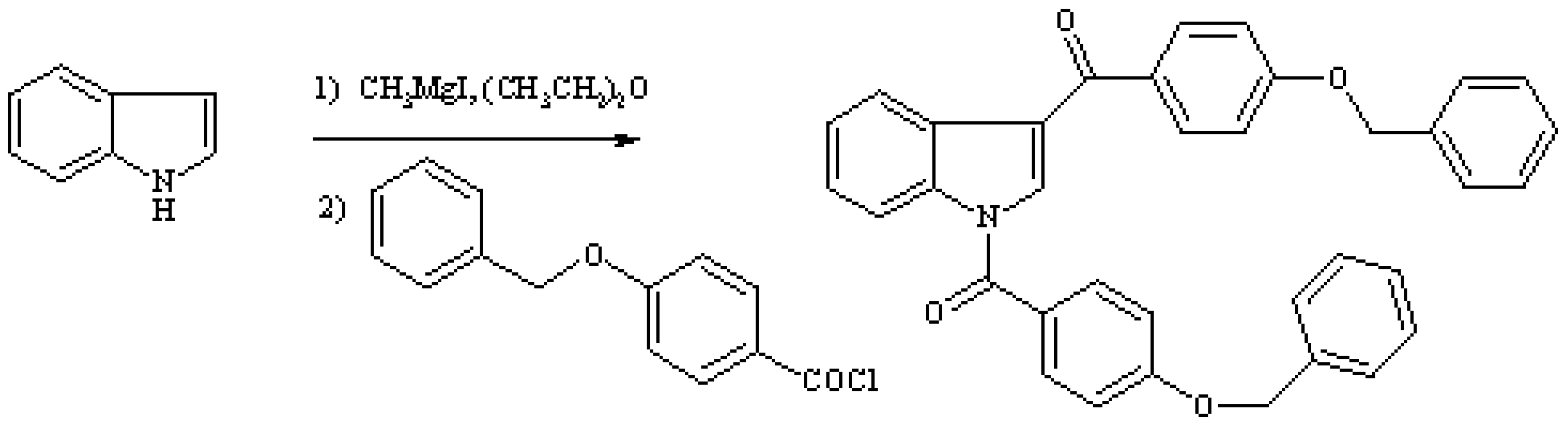
1,3-Di(4-benzyloxybenzoyl)indole
Supplementary materials
Supplementary File 1Supplementary File 2Acknowledgments
References and Notes
- Lin, S.-K.; Rasetti, V. Synthesis of benzospiro[5,6]undecane derivatives as inhibitors of steroid 5-a-reductase. Helv. Chim. Acta. 1995, 78, 857–865. [Google Scholar] [CrossRef]
- Sample Availability: Available from MDPI, 0.1745g, MDPI 9940.
© 1997 MDPI. All rights reserved
Share and Cite
Lin, S.-K. 1,3-Di(4-benzyloxybenzoyl)indole. Molecules 1997, 2, M1. https://doi.org/10.3390/M1
Lin S-K. 1,3-Di(4-benzyloxybenzoyl)indole. Molecules. 1997; 2(4):M1. https://doi.org/10.3390/M1
Chicago/Turabian StyleLin, Shu-Kun. 1997. "1,3-Di(4-benzyloxybenzoyl)indole" Molecules 2, no. 4: M1. https://doi.org/10.3390/M1
APA StyleLin, S.-K. (1997). 1,3-Di(4-benzyloxybenzoyl)indole. Molecules, 2(4), M1. https://doi.org/10.3390/M1



