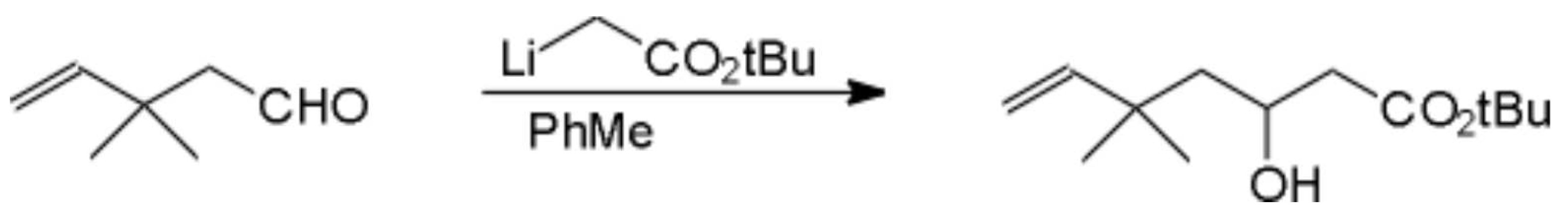
tert-Butyl-3-hydroxy-5,5-dimethyl-6-heptenoate
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes
- Sakan, K.; Smith, D. A.; Babirad, S. A.; Fronczek, F. R.; Houk, K. N. J. Org. Chem. 1991, 56, 2311.
- Smith, D. A.; Sakan, K.; Houk, K. N. Tetrahedron Lett. 1986, 27, 4877.
- Sakan, K.; Smith, D. A. Tetrahedron Lett. 1984, 25, 2081.
- Gadwood, R. C.; Lett, R. M.; Wissinger, J. E. J. Am. Chem. Soc. 1986, 108, 6343.
- Boeckman, R. K., Jr.; Ko, S. S. J. Am. Chem. Soc. 1982, 104, 1033.
- Rathke, M. W.; Sullivan, D. F. J. Am. Chem. Soc. 1973, 95, 3050.
- Sample Availability: No sample available.
© 1997 MDPI. All rights reserved
Share and Cite
Smith, D.A. tert-Butyl-3-hydroxy-5,5-dimethyl-6-heptenoate. Molecules 1997, 2, M29. https://doi.org/10.3390/M29
Smith DA. tert-Butyl-3-hydroxy-5,5-dimethyl-6-heptenoate. Molecules. 1997; 2(10):M29. https://doi.org/10.3390/M29
Chicago/Turabian StyleSmith, Douglas A. 1997. "tert-Butyl-3-hydroxy-5,5-dimethyl-6-heptenoate" Molecules 2, no. 10: M29. https://doi.org/10.3390/M29
APA StyleSmith, D. A. (1997). tert-Butyl-3-hydroxy-5,5-dimethyl-6-heptenoate. Molecules, 2(10), M29. https://doi.org/10.3390/M29



