Abstract
Natural abundance 17O NMR spectra of a series of 5-alkylaminomethylene-2,2-dimethyl-1,3- dioxane-4,6-diones, recorded in acetonitrile solution, are reported. The δ(17O) values correlate well with those of 4-alkylaminobut-3-en-2-ones and with the pKa values of amines. The effects of the N-alkyl groups on the 17O shift values is diminished, owing to the resonance effects of the alkoxy groups. The shift difference (ΔδHB) between the two carbonyl groups is mainly attributed to the intramolecular hydrogen bonding and depends on the donor property of the amino group.
Introduction
Enamino-diesters [1] are useful starting materials for the synthesis of heterocyclic compounds [1], and antimalarial drug choroquine and similar products of medicinal interest [2]. The diacylvinyl groups have been used to protect the amino group of amino acids during the systhesis of peptides and penicillin derivatives [3], and amino sugars during Fischer glycosidation [4]. The properties of enamino-diesters have been investigated by UV [5], IR [6], and 1H [6a,7], 13C [8], 15N NMR [9], and X-ray crystallography [10]. These studies confirmed the presence of an intramolecular C=O ... HN bond in primary and secondary enamino-diesters.
17O NMR is a particularly useful tool for the study of intramolecular hydrogen bonding in organic compounds [11,12] Recently the 17O NMR spectra of enaminones and enamino-diketones have been investigated [13]. The shielding of the carbonyl O-atom by intramolecular hydrogen-bonding (ΔδHB), ranging from –14 to –47 ppm, was sensitive to the nature, number and position of the substituents. 17O NMR investigations of enamino-diesters have not been reported.
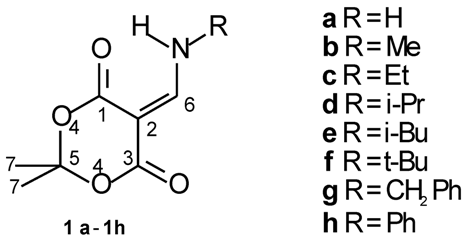
To evaluate the sensitivity of the C=O...H-N type of intramolecular hydrogen bonding on the 17O shift value of the hydrogen bonded carbonyl group, the 17O NMR spectra of a series of 5-alkylaminomethylene-2,2-dimethyl- 1,3-dioxane-4,6-diones (1a-1h) have been investigated.
Results and Discussion
The 17O-NMR data of enamino-diesters 1a-1h are listed in Table 1. The 17O NMR spectra of these types of compounds exhibit three 17O signals: alkoxy O-4 atom appears at 185-189 ppm and two carbonyl O atoms are at 304-314 and 317-326 ppm. The 1H-N signal appears at 9.2– 11.2 ppm (Table 1), indicating the existence of strong intramolecular hydrogen bonding in molecules. On the basis of the shielding effects of intramolecular hydrogen bonding upon the 17O shift value [11,12], the signal at 304-314 ppm is assigned to the hydrogen bonded carbonyl O-1 atom and the signal at 317-326 ppm to the free carbonyl O-3 atom.

Table 1.
17O, 13C and 1H NMR data (ppm) of 1a-1h.
| Compd | 17O-1 a | 17O-3 a | 17O-4 a | ΔδHB b | 1H-N c | 13C-2 c |
|---|---|---|---|---|---|---|
| 1a | 307.1 (170) | 324.2 (120) | 187.7 (350) | 17.1 | 9.18 | 104.93 |
| 1b | 304.8 (210) | 317.7 (190) | 186.0 (300) | 12.9 | 9.46 | 104.62 |
| 1c | 304.7 (240) | 318.5 (200) | 185.9 (330) | 13.8 | 9.54 | 104.62 |
| 1d | 305.0 (400) | 318.1 (200) | 185.5 (340) | 13.1 | 9.52 | 104.57 |
| 1e | 304.5 (340) | 317.4 (240) | 185.8 (390) | 12.9 | 9.58 | 104.67 |
| 1f | 305.5 (220) | 317.5 (350) | 185.4 (430) | 12.0 | 9.84 | 104.58 |
| 1g | 305.3 (420) | 320.6 (350) | 187.2 8450) | 15.3 | 9.79 | 104.72 |
| 1h | 314.0 (360) d | 325.5 (320) | 188.4 (510) | 11.5 | 11.24 | 105.13 |
- a)
- 0.5 M Acetonitrile solution at 40°C; linewidth at half-height in parentheses.
- b)
- ΔδHB = δ(17O-3) - δ(17O-1).
- c)
- CDCl3 solution, relative to internal TMS.
- d)
- Data taken from ref 13b.
The results in Table 1 show that the δ(17O) values are influenced by the nature of the nitrogen substitutents. As previously observed for enaminones and enaminodiketones [13], the 17O shift values of the carbonyl O atoms of enamino-diesters with N-alkyl substituents (1b-1g) are very similar. A phenyl group at the N atom (1h) causes a deshielding (ca. 8 ppm) of the two carbonyl O atoms. Acceptable correlations between the carbonyl O-1 and O-3 in enamino-diesters (1b-1h) are obtained with those of the E- and Z-forms of the corresponding MeC(O)CH=CHNHR (2) [13a] [Eqns (1) and (2) respectively].
δ(17O-1)(1) = 152.0 + 0.34 δ(17O) [(Z)-2]
(r = 0.972, n = 7, SD = 0.9)
(r = 0.972, n = 7, SD = 0.9)
δ(17O-3)(1) = 189.7 + 0.28 δ(17O) [(E)-2]
(r = 0.988, n = 7, SD = 0.5)
(r = 0.988, n = 7, SD = 0.5)
In the correlations, enamino-diesters with a primary amino group (1a) were excluded because of the influence due to intermolecular hydrogen bonding[13]. The slopes of the correlations show that the substituent effects on the shielding of O-atoms in enamino-diesters are much less sensitive than those in enaminones and enamino-diketones[13].
The δ(17O) values of the carbonyl O atoms, as those noted for enaminones and enamino-diketones [13], are dependent on the donor power of the amines. The 17O data of enaminones and enamino-diketones have been shown to correlate with the pKa values of the amines [13]. Reasonable correlations between the δ(17O), δ(13C-2) and δ(1HN) values of enamino-diesters (1) and pKa values [14] of the corresponding amines were observed [Eqns (3)-(7)]. The small negative slopes (–1.28 to –1.47) of the correlations for δ(17O) values, compared with those noted for enaminones (ca. –4.5) and enamino-diketones (ca. – 2.9) [13], show that the electron donating alkyoxy group at the carbonyl carbon diminishes the shielding effect of the amine group to the carbonyl O-atom, and also indicate that the n,π-conjugation between amino group and C=C double bond is reduced. This can be attributed to the resonance effects between the carbonyl and alkyloxy groups. The existence of secondary electron donating groups on the carbonyl carbon diminishes the shielding of the electron donating group to the carbonyl O-atom. This has been previously noted in the series of acetone (569 ppm), N,N-dimethylacetamide (338 ppm) and tetramethylurea (273 ppm) [15] and also in the series of but-3-en-2-one (563 ppm), ethyl acrylate (338 ppm) and ethyl 3- dimethylaminoprop-2-enoate (295 ppm) [15].
δ(17O-1) = 320.7 – 1.47 pKa
(r = 0.979, N = 8, SD = 0.7)
(r = 0.979, N = 8, SD = 0.7)
δ(17O-3) = 331.6 – 1.28 pKa
(r = 0.977, N = 7, SD = 0.7, omitting 1a)
(r = 0.977, N = 7, SD = 0.7, omitting 1a)
δ(17O-4) = 199.9 – 1.33 pKa
(r = 0.966, N = 7, SD = 0.2, omitting 1h)
(r = 0.966, N = 7, SD = 0.2, omitting 1h)
δ(1HN) = 12.47 – 0.27 pKa
(r = 0.976, N = 7, SD = 0.15, omitting 1a)
(r = 0.976, N = 7, SD = 0.15, omitting 1a)
δ(13C-2) = 105.53 – 0.087 pKa
(r = 0.991, N = 7, SD = 0.03, omitting 1a)
(r = 0.991, N = 7, SD = 0.03, omitting 1a)
17O NMR data has been shown to be sensitive to intramolecular hydrogen bonding [11]. The shielding in the 17O shift value by the hydrogen bonding components (ΔδHB value) has been shown to depend on the H-donor and H-acceptor groups. The ΔδHB values of –50, –30, –19 and –10 ppm have been reported for ketone [12f,g] aldehyde [12h] and amide [12a,j] and ester [12k] carbonyl oxygens, respectively; the difference between these values has been attributed to differences in basicity of the carbonyl groups. For the N–H…O=C type of intramolecular hydrogen bonding in enaminones and enamino-diketones ΔδHB values range from –14 to –47 ppm [13].
It has been demonstrated that the 17O NMR parameters are sensitive to electronic effects, torsional angles, steric interactions and intramolecular hydrogen bonding [11,12] Therefore, to evaluate the ΔδHB values these factors should be taken into account. IR [6a] and X-ray [10] analyses show that the conjugated system in hydrogen bonded enamino-diesters (1) is essentially planar, and that electron delocalization affects both carbonyl groups almost equally. This demonstrates that the contribution of electronic and torsional effects to observed chemical shifts can be considered negligible. Thus, the 17O chemical shift differences (ΔδHB)between the carbonyl groups O-1 and O-3 in enamino-diesters with primary or secondary amino groups are attributed mainly to the intramolecular hydrogen bonding.
The ΔδHB values (Tables 1) for enamino-diesters 1a-1h, ranging from –11 to –17 ppm, are much smaller than those found for 2,2-diacetyl-enamines (ca. -30 ppm) [13b]. It is well known that ester carbonyl groups are less basic than ketones carbonyls [16], and consequently ester carbonyls are expected to be poorer hydrogen bond acceptors than ketone carbonyls. The small ΔδHB values is consistent with the lower basicity of the ester carbonyl groups. The ΔδHB values, –4 to –13 ppm have been previously noted for hydroxypyridine carboxy esters and benzoates [12k].
Conclusion
The results of this paper demonstrate that the effects of the N-alkyl substituents on 17O shift values of the carbonyl groups in enamino-diesters are diminished, in comparison to those in enaminones and enamino-diketones. This is attributed to the resonance between the carbonyl and alkyloxy groups. 17O NMR data show clearly the existence of intramolecular hydrogen bonding in enamino-diesters. The shielding effect of intramolecular hydrogen bonding of a NH to an ester carbonyl, ranging from –11 to –17 ppm, is much smaller than that for analogous diacetyl-enamine carbonyl groups (ca. –30 ppm). This is because of the lower basicity of the ester carbonyl groups.
Experimental
General
1H NMR and 13C NMR spectra (δ, in ppm; J, in Hz; relative to internal TMS in CDCl3 solns. at 20°C) were recorded on Bruker WH-250 and Bruker Advance DPX- 400 spectrometers, MS (electron impact mass spectrum; m/z in % of base peak) on a Nermag R-10-10C spectrometer. Melting points (M. p.) were observed under a microscope using a Mettler FP-52 instrument.
17O NMR Spectroscopy
17O NMR spectra were recorded on a Bruker-WH-360 spectrometer, equipped with a 10-mm probe, at 48.8 MHz, in the Fourier transform (FT) mode without lock. System control, data acquisitions, and data managements were performed by an Aspect-2000 microcomputer. Instrumental settings: spectral width 50 kHz (1025 ppm), 8 K data points, pulse width 33 μs, acquisition time 20 ms, preacquisition delay 5 μs, 150000 – 300000 scans, sample spinning 30 Hz. An even number (12-28) left-shifts (LS) were applied to the FID signal; the latter was zero-filled to 8 K words and exponentially multiplied with 100-Hz line-broadening factor (LB) before being subjected to the FT. The chemical shifts δ(17O), measured in 0.5 M acetonitrile solution at 40°C at natural isotopic abundance, are reported relative to δ(17O)(H2O) (= 0.0 ppm); dioxane (δ(17O) = 0.0 ppm) was used as an external standard; downfield shifts are positive. The general reproducibility of chemical shift values is ca. + 1 ppm (+ 0.2 ppm within the same series).
General procedure
Compounds 1a-1h were prepared according to the literature procedure [17,18], A solution of 5-methoxymethylene-2,2-dimethyl-1,3-dioxane-4,6-diones [17] (10 mmol) and the appropriate amine (10 mmol) (in cases of gaseous amine, ammonia or methylamine ethanol solution or ethylamine aqueous solution was applied) in acetonitrile (10 ml) was left at room temperature for 30 min. Evaporation of the solvent under reduced pressure gave the product as a crystalline solid, which was filtered and washed with ethyl ether.
5-Aminomethylene-2,2-dimethyl-1,3-dioxane-4,6-dione (1a)
M.p. 211.3-212.4 °C (lit. [17] 214-215 °C); 1H-NMR (CDCl3), 9.18 (br, 1H, NH), 8.27 (dd, J = 15.8, 8.5, 1H, H- 6), 6.55 (br, 1H, NH), 1.73 (s, 6H, H-7); 13C-NMR (CDCl3), 165.23 (C-1), 104.93 (C-2), 163.83 (C-3), 87.03 (C-5), 158.82 (C-6), 27.02 (C-7); EI-MS 171 (M+, 1), 156 (1), 114 (3), 85 (2), 70 (28), 69 (100), 59 (25), 53 (5).
5-Methylaminomethylene-2,2-dimethyl-1,3-dioxane-4,6- dione (1b)
M.p. 176.4-176.9°C (lit. [6a] 175-176 °C); 1H-NMR (CDCl3) 9.46 (br, 1H, NH), 8.11 (d, J = 15.0, 1H, H-6), 3.27 (d, J = 5.0, 3H, NMe), 1.70 (s, 6H, H-7); 13C-NMR (CDCl3), 165.71 (C-1), 104.62 (C-2), 163.99 (C-3), 84.44 (C-5), 160.97 (C-6), 26.81 (C-7), 36.71 (NMe); EI-MS 185 (M+, 2), 128 (3), 99 (3), 84 (15), 83 (55), 71 (12), 69 (6), 59 (10), 55 (100), 54 (64), 53 (38).
5-Ethylaminomethylene-2,2-dimethyl-1,3-dioxane-4,6- dione (1c)
M.p. 112.9-113.6 °C; 1H-NMR (CDCl3) 9.54 (br, 1H, NH), 8.14 (d, J = 15.0, 1H, H-6), 3.52 (qd, J = 7.2, 5.3, 2H, NEt), 1.71 (s, 6H, H-7), 1.36 (t, J = 7.2, 3H, NEt); 13C-NMR (CDCl3) 165.70 (C-1), 104.62 (C-2), 164.08 (C-3), 84.26 (C-5), 159.28 (C-6), 26.84 (C-7), 45.22 and 15.57 (NEt); EI-MS 199 (M+, 1), 142 (1), 97 (4), 84 (6), 82 (3), 71 (12), 70 (8), 69 (83), 68 (100), 67 (12), 59 (6), 56 (8), 54 (14), 53 (31).
5-(1-Methylethylaminomethylene)-2,2-dimethyl-1,3- dioxane-4,6-dione (1d)
M.P. 104.4-105.0 °C; 1H-NMR (CDCl3) 9.52 (br, 1H, NH), 8.16 (d, J = 15.0, 1H, H-6), 3.73 (m, 1H, NCH), 1.70 (s, 6H, H-7), 1.36 (d, J = 6.7, 6H, NCHMe2); 13C-NMR (CDCl3) 165.65 (C-1), 104.57 (C-2), 164.14 (C-3), 84.02 (C-5), 157.49 (C-6), 26.85 (C-7), 52.18 and 23.09 (NCHMe2); EI-MS 213 (M+, 1), 156 (1), 111 (5), 96 (11), 85 (9), 83 (9), 82 (43), 70 (30), 69 (100), 68 (85), 67 (12), 59 (20), 58 (8), 54 (13), 53 (44).
5-(2-Methylpropylaminomethylene)-2,2-dimethyl-1,3- dioxane-4,6-dione (1e)
M.p. 151.1-151.8 °C; 1H-NMR (CDCl3) 9.58 (br, 1H, NH), 8.09 (d, J = 14.8, 1H, H-6), 3.27 (t, J = 6.5, 2H, NCH2), 1.93 (m, 1H, CHMe2), 1.71 (s, 6H, H-7), 0.99 (d, J = 6.7, 6H, CHMe2); 13C-NMR (CDCl3), 165.79 (C-1), 104.67 (C-2), 164.06 (C-3), 84.27 (C-5) 160.00 (C-6), 26.87 (C-7), 58.04, 29.30 and 19.60 (NCH2CHMe2); EI-MS 227 (M+, 2), 170 (3), 145 (1), 126 (10), 125 (6), 96 (3), 85 (2), 84 (6), 83 (32), 82 (26), 70 (100), 69 (48), 68 (27), 67 (5), 59 (12), 58 (15), 57 (15), 56 (23), 55 (30), 54 (33), 53 (80).
5-(Dimethylethylaminomethylene)-2,2-dimethyl-1,3- dioxane-4,6-dione (1f)
M.p. 148.2-149.1 °C (lit. [18] 151-153 °C); 1H-NMR (CDCl3) 9.84 (br, 1H, NH), 8.22 (d, J = 15.3, 1H, H-6), 1.71 (s, 6H, H-7), 1.42 (s, 9H, CMe3); 13C-NMR (CDCl3) 165.73 (C-1), 104.58 (C-2), 164.24 (C-3), 83.97 (C-5), 155.44 (C-6), 26.86 (C-7), 55.10 and 29.62 (NCMe3); EI-MS 227 (M+, 0.5), 154 (1), 114 (3), 110 (2), 85 (2), 84 (2), 83 (3), 82 (6), 80 (7), 70 (88), 69 (100), 68 (12), 67 (6), 59 (25), 58 (24), 57 (91), 56 (15), 55 (10), 54 (3), 53 (16).
5-(benzylaminomethylene)-2,2-dimethyl-1,3-dioxane-4,6- dione (1g)
M.p. 164.8-166.5 °C; 1H-NMR (CDCl3) 9.79 (br, 1H, NH), 8.22 (d, J = 14.7, 1H, H-6), 7.39 (m, 3H), 7.28 (m, 2H), 4.61 (d, J = 6.0, 2H, NCH2), 1.71 (s, 6H, H-7); 13C-NMR (CDCl3) 165.60 (C-1), 104.72 (C-2), 163.94 (C-3), 84.92 (C-5), 159.63 (C-6), 26.89 (C-7), 134.90 (C, Ph), 129.22 (2 CH, Ph), 127.72 (2 CH, Ph), 128.73 (CH, Ph), 54.04 (NCH2); EI-MS 262 (M++1, 5), 261 (M+, 25), 246 (2), 204 (15), 203 (23), 174 (28), 159 (14), 130 (75), 104 (17), 102 (27), 91 (100), 86 (8), 85 (4), 84 (12), 83 (2), 77 (5), 70 (4), 69 (4), 66 (9), 65 (14), 59 (7), 58 (9), 57 (6), 56 (4), 55 (4), 54 (4), 53 (6), 52 (10), 51 (10).
5-(Anilinomethylene)-2,2-dimethyl-1,3-dioxane-4,6-dione (1h)
M.p. 155.4-156.1°C (lit. [6a] 155-156 °C); 1H-NMR (CDCl3) 11.24 (d, J = 14.7, 1H, NH), 8.65 (d, J = 14.7, 1H, H-6), 7.44 (m, 2H), 7.26 (m, 3H), 1.75 (s, 6H, H-7); 13C-NMR (CDCl3) 165.51 (C-1), 105.13 (C-2), 163.52 (C-3), 87.23 (C-5), 152.62 (C-6), 27.03 (C-7), 137.79 (C, Ph), 130.08 (2 CH, Ph), 126.82 (CH, Ph), 118.03 (2 CH, Ph); EI-MS 248 (M++1, 2), 247 (M+, 12), 189 (26), 172 (3), 161 (3), 144 (64), 117 (100), 116 (9), 104 (7), 93 (14), 90 (28), 89 (17), 86 (2), 84 (2), 77 (26), 69 (2), 66 (3), 65 (4), 59 (4), 58 (4), 54 (4), 53 (6), 52 (8), 51 (9).
References
- Rappoport, Z. (Ed.) The Chemistry of Enamines; John Wiley & Sons: Chichester-New York-Brisbane-Toronto-Singapore, 1994; Part 1.
- Sweeney, T. R.; Strube, R. E. Burger‘s Medicinal Chemistry, 4th edn; Wolff, M. E., Ed.; Wiley: New York, 1979; Part II; p. 333. [Google Scholar] [PubMed]
- Dane, E.; Drees, F.; Konard, P.; Dockner, T. Angew. Chem. 1962, 74, 873. Dane, E.; Drees, F.; Konard, P.; Dockner, T. Belg. Pat. 616 915; Chem. Abstr. 1963, 59, 1754. Dane, E.; Dockner, T. Angew. Chem. 1964, 76, 342. [PubMed]
- Garcia-Martin, M. G.; Gasch, C.; Gómez-Sánchez, A.; Diánez, M. J.; Castro, A. L. Carbohydr. Res. 1987, 162, 181. Gómez-Sánchez, A.; Garcia-Martin, M. G.; Pascual, C.; Diánez, M. J.; Castro, A. L. Carbohydr. Res. 1986, 149, 329. Gómez-Sánchez, A.; Moya, P. B.; Bellanato, J. Carbohydr. Res. 1984, 135, 101. [PubMed]
- Uray, G.; Wolfbeis, O. S.; Junek, H. J. Mol. Struct. 1979, 54, 77. [CrossRef]
- Gómez-Sánchez, A.; Gracia-Martin, M.; Borrachero, P.; Bellanato, J. J. Chem. Soc. Perkin Trans. 2 1987, 301. Gómez-Sánchez, A.; Sempere, E.; Bellanato, J. J. Chem. Soc. Perkin Trans. 2 1981, 561. Smith, D.; Taylor, P. J. J. Chem. Soc. Perkin Trans. 2 1979, 1376.
- Couchouron, B.; Le Saint, J.; Courtot, P. Bull. Soc. Chim. Fr. 1983, II-66.
- Bogdanov, V. S.; Krasnaya, Z. A.; Stytsenko, T. S. Izv. Akad. Nauk SSSR Ser. Khim. 1990, 1304. Bogdanov, V. S.; Ugrak, B. I.; Krasnaya, Z. A.; Stytsenko, T. S. Izv. Akad. Nauk SSSR Ser. Khim. 1990, 356.
- Kreutzmann, J.; Michalik, M.; Thomas, B. Z. Chem. 1986, 26, 404.
- Diánez, M. J.; López-Castro, A. Acta Cryst. C 1991, 47, 1028. Blake, A. J.; Hunter, G. A.; McNab, H. J. Chem. Res. (S) 1989, 118. Diánez, M. J.; López-Castro, A.; Márquez, R. Acta Cryst. C 1987, 43, 558. Shmueli, U.; Shanan-Atidi, H.; Horwitz, H.; Shvo, Y. J. Chem. Soc. Perkin Trans. 2 1973, 657.
- Boykin, W. D. (Ed.) 17O NMR Spectroscopy in Organic Chemistry; CRC Press: Boca Raton, FL, 1991; and references cited therein.
- Zhuo, J.-C.; Wyler, H.; Péchy, P.; Dahn, H. Helv. Chim. Acta. 1994, 77, 317. Jaccard, G.; Carrupt, P.-A.; Lauterwein, J. Magn. Reson. Chem. 1988, 26, 239. Baumstark, A. L.; Grahan, S. S.; Boykin, D. W. Tetrahedron Lett. 1990, 31, 957. Baumstark, A. L.; Grahan, S. S.; Boykin, D. W. J. Chem. Soc. Chem. Comm. 1989, 767. Jaccard, G.; Lauterwein, J. Helv. Chim. Acta. 1986, 69, 1469. Boykin, D. W.; Baumstark, A. L.; Beeson, M. J. Org. Chem. 1991, 56, 1969. Baumstark, A. L.; Boykin, D. W. New J. Chem. 1992, 16, 357. Boykin, D. W.; Chandrasekaran, S.; Baumstark, A. L. Mag. Reson. Chem. 1993, 31, 489. Boykin, D. W.; Kumar, A. J. Mol. Struct. 1993, 298, 121. Nowak-Wydra, B.; Allison, L. W.; Kumar, A.; Boykin, D. W. J. Chem. Research (S). 1991, 490. Boykin, D. W.; Kumar, A. J. Heterocycl. Chem. 1992, 29, 1. [PubMed]
- Zhuo, J.-C. Magn. Reson. Chem. 1997, 35, 21. Zhuo, J.-C. Magn. Reson. Chem. 1997, 35. in press.
- Smith, J. W. The Chemistry of the Emino Group; Patai, S., Ed.; Wiley: Chichester, 1968; Charp. 4. [Google Scholar]
- Zhuo, J.-C. Magn. Reson. Chem. 1996, 34, 595. [CrossRef]
- March, J. Advanced Organic Chemistry, 4th Ed ed; John Wiley and Sons: New York, 1992; p. 248. [Google Scholar]
- Bihlmayer, G. A.; Derflinger, G.; Derkosch, J.; Polansky, O. E. Monatsh. Chem. 1967, 98, 564.
- McNab, H.; Monahan, L. C. J. Chem. Soc. Perkin Trans. 1 1988, 863.
- Sample Availability: Most of the samples published here and in ref. 12a, 13, 15 are available from MDPI.
© 1997 MDPI. All rights reserved
