Simple Rain-Shelter Cultivation Prolongs Accumulation Period of Anthocyanins in Wine Grape Berries
Abstract
:1. Introduction
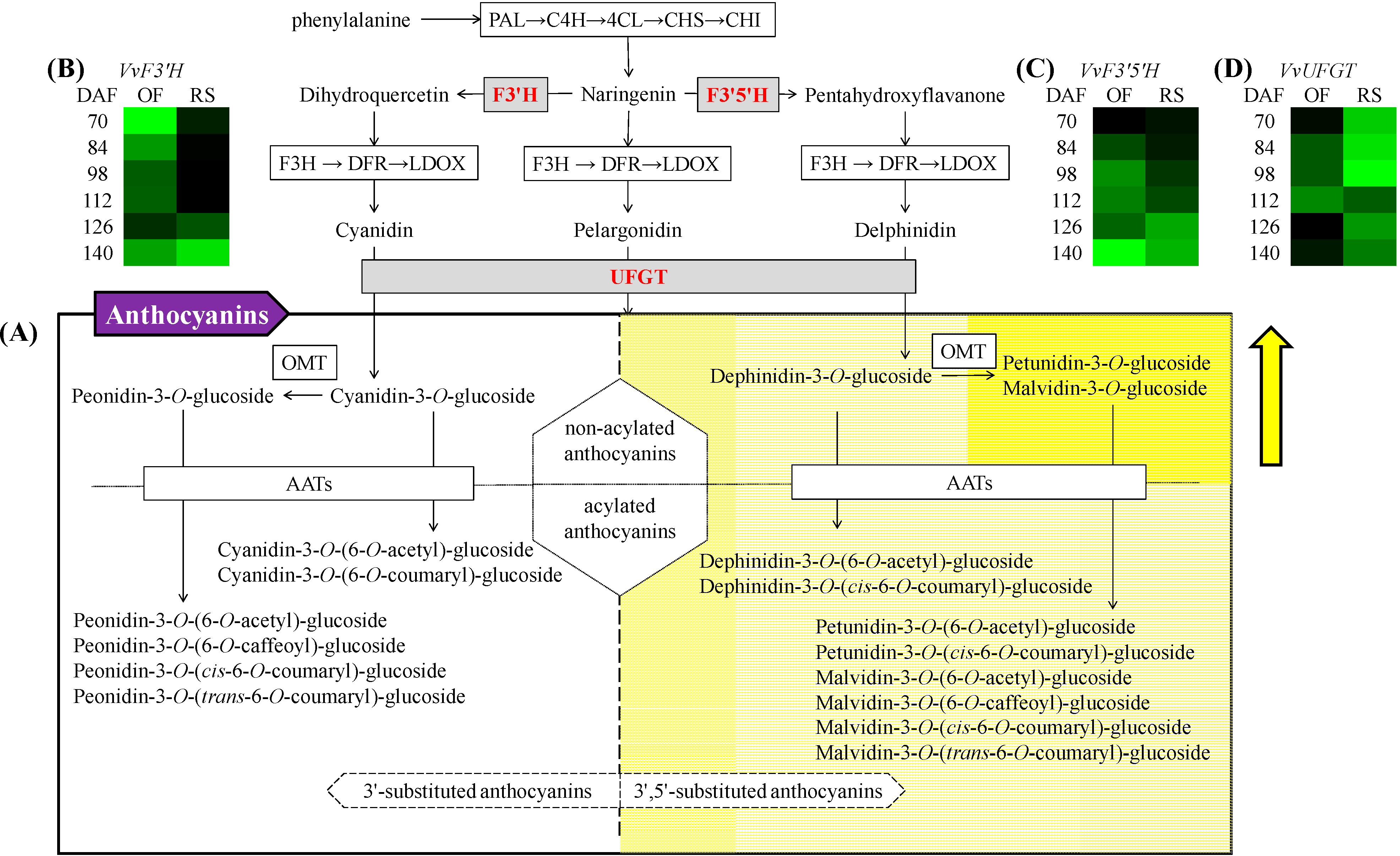
2. Results and Discussion
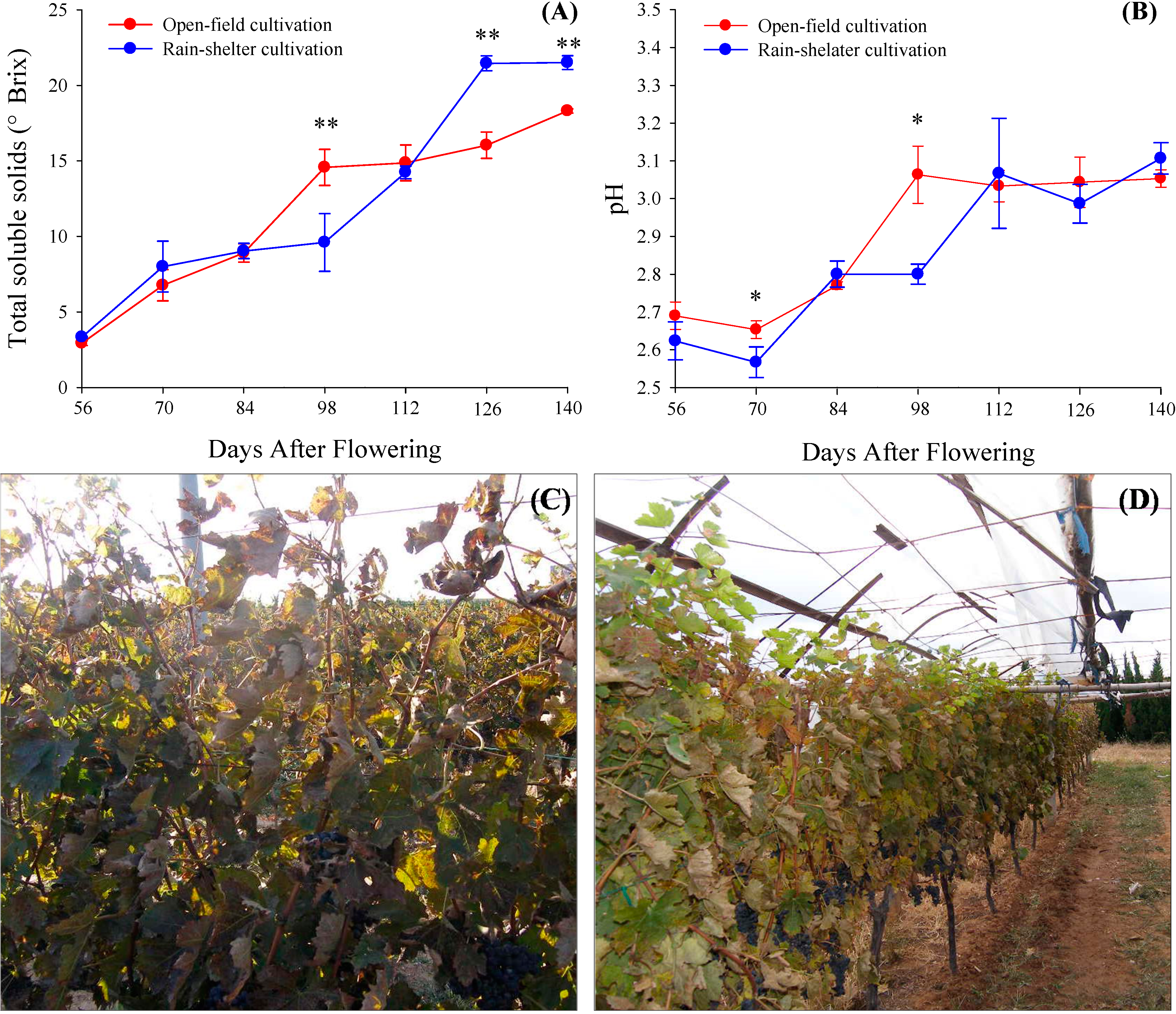
2.1. Total Soluble Solid and pH Value
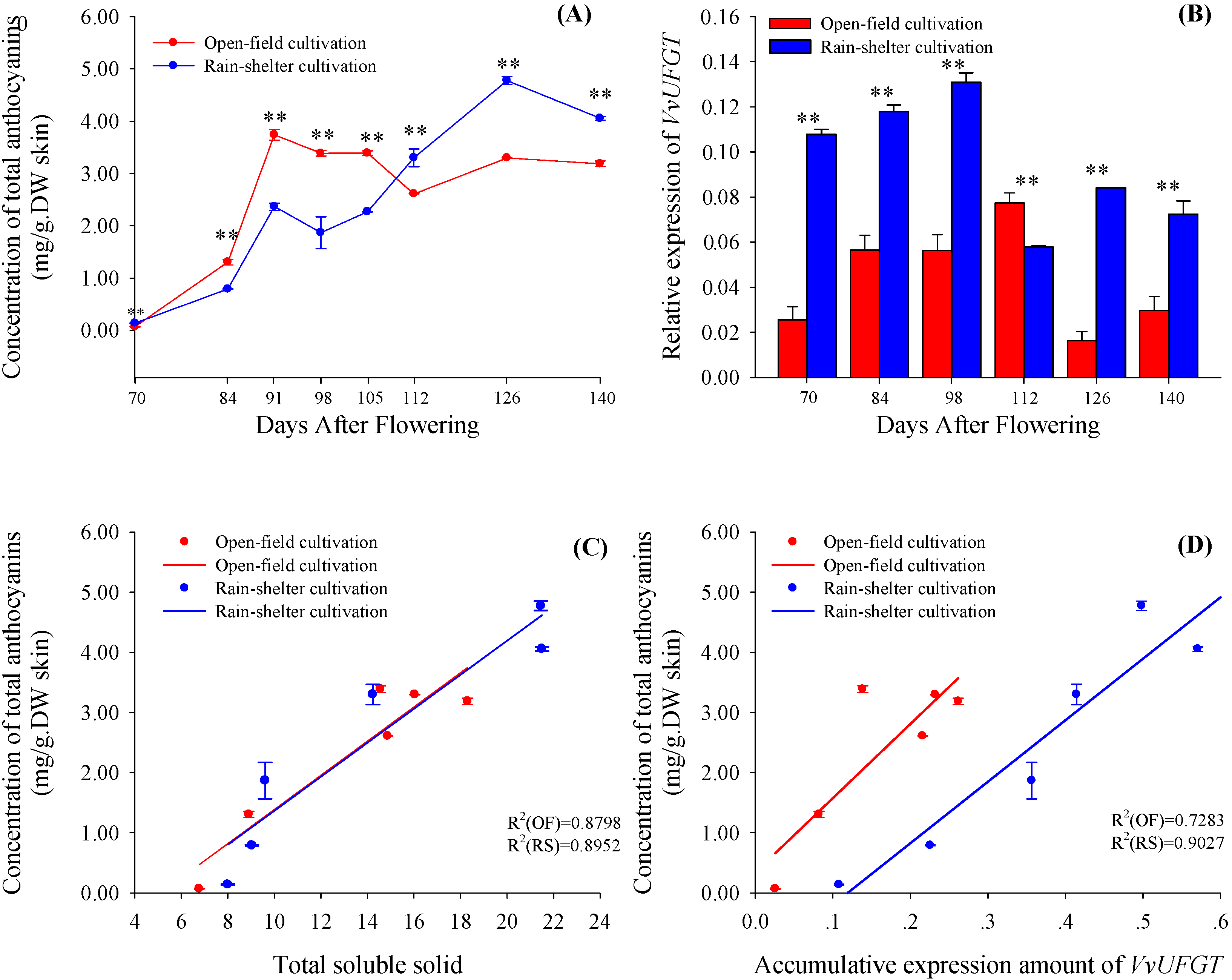
2.2. Accumulation of Anthocyanins and Expression of VvUFGT
| Anthocyanins (mg/kg DW skin) | 70 DAF | 84 DAF | 91 DAF | 98 DAF | 105 DAF | 112 DAF | 126 DAF | 140 DAF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rain-Shelter | Open-Field | Rain-Shelter | Open-Field | Rain-Shelter | Open-Field | Rain-Shelter | Open-Field | Rain-Shelter | Open-Field | Rain-Shelter | Open-Field | Rain-Shelter | Open-Field | Rain-Shelter | Open-Field | ||
| Dephinidin-3-O-glucoside | 14.5 ± 0.4a | 7.8 ± 0.9b | 77.6 ± 0.9b | 172.3 ± 5.5a | 212.4 ± 7.2b | 420.8 ± 18.6a | 94.8 ± 13.2b | 238.4 ± 7.2a | 149.4 ± 3.2b | 286.4 ± 8.0a | 191.7 ± 4.9a | 174.0 ± 4.9b | 491.5 ± 26.2a | 268.7 ± 11.6b | 403.7 ± 13.6a | 181.2 ± 7.1b | |
| Petunidin-3-O-glucoside | 9.8 ± 0.4a | 5.3 ± 0.7b | 50.3 ± 3.5b | 95.5 ± 3.8a | 146.5 ± 11.4b | 252.4 ± 8.4a | 75.5 ± 12.4b | 166.5 ± 11.5a | 106.1 ± 9.9b | 189.4 ± 5.2a | 140.6 ± 5.0a | 109.9 ± 2.8b | 303.8 ± 17.3a | 164.5 ± 7.7b | 231.6 ± 1.6a | 122.1 ± 10.7b | |
| Malvidin-3-O-glucoside | 34.8 ± 2.0a | 12.9 ± 0.8b | 280.9 ± 1.0b | 392.6 ± 13a | 895.3 ± 21.2b | 1291.2 ± 32.2a | 720.8 ± 103.0b | 1329.1 ± 22.6a | 882.9 ± 0.9b | 1252.6 ± 25.7a | 1366.1 ± 59.2a | 998.3 ± 1.8a | 1831 ± 23.2a | 1291.3 ± 1.6b | 1595.4 ± 1.3a | 1323.3 ± 5.6b | |
| Dephinidin-3-O-(6-O-acetyl)-glucoside | 8.1 ± 0.4a | 3.8 ± 0.1b | 28.8 ± 1.1b | 51.8 ± 1.7a | 73.9 ± 0.2b | 121.8 ± 0.8a | 47.2 ± 2.4b | 85.5 ± 3.9a | 59.6 ± 0.4b | 95.6 ± 0.8a | 79.7 ± 3.4a | 72.8 ± 1.7a | 148.6 ± 0.2a | 90.5 ± 4.8b | 135.9 ± 3.9a | 82.3 ± 0.6b | |
| Petunidin-3-O-(6-O-acetyl)-glucoside | 6.1 ± 0.6a | 3.4 ± 0.0b | 19.1 ± 0.2b | 35.3 ± 1.8a | 53.0 ± 4.1b | 87.0 ± 1.7a | 29.5 ± 4.2b | 61.8 ± 2.5a | 41.3 ± 3.0b | 68.5 ± 0.1a | 54.8 ± 3.7a | 39.1 ± 2.3b | 104.9 ± 0.9a | 52.0 ± 1.5b | 89.5 ± 5.3a | 41.1 ± 4.2b | |
| Malvidin-3-O-(6-O-acetyl)-glucoside | 28.0 ± 3.1a | 10.9 ± 0.5b | 153.0 ± 8.2b | 196.6 ± 6.1a | 475.2 ± 2.7b | 647.8 ± 10.3a | 472.2 ± 4.6b | 782.6 ± 3.3a | 516.3 ± 13.8b | 709.5 ± 0.8a | 782.3 ± 48.2a | 582.4 ± 3.0b | 883.6 ± 1.7a | 673.8 ± 2.4b | 836.4 ± 10.9a | 700.2 ± 10.1b | |
| Dephinidin-3-O-(cis-6-O-coumaryl)-glucoside | - | - | 4.9 ± 0.1b | 9.4 ± 0.8a | 16.6 ± 1.7b | 27.3 ± 3.7a | 10.2 ± 4.8b | 20.4 ± 0.9a | 13.8 ± 0.8b | 22.8 ± 0.3a | 17.4 ± 1.8a | 14.6 ± 0.1a | 30.4 ± 0.5a | 18.5 ± 0.3b | 25.6 ± 1.6a | 16.0 ± 0.4b | |
| Malvidin-3-O-(6-O-caffeoyl)-glucoside | - | - | - | - | - | - | - | - | 5.3 ± 0.5 | - | 7.4 ± 0.8 | - | 7.7 ± 0.5 | - | 11.4 ± 1.7a | 9.4 ± 0.2b | |
| Petunidin-3-O-(cis-6-O-coumaryl)-glucoside | - | - | 4.4 ± 0.3b | 7.9 ± 0.8a | 12.7 ± 0.3b | 19.4 ± 0.8a | 7.8 ± 1.2b | 15.3 ± 0.4a | 9.8 ± 0.4b | 18.0 ± 0.0a | 11.2 ± 1.4a | 12.1 ± 0.3a | 21.5 ± 0.5a | 14.0 ± 0.1b | 16.8 ± 1.4a | 11.8 ± 0.4b | |
| Malvidin-3-O-(cis-6-O-coumaryl)-glucoside | - | - | 5.9 ± 0.3a | 7.7 ± 1.1a | 17.1 ± 1.2a | 21.1 ± 1.1a | 17.9 ± 2.3b | 25.0 ± 0.5a | 18.4 ± 0.2a | 20.7 ± 0.4a | 26.2 ± 3.2a | 25.4 ± 1.0a | 17.2 ± 1.4b | 23.6 ± 1.9a | 16.7 ± 0.9b | 27.8 ± 1.4a | |
| Malvidin-3-O-(trans-6-O-coumaryl)-glucoside | 4.5 ± 0.2a | 3.0 ± 0.3b | 38.6 ± 4.3b | 52.5 ± 5.3a | 156.5 ± 1.0b | 221.2 ± 6.8a | 163.1 ± 29.1b | 274 ± 5.3a | 175.8 ± 11.3b | 250.2 ± 2.7a | 252.1 ± 18.4a | 255.3 ± 6.5a | 294.3 ± 3.3a | 267.4 ± 9.9a | 249.5 ± 2b | 330.2 ± 9.1a | |
| Cyanidin-3-O-glucoside | 6.7 ± 0.2a | 4.9 ± 0.5a | 24.9 ± 2.2b | 64.0 ± 2.4a | 51.2 ± 2.2b | 122.2 ± 3.9a | 26.6 ± 4.1b | 47.7 ± 9.1a | 35.0 ± 4.9b | 71.7 ± 1.1a | 43.2 ± 1.5a | 35.1 ± 3.4b | 106.2 ± 2.9a | 59.9 ± 1.5b | 68.1 ± 3.6a | 33.2 ± 2.9b | |
| Peonidin-3-O-glucoside | 13.0 ± 0.5a | 7.8 ± 0.5b | 61.0 ± 0.3b | 129.2 ± 4.6a | 148.0 ± 6.6b | 299.2 ± 6.9a | 108.9 ± 17.9b | 188.7 ± 5.3a | 140.1 ± 2.8b | 225.8 ± 4.0a | 178.5 ± 7.8a | 160.1 ± 1.2b | 316 ± 6.8a | 213.7 ± 0.8b | 212.3 ± 3.9a | 176.0 ± 5.7b | |
| Cyanidin-3-O-(6-O-acetyl)-glucoside | 3.5 ± 0.2a | 2.6 ± 0.0a | 8.0 ± 0.6b | 17.8 ± 0.2a | 17.7 ± 2.6b | 33.3 ± 0.6a | 12.7 ± 4.6a | 21.6 ± 2.4a | 15.9 ± 2.7b | 24.9 ± 0.2a | 21.3 ± 1.9a | 13.5 ± 1.3b | 34.5 ± 0.3a | 19.6 ± 1.3b | 27.1 ± 3.0a | 17.0 ± 2.6b | |
| Peonidin-3-O-(6-O-acetyl)-glucoside | 7.4 ± 1.0a | 4.4 ± 0.3b | 21.3 ± 0.6b | 41.6 ± 1.9a | 53.3 ± 1.5b | 97.5 ± 0.9a | 46.9 ± 0.9b | 75.1 ± 0.8a | 56.1 ± 0.6b | 83.8 ± 0.5a | 70.2 ± 4.1a | 61.5 ± 1.1b | 101.7 ± 0.1a | 72.7 ± 0.1b | 75.8 ± 2.5a | 63.9 ± 1.4b | |
| Peonidin-3-O-(6-O-caffeoyl)-glucoside | - | - | - | - | - | - | - | - | - | - | 5.5 ± 0.2 | - | 5.3 ± 0.3 | - | 5.8 ± 1.1 | Trace | |
| Cyanidin-3-O-(6-O-coumaryl)-glucoside | - | - | - | 7.7 ± 0.7 | 7.2 ± 0.4b | 15.5 ± 0.3a | 4.7 ± 0.7b | 8.2 ± 0.0a | 6.2 ± 0.0b | 12.7 ± 1.5a | 7.2 ± 0.7 | 6.9 ± 0.1 | 11.5 ± 0.4a | 10.8 ± 1.3a | 8.4 ± 0.8a | 6.4 ± 0.2a | |
| Peonidin-3-O-(cis-6-O-coumaryl)-glucoside | - | - | 2.5 ± 0.1a | 4.0 ± 0.4a | 4.4 ± 0.3b | 7.0 ± 0.4a | 4.4 ± 0.2a | 5.9 ± 0.1a | 5.1 ± 0.2a | 6.0 ± 0.6a | 6.3 ± 1.2a | 5.5 ± 0.3a | 5.7 ± 0.9a | 6.1 ± 0.5a | 5.3 ± 1.1a | 5.5 ± 0.2a | |
| Peonidin-3-O-(trans-6-O-coumaryl)-glucoside | 2.7 ± 0.2a | 2.3 ± 0.1a | 8.9 ± 1.0b | 19.4 ± 2.4a | 26.7 ± 0.2b | 55.2 ± 3.0a | 24.0 ± 4.0b | 42.5 ± 0.5a | 31.2 ± 1.9b | 52.0 ± 0.0a | 38.9 ± 4.1a | 43.6 ± 0.1a | 59.2 ± 1.1a | 50.6 ± 2.6b | 41 ± 0.3b | 48.0 ± 3.3a | |
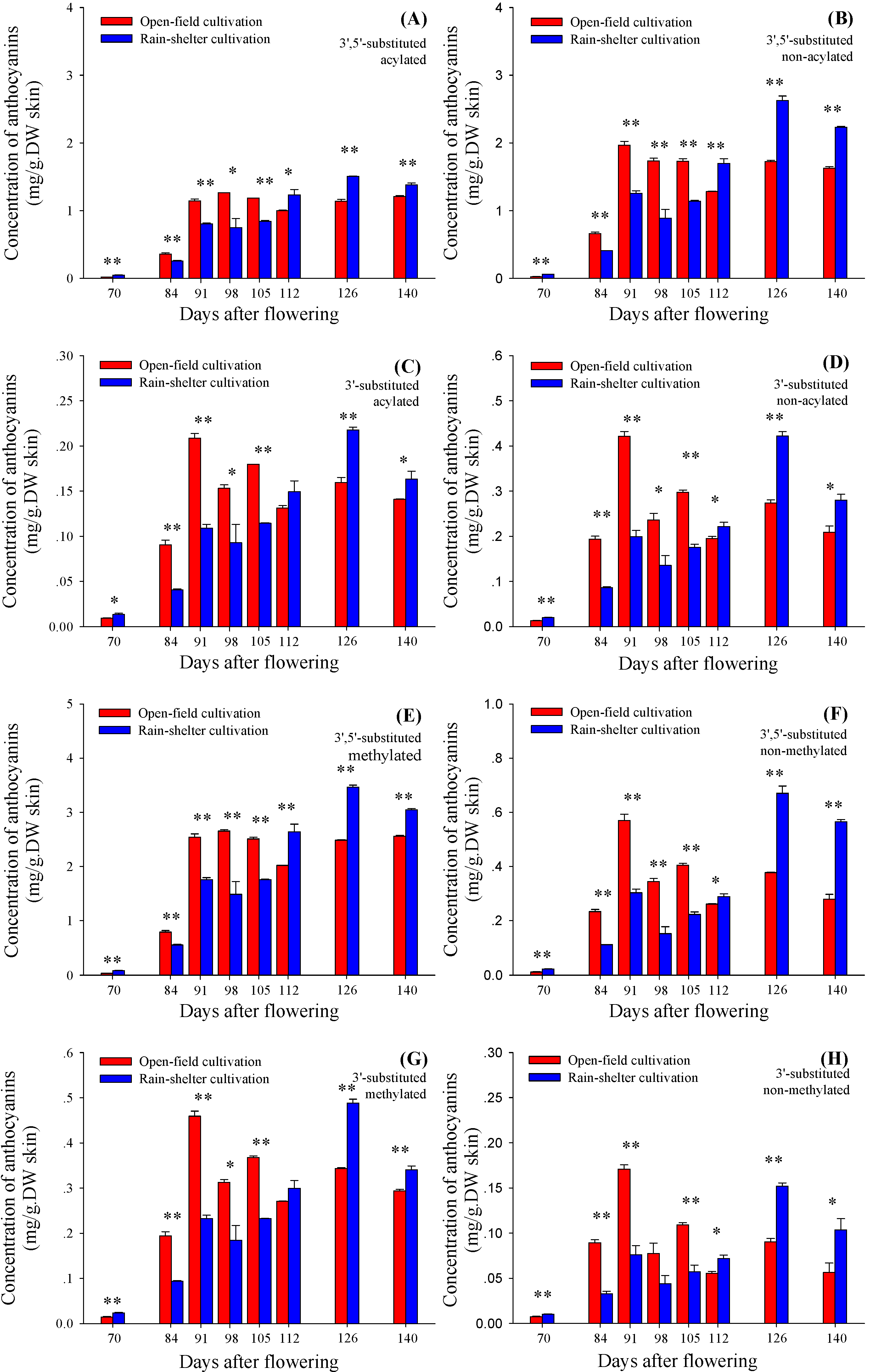
2.3. 3'5'-Substituted and 3'-Substituted Anthocyanins
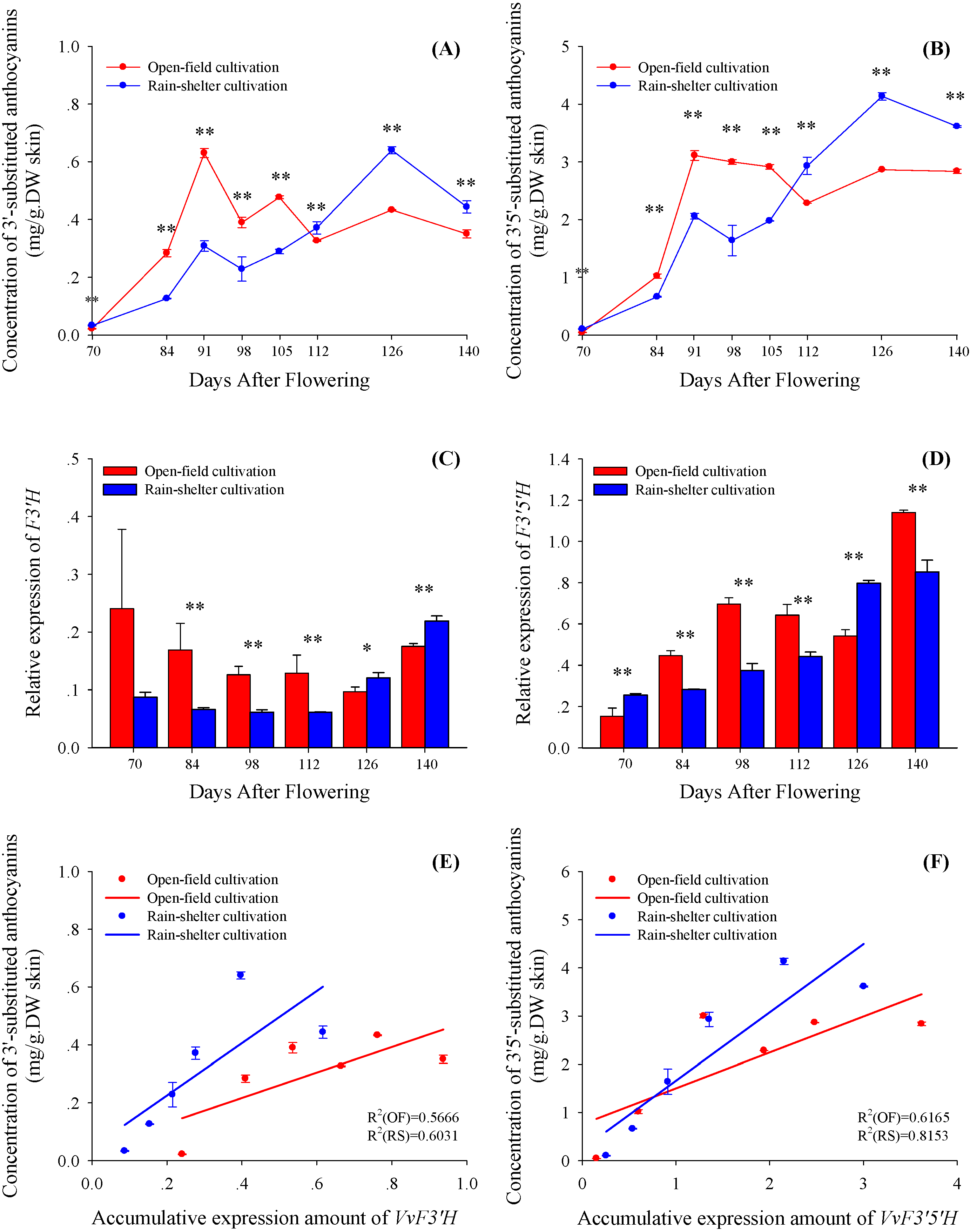
2.4. Acylating and Methylating Modification of 3'5'-Substituted and 3'-Substituted Anthocyanins
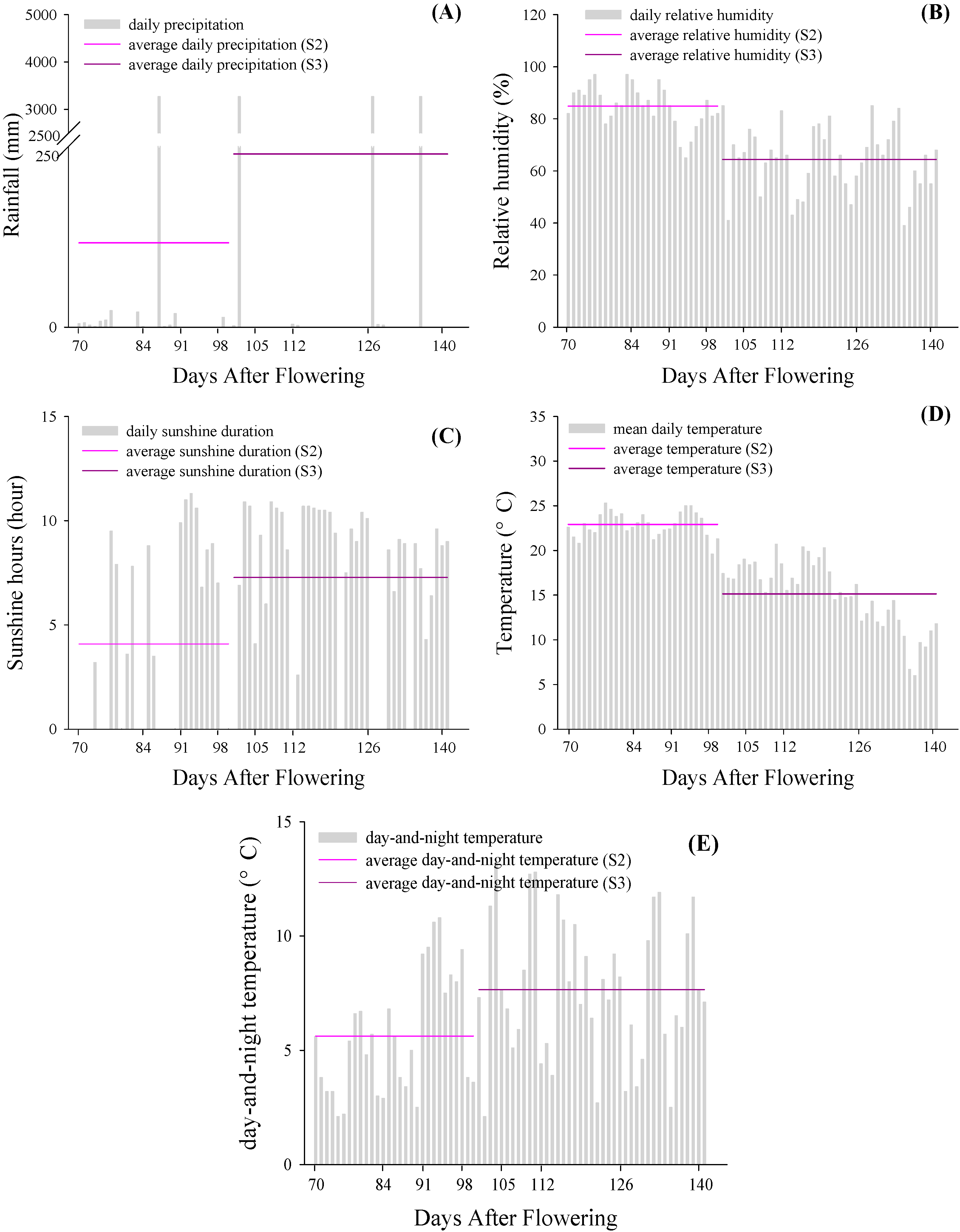
2.5. Climate Characters
3. Experimental Section
3.1. Field Treatment and Sample Collection
3.2. Determination of Physicochemical Parameters
3.3. Extraction and Determination of Anthocyanins from Berry Skins
3.4. Analysis of Transcript Level by Real-Time PCR
| Genes | Genbank Accession | Primer Sequences (5'–3') | Size of PCR Product (bp) |
|---|---|---|---|
| VvF3'H | AJ880357 | F: CCAAGTTTTCGGGAAGTAAATG | 171 |
| VvF3'5'H | AJ880356 | F: GCATGGATGCAGTTAAGTAGAAAA | 113 |
| VvUFGT | AF000372 | F: GGGATGGTAATGGCTGTGG | 253 |
| VvUbiquitin | BN000705 | F: GTGGTATTATTGAGCCATCCTT | 182 |
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Staff, W.-S. Chinese wine. Available online: http://www.wine-searcher.com/regions-china (accessed on 3 March 2013).
- Meng, J.-F.; Ning, P.-F.; Xu, T.-F.; Zhang, Z.-W. Effect of rain-shelter cultivation of Vitis vinifera cv. Cabernet gernischet on the phenolic profile of berry skins and the incidence of grape diseases. Molecules 2012, 18, 381–397. [Google Scholar]
- Chavarria, G.; dos Santos, H.P.; Sônego, O.R.; Marodin, G.A.B.; Bergamaschi, H.; Cardoso, L.S. Incidence of diseases and needs of control in overhead covered grapes. Rev. Bras. Frutic. 2007, 29, 477–482. [Google Scholar]
- Cortell, J.M.; Halbleib, M.; Gallagher, A.V.; Righetti, T.L.; Kennedy, J.A. Influence of vine vigor on grape (Vitis vinifera L. Cv. Pinot Noir) anthocyanins. 1. Anthocyanin concentration and composition in fruit. J. Agric. Food Chem. 2007, 55, 6575–6584. [Google Scholar]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry temperature and solar radiation alter acylation, proportion, and concentration of anthocyanin in merlot grapes. Am. J. Enol. Viticult. 2008, 59, 235–247. [Google Scholar]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar]
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA are involved in phenol metabolism of Vitis vinifera L. Increasing biosynthesis of berry skin polyphenols. J. Agric. Food Chem. 2011, 59, 4874–4884. [Google Scholar]
- Tangolar, S.G.; Tangolar, S.; Blllr, H.; Ozdemir, G.; Sabir, A.; Cevlk, B. The effects of different irrigation levels on yield and quality of some early grape cultivars grown in greenhouse. Asian J. Plant Sci. 2007, 6, 643–647. [Google Scholar]
- Fanizza, G.R.L. The effect of vineyard overhead plastic sheet covering on some morphological and physiological characteristics in the table grape cv. Regina dei Vigneti Vitis vinifera L. Agric. Mediterr. 1991, 121, 239–243. [Google Scholar]
- Júnior, M.J.P.; Hernandes, J.L.; de Souza Rolim, G.; Blain, G.C. Microclimate and yield of “niagara rosada” grapevine grown in vertical upright trellis and “y” shaped under permeable plastic cover overhead. Rev. Bras. Frutic. 2011, 33, 511–518. [Google Scholar]
- Chavarria, G.; dos Santos, H.P.; Zanus, M.C.; Marodin, G.A.B.; Zorzan, C. Plastic cover use and its influences on physical-chemical characteristics in must and wine. Rev. Bras. Frutic. 2011, 33, 809–815. [Google Scholar]
- Guo, Y.-P.; Guo, D.-P.; Zhou, H.-F.; Hu, M.-J.; Shen, Y.-G. Photoinhibition and xanthophyll cycle activity in bayberry (Myrica rubra) leaves induced by high irradiance. Photosynthetica 2006, 44, 439–446. [Google Scholar]
- Sasaki, H.; Yano, T.; Yamasaki, A. Reduction of high temperature inhibition in tomato fruit set by plant growth regulators. JARQ-Jpn. Agr. Res. Q. 2005, 39, 135–138. [Google Scholar]
- Yoon, J.; Ji, J.; Lim, S.; Lee, K.; Kim, H.; Jeong, H.; Lee, J. Changes in selected components and antioxidant and antiproliferative activity of peppers depending on cultivation. J. Korean Soc. Food Sci. Nutr. 2010, 39, 731–736. [Google Scholar]
- Larsson, S.; Górny, A. Grain yield and drought resistance indices of oat cultivars in field rain shelter and laboratory experiments. J. Agron. Crop Sci. 1988, 161, 277–286. [Google Scholar]
- Kim, J.; Jo, J.; Kim, H.; Sub-Station, N.; Ryou, M.; Kim, J.; Hwang, H.; Hwang, Y. Growth and fruit characteristics of blueberry “northland” cultivar as influenced by open field and rain shelter house cultivation. J. Biol. Environ. Control 2011, 20, 387–393. [Google Scholar]
- Polat, A.A.; Durgac, C.; Caliskan, O. Effect of protected cultivation on the precocity, yield and fruit quality in loquat. Sci. Hortic. 2005, 104, 189–198. [Google Scholar]
- Heraud, P.; Beardall, J. Changes in chlorophyll fluorescence during exposure of dunaliella tertiolecta to UV radiation indicate a dynamic interaction between damage and repair processes. Photosynth. Res. 2000, 63, 123–134. [Google Scholar]
- Pfündel, E.E. Action of UV and visible radiation on chlorophyll fluorescence from dark-adapted grape leaves (Vitis vinifera L.). Photosynth. Res. 2003, 75, 29–39. [Google Scholar]
- Boss, P.K.; Davies, C.; Robinson, S.P. Anthocyanin composition and anthocyanin pathway gene expression in grapevine sports differing in berry skin colour. Aust. J. Grape Wine Res. 1996, 2, 163–170. [Google Scholar]
- Bridle, P.; Timberlake, C. Anthocyanins as natural food colours-selected aspects. Food Chem. 1997, 58, 103–109. [Google Scholar]
- Kim, B.; Lee, H.; Park, Y.; Lim, Y.; Ahn, J.-H. Characterization of an O-methyltransferase from soybean. Plant Physiol. Biochem. 2006, 44, 236–241. [Google Scholar]
- Suelves, M.; Puigdomènech, P. Specific mrna accumulation of a gene coding for an O-methyltransferase in almond (Prunus amygdalus, batsch) flower tissues. Plant Sci. 1998, 134, 79–88. [Google Scholar]
- Bakker, J.; Timberlake, C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar]
- Chavarria, G.; dos Santos, H.P.; Zanus, M.C.; Marodin, G.A.B.; Chalaça, M.Z.; Zorzan, C. Grapevine maturation of moscato giallo under plastic cover. Rev. Bras. Frutic. 2010, 32, 151–160. [Google Scholar]
- Li, Z.; Pan, Q.; Jin, Z.; Mu, L.; Duan, C. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in china. Food Chem. 2011, 125, 77–83. [Google Scholar]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Viticult. 2002, 53, 171–182. [Google Scholar]
- Liang, N.-N.; Pan, Q.-H.; He, F.; Wang, J.; Reeves, M.J.; Duan, C. Phenolic profiles of Vitis davidii and Vitis quinquangularis species native to China. J. Agric. Food Chem. 2013, 61, 6016–6027. [Google Scholar]
- Revilla, E.; García-Beneytez, E.; Cabello, F. Anthocyanin fingerprint of clones of tempranillo grapes and wines made with them. Aust. J. Grape Wine Res. 2009, 15, 70–78. [Google Scholar]
- Wang, H.; Race, E.J.; Shrikhande, A.J. Anthocyanin transformation in Cabernet Sauvignon wine during aging. J. Agric. Food Chem. 2003, 51, 7989–7994. [Google Scholar]
- Villiers, A.D.; Vanhoenacker, G.; Majek, P.; Sandra, P. Determination of anthocyanins in wine by direct injection liquid chromatography-diode array detection-mass spectrometry and classification of wines using discriminant analysis. J. Chromatogr. A 2004, 1054, 195–204. [Google Scholar]
- Han, F.-L.; Zhang, W.-N.; Pan, Q.-H.; Zheng, C.-R.; Chen, H.-Y.; Duan, C.-Q. Principal component regression analysis of the relation between cielab color and monomeric anthocyanins in young Cabernet Sauvignon wines. Molecules 2008, 13, 2859–2870. [Google Scholar]
- García-Beneytez, E.; Cabello, F.; Revilla, E. Analysis of grape and wine anthocyanins by HPLC-MS. J. Agric. Food Chem. 2003, 51, 5622–5629. [Google Scholar]
- Downey, M.O.; Rochfort, S. Simultaneous separation by reversed-phase high-performance liquid chromatography and mass spectral identification of anthocyanins and flavonols in Shiraz grape skin. J. Chromatogr. A 1201, 43–47. [Google Scholar]
- Núñez, V.; Monagas, M.; Gomez-Cordoves, M.; Bartolomé, B. Vitis vinifera L. cv. Graciano grapes characterized by its anthocyanin profile. Postharvest Biol. Technol. 2004, 31, 69–79. [Google Scholar]
- Zhang, Z.-Z.; Che, X.-N.; Pan, Q.-H.; Li, X.-X.; Duan, C.-Q. Transcriptional activation of flavan-3-ols biosynthesis in grape berries by UV irradiation depending on developmental stage. Plant Sci. 2013, 208, 64–74. [Google Scholar]
- Bogs, J.; Ebadi, A.; McDavid, D.; Robinson, S.P. Identification of the flavonoid hydroxylases from grapevine and their regulation during fruit development. Plant Physiol. 2006, 140, 279–291. [Google Scholar]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.-M.; Robinson, S.P.; Barrieu, F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, X.-X.; He, F.; Wang, J.; Li, Z.; Pan, Q.-H. Simple Rain-Shelter Cultivation Prolongs Accumulation Period of Anthocyanins in Wine Grape Berries. Molecules 2014, 19, 14843-14861. https://doi.org/10.3390/molecules190914843
Li X-X, He F, Wang J, Li Z, Pan Q-H. Simple Rain-Shelter Cultivation Prolongs Accumulation Period of Anthocyanins in Wine Grape Berries. Molecules. 2014; 19(9):14843-14861. https://doi.org/10.3390/molecules190914843
Chicago/Turabian StyleLi, Xiao-Xi, Fei He, Jun Wang, Zheng Li, and Qiu-Hong Pan. 2014. "Simple Rain-Shelter Cultivation Prolongs Accumulation Period of Anthocyanins in Wine Grape Berries" Molecules 19, no. 9: 14843-14861. https://doi.org/10.3390/molecules190914843
APA StyleLi, X.-X., He, F., Wang, J., Li, Z., & Pan, Q.-H. (2014). Simple Rain-Shelter Cultivation Prolongs Accumulation Period of Anthocyanins in Wine Grape Berries. Molecules, 19(9), 14843-14861. https://doi.org/10.3390/molecules190914843







