMR: 1.28 (s, 6H, 3-CH3), 2.28 (s, 3H, 2-CH3), 7.20−7.60 (m, 3H, aromatic hydrogen).
Synthesis of indolenium iodide 4
A solution of indolenine and iodomethane in anhydrous trichloromethane was heated for 8-12 h at 90-100°C in a sealed tube. The trichloromethane was removed by evaporation. The solid was washed with water and diethyl ether respectively. and recrystallized from ethanol. The purity of the salts 4 was tested by elemental analysis. The infrared spectroscopy of the salt molecules was also measured. The IR spectra showed typical aromatic absorption (benzen 1600−1610, 1500, 1400, 800 or so,) The main fragment peak value of Mass Spectra are shown in Table 1. which are much correspond to the structures of 4.
Preparation of 2-chloro-formyl-3-hydroxy-methylenecyc- lohexene (compound A in Scheme 2)
This compound was made by the reported procedure [13]. 1HNMR (CDCl3): δ: 1.58 (q, 2H), 2.40 (t, 4H), 9.5-10 (s,1H), 7.6 (s, 1H), 3.4 (s,1H). Anal. Calcd. For C8H9ClO2: C, 55.7;H, 5.3; Cl, 20.5. Found: C, 55.4; H, 5.4; Cl, 20.4. IR: 3200 (CHO, OH), 1600 (C=C), 1520 (1470 - 1465 ), 1200.
General procedure for the preparation of dyes 5a-5f
To a mixture of indolinium salt and 2-chloro -formyl-3- hydroxymethylene-cyclohexene sodium acetate was added. The mixture was refluxed under the conditions given in Table 2. The progress of the reaction was monitored by measuring the Vis-near-IR spectroscopy After the mixture was allowed to cool to room temperature, filtered, washed with acetone and a small amount of cooled ethanol. and dried at room temperature to give the crude dye. The analytical samples were obtained by recrystallization twice from methanol. In the case of dye 5e, the crude product was recrystallized from ethanol and acetonitrile. The analytical data and physical properties of dye 5a to dye 5f are given in Table 2, Table 3 and Table 4.
References
- Robert, G.; David, W.; Jon, B.; Paul, R.; Heptinstall, J. Dyes and Pigments 1996, 30, 321.
- Alipowsk, N.; Patonay, G. Synth. Commun. 1993, 23, 3087.
- Patonay, G.; Middendorpp, L. EP 0533 302A1, 2 March 1993.
- Middendorf, L. R.; Bruce, J. C.; Bruce, R. C.; Eckles, R. D.; Grone, P. L.; Roemer, S. C.; Sloniler, G. D.; Steffens, D. L.; Sutter, S. L.; Brumbaugh, J. A.; Patonay, G. Electrophoresis 1992, 13, 487. [PubMed]
- Willoams, R. J.; Lipowska, M.; Patonay, G.; Strekowski, L. Anal. Chem. 1993, 165, 601.
- Mujumdar, R. B.; Ernst, L. A.; Mujumdar, S. R.; Lewis, C. J.; Waggoner, A. S. Bioconjugate Chem. 1993, 4, 105.
- Mess, C. E. K.; James, T. H. The Theory of the Photographic ProcessMacmillan Co.: New York, 4th ed.; 1977. [Google Scholar]
- Peng, B. X.; Li, Q. J. Photogr. Sci. Photochem. 1994, 12, 150–165.
- (a) Higela, S.; Suzuki, Y.; Shiojima, I. JP 62232461, 1987. (b) Sato, G.; Ishizaka, Y. 6333477, 1988. (c) Ricoh Co., Ltd. JP 6078787, 1985. (d) Oba, H.; Sato, T.; Umehara, M.; Abe, M. JP 61248789, 1986.
- France, M. H. The Cyanine Dyes and Related Compounds; Wiley: New York, 1964. [Google Scholar]
- Robinson, B. Chem. Rev. 1962, 63, 373, and the references cited therein.
- Li, Q.; Peng, B. X. Youji Huaxue 1995, 15, 275–278.
- Reynolds, G. A.; Drexhage, K. H. J. Org. Chem. 1977, 42, 885.
- Sample Availability: Supporting samples are available from MDPI: 5a, MDPI 11848; 5b , MDPI 11849; 5c, MDPI 11850; 5d, MDPI 11852; 5e, MDPI 11853; 5f, MDPI 11851; 5g, MDPI 11846; 5h, MDPI 11847; 4b , MDPI 11855; 4c, MDPI 11856; 4d, MDPI 11855.
© 1997 MDPI. All rights reserved
