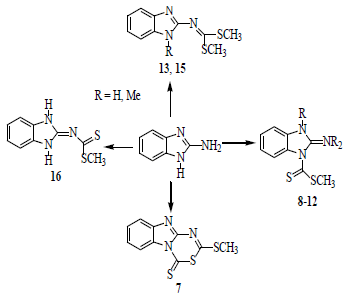
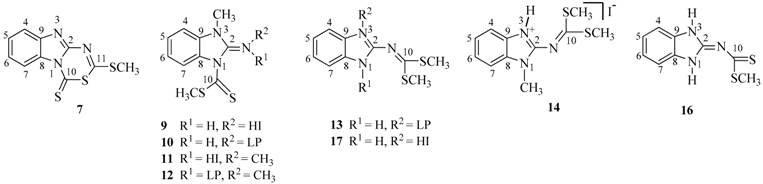
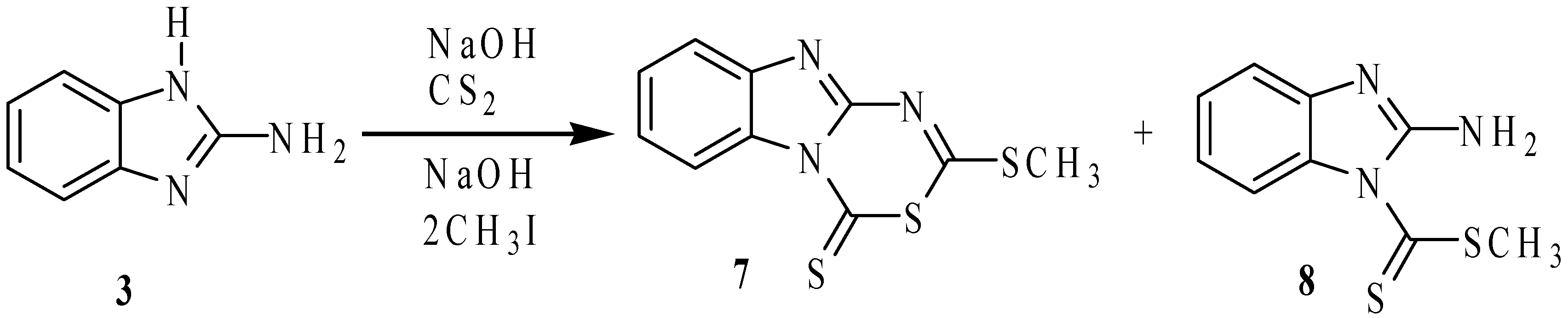
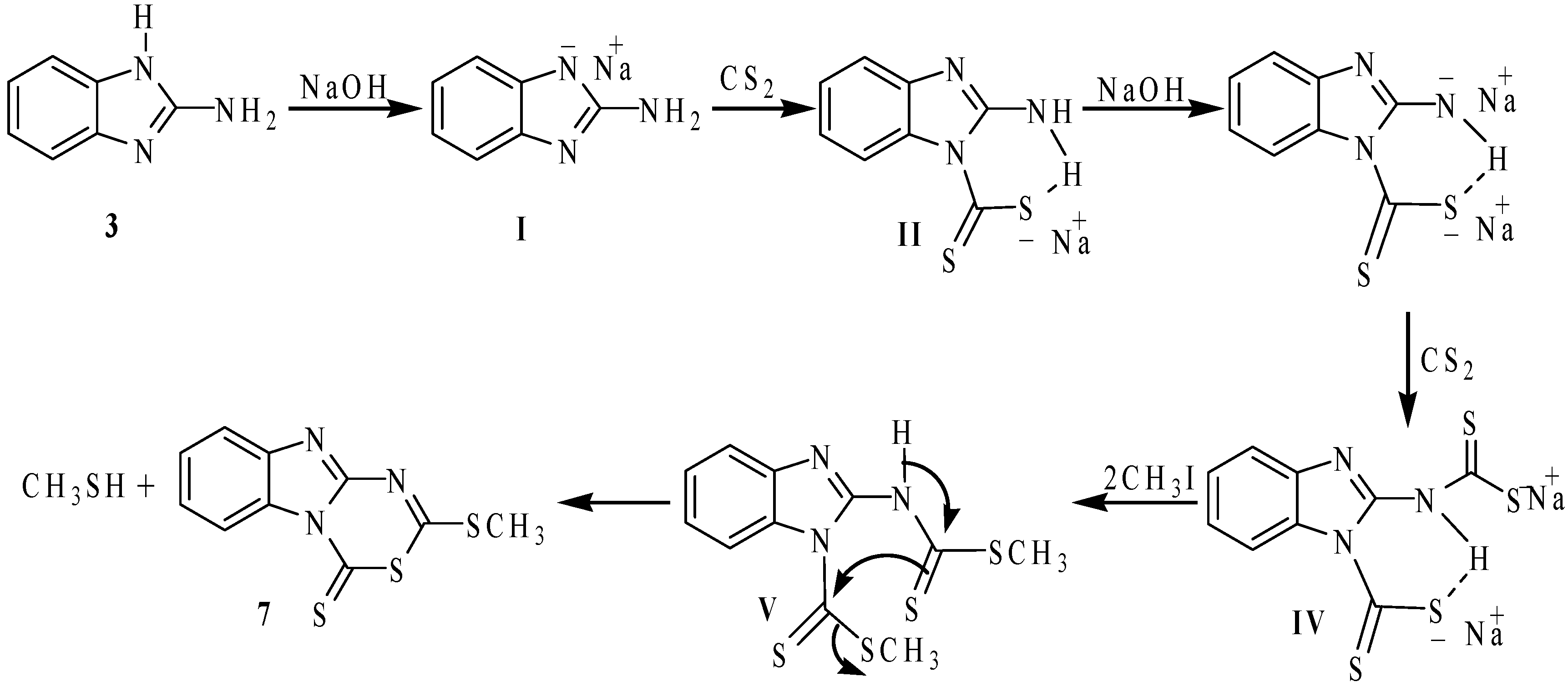
Synthesis and Structure of Sulfur Derivatives from 2-Aminobenzimidazole
Abstract
:1. Introduction

2. Results and Discussion
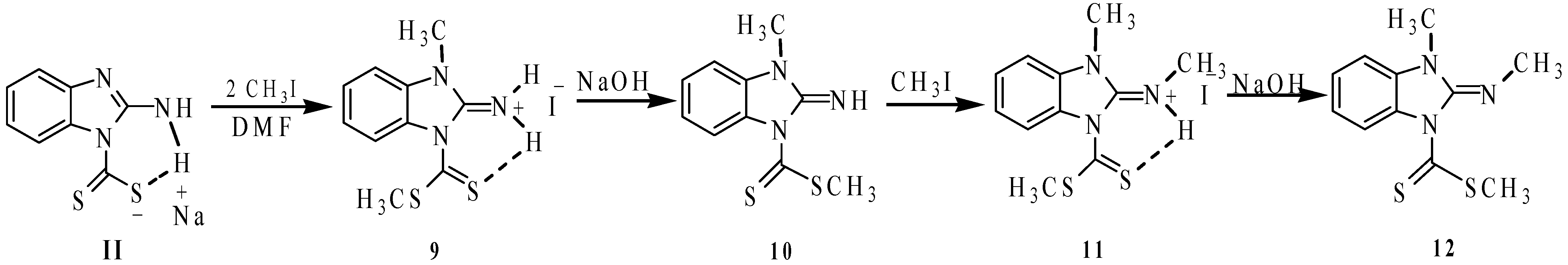
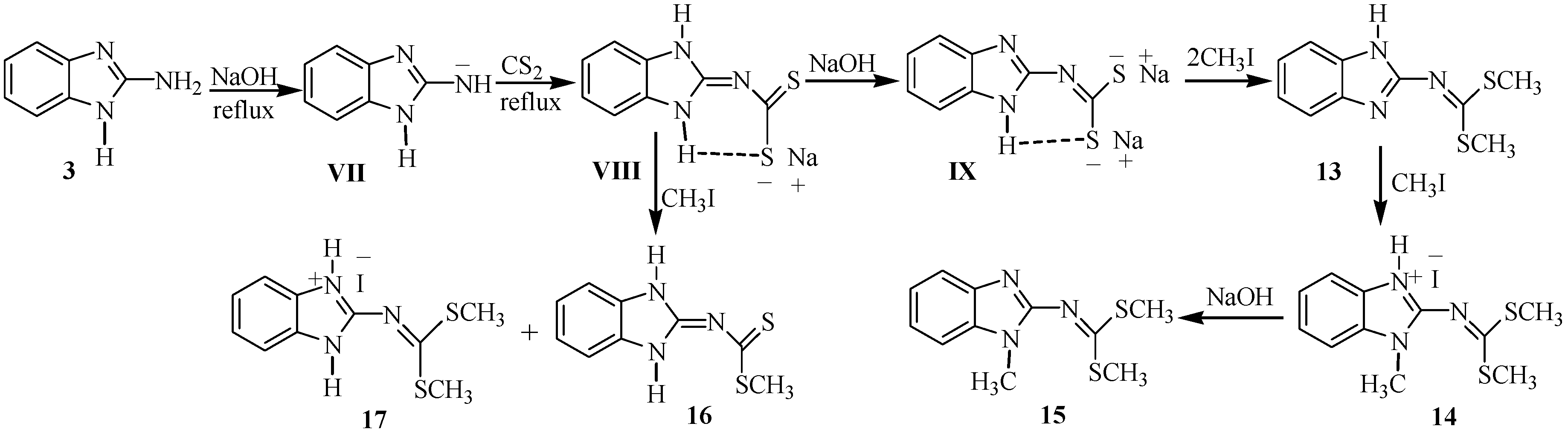
2.1. Synthesis

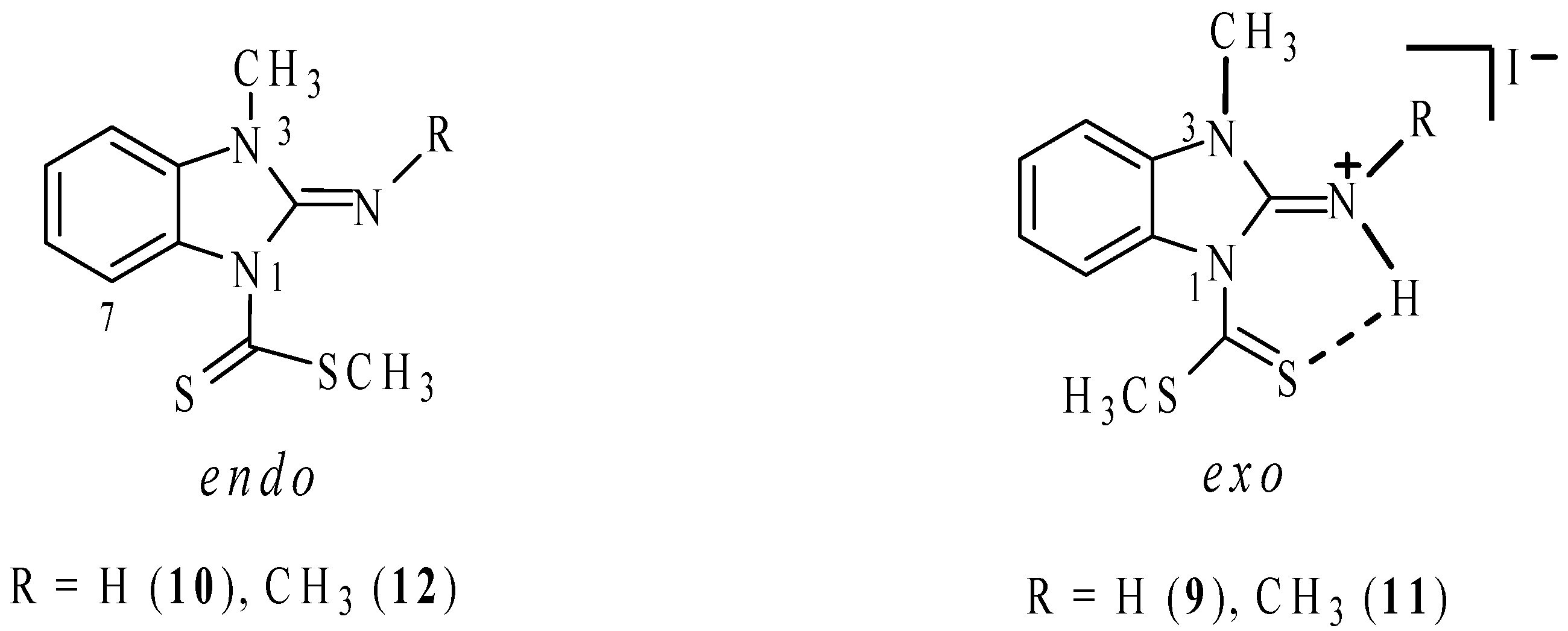
2.2. Molecular Structure in Solution by NMR
| Comp. | H4 | H5 | H6 | H7 | NH | NCH3 | SCH3 |
|---|---|---|---|---|---|---|---|
| 7 b | 7.82 | 7.43 | 7.54 | 9.00 | – | – | 2.80 |
| 9 a | 7.58 | 7.46 | 7.31 | 7.24 | 9.3 | 3.67 | 2.91 |
| 10 b | 6.85 | 7.19 | 6.98 | 8.10 | 7.3 | 3.41 | 2.78 |
| 11 a | 7.59 | 7.41 | 7.31 | 7.61 | 9.1 | 3.71, 3.10 | 2.96 |
| 12 b | 6.77 | 6.93 | 7.11 | 8.12 | – | 3.40, 3.25 | 2.71 |
| 13 b | 7.50 | 7.10 | 7.11 | 7.32 | 12.2 | – | 2.57 |
| 14 a | 7.78 | 7.47 | 7.45 | 7.60 | 8.6 | 3.77 | 2.73 |
| 16 | 7.24 | 7.49 | 7.49 | 7.24 | 13.0 | – | 2.44 |
| 17 | 7.10 | 7.40 | 7.40 | 7.10 | – | – | 2.55 |
| Comp. | C2 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | NCH3 | SCH3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 a | 172.3 | 127.9 | 120.4 | 125.8 | 118.0 | 131.8 | 142.4 | 182.8 | 172.4 | – | 14.3 |
| 9 b | 148.6 | 125.6 | 111.9 | 124.8 | 111.6 | 129.4 | 131.0 | 200.3 | – | 30.6 | 22.8 |
| 10 a | 151.5 | 125.9 | 122.3 | 121.4 | 118.9 | 129.1 | 132.7 | 201.6 | – | 28.5 | 20.4 |
| 11 a | 149.0 | 125.7 | 124.8 | 111.3 | 111.3 | 129.7 | 131.1 | 201.4 | – | 31.6, 31.3 | 23.0 |
| 12 b | 145.5 | 124.4 | 120.1 | 112.5 | 106.3 | 131.0 | 134.5 | 203.3 | – | 36.0, 29.8 | 21.5 |
| 13 a | 153.2 | 118.9 | 121.8 | 118.9 | 111.1 | 133.0 | 143.0 | 173.5 | – | – | 16.1 |
| 14 a | 149.2 | 114.7 | 125.3 | 125.8 | 114.7 | 130.8 | 132.1 | 185.8 | – | 30.7 | 16.7 |
| 16 b | 151.2 | 112.8 | 124.2 | 124.2 | 112.8 | 129.1 | 129.1 | 205.6 | – | – | 18.6 |
| 17 a | 152.9 | 114.8 | 121.8 | 121.8 | 114.8 | 138.0 | 138.0 | 173.4 | – | – | 15.9 |
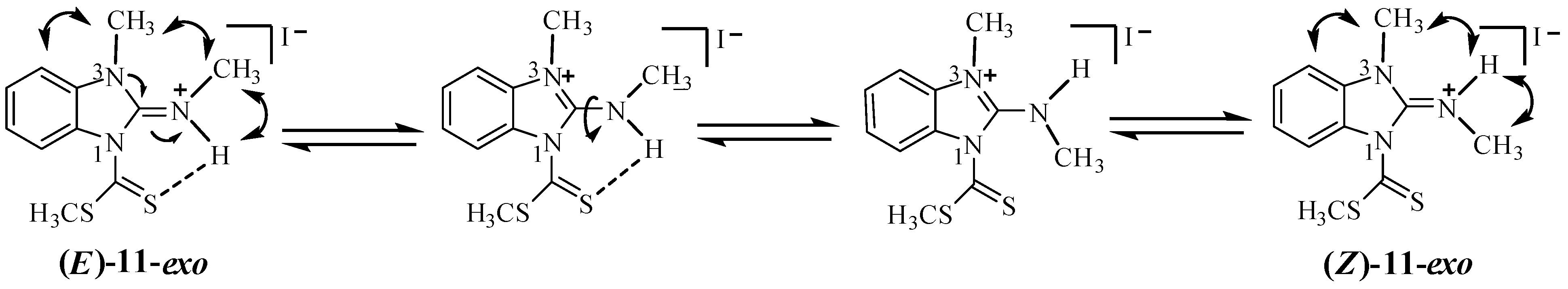
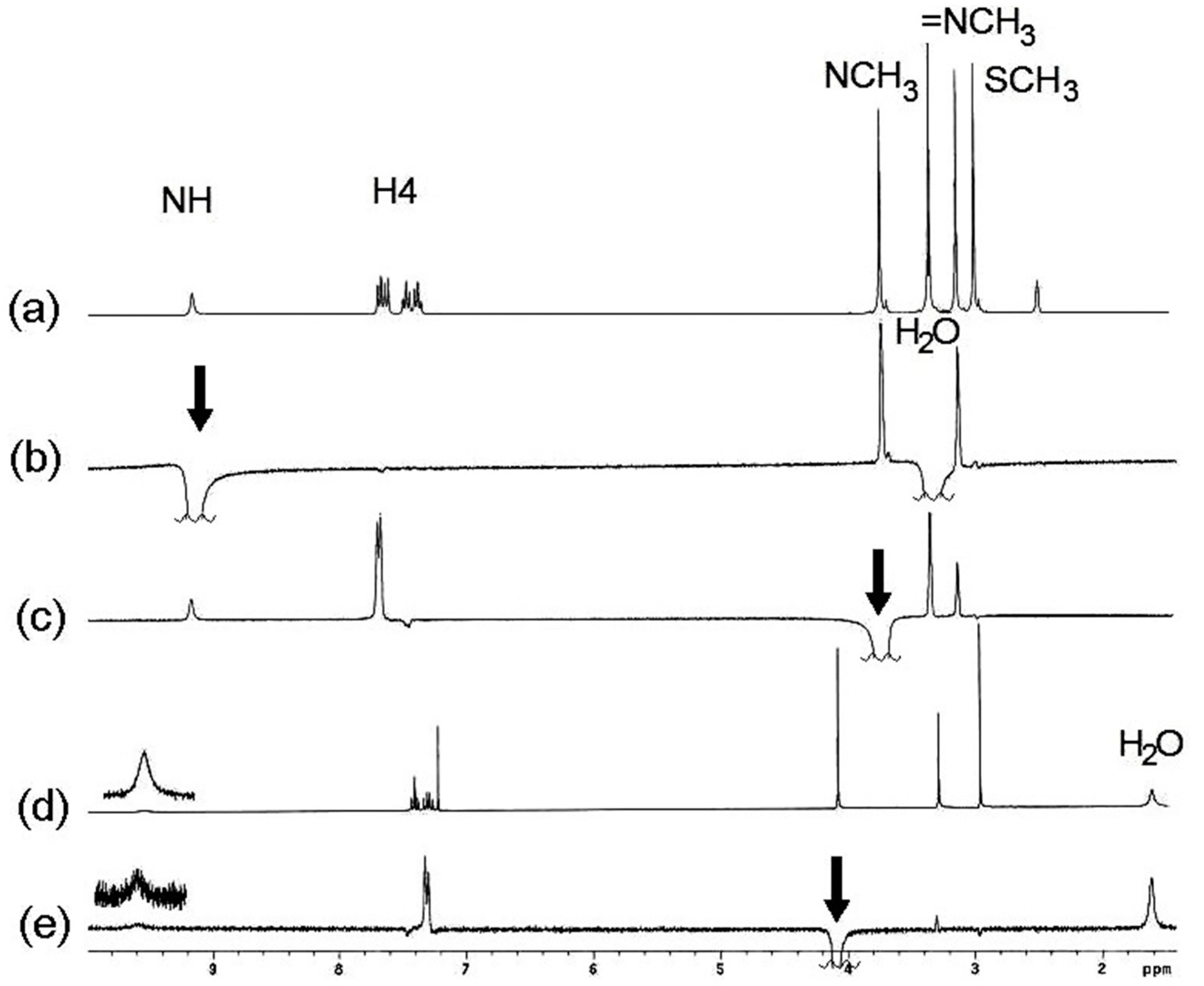
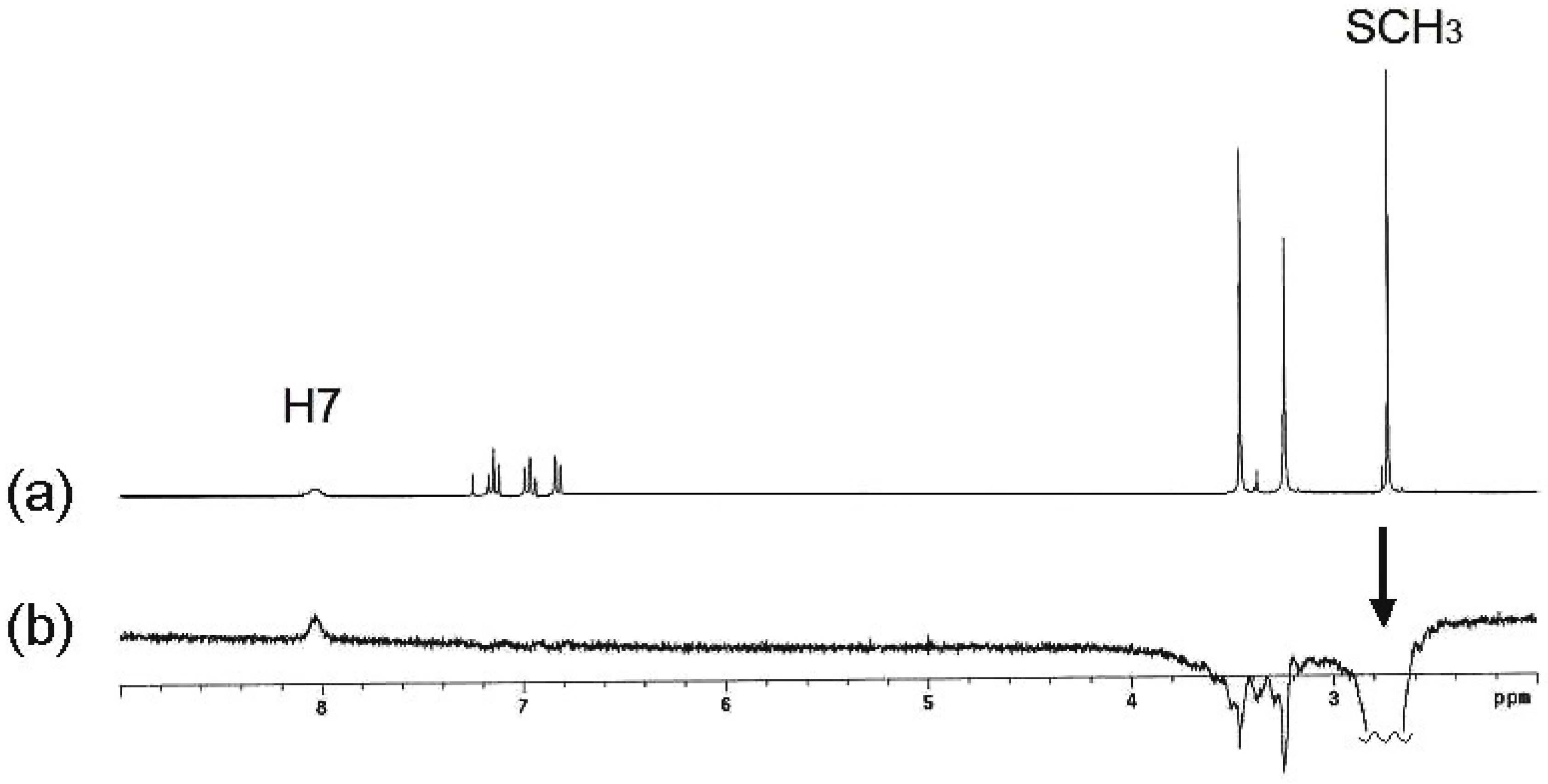
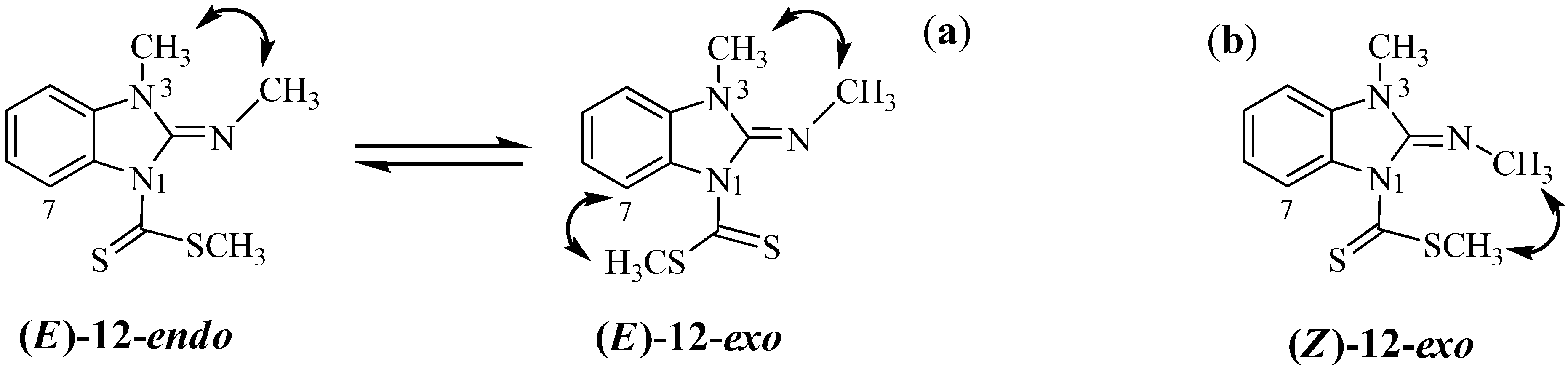
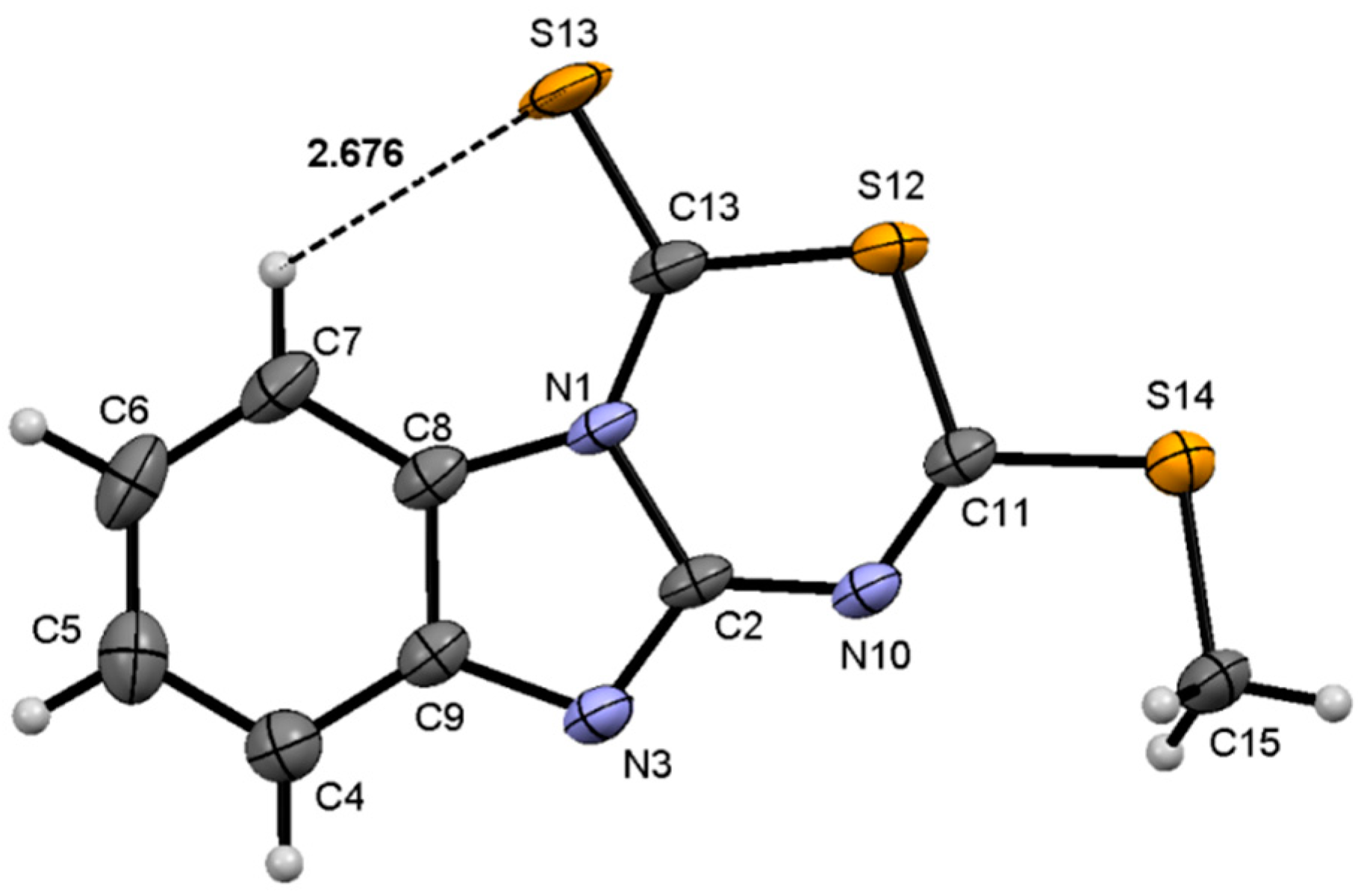
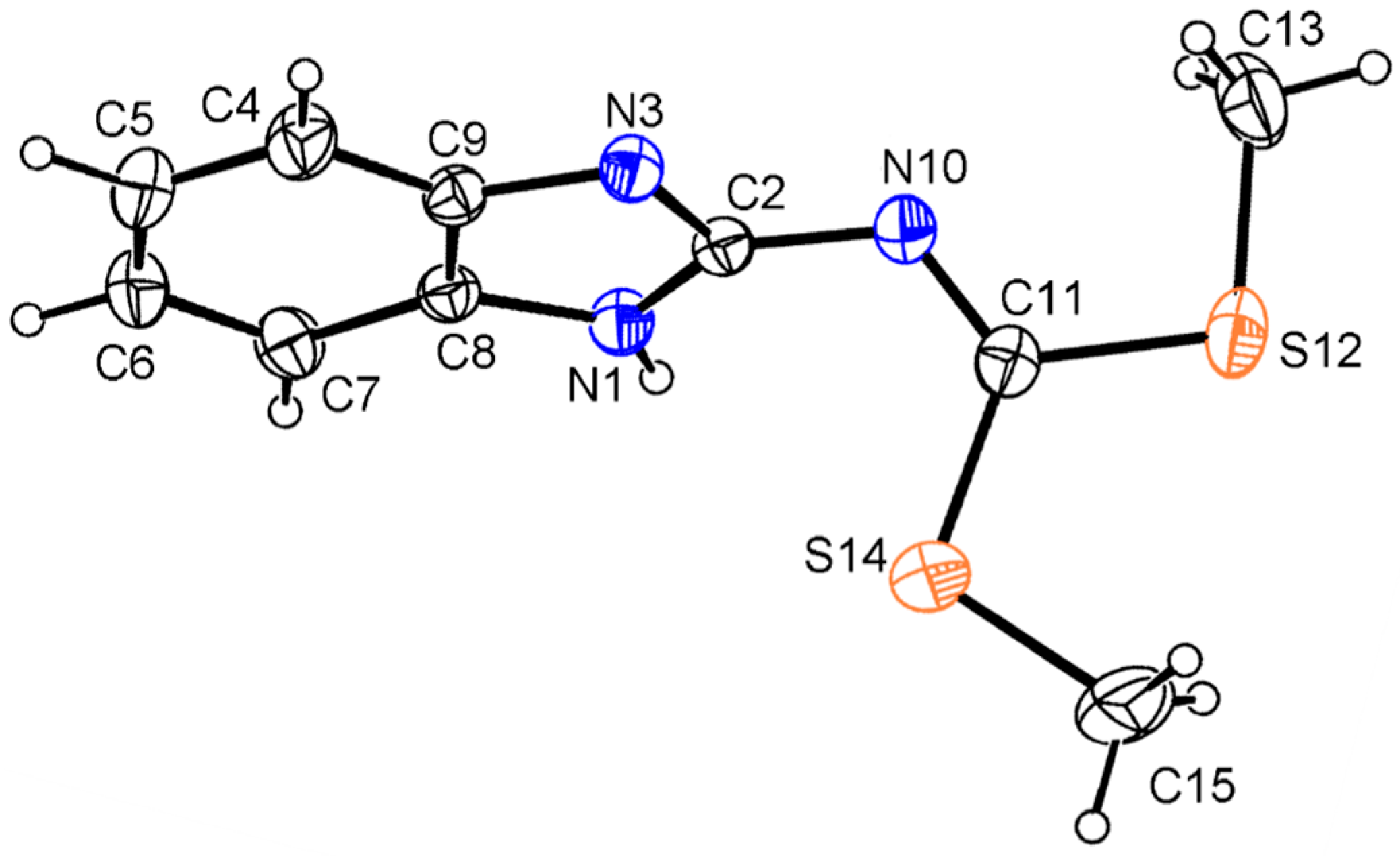
2.3. Molecular Structure of Compounds 7, 13 and 14 by X-Ray Diffraction

3. Experimental Section
3.1. General Procedures
| Comp. | Yield (%) | Physical Appearance | M.p. (°C) | (cm−1) | m/z (%M+) | Elemental AnalysisFound (Calculated) | ||
|---|---|---|---|---|---|---|---|---|
| C | H | N | ||||||
| 6 | 95 | white crystals | 263–264 | 3273, 1647 | 37.73 (37.40) | 4.33 (4.18) | 13.96 (14.54) | |
| 7 | 40 | yellow crystals | 134–135 | 1607, 1542 | 265(70) | 46.30 (45.26) | 2.72 (2.66) | 15.20 (15.83) |
| 9 | 80 | yellow powder | 198–199 | 3273, 3206, 1647 | 237+HI(61) | 32.95 (32.87) | 3.39 (3.28) | 11.29 (11.50) |
| 10 | 90 | yellow powder | 66–67 | 237(61) | 49.93 (50.60) | 4.78 (4.67) | 17.29 (17.70) | |
| 11 | 92 | yellow powder | 148–150 | 3276, 3114 1646 | 34.25 (34.83) | 3.79 (3.69) | 10.66 (11.08) | |
| 12 | 86 | yellow powder | 85–86 | 53.05 (52.58) | 5.12 (5.18) | 16.15 (16.73) | ||
| 13 | 25 | white crystals | 179–180 | 3469, 1624 1554 | 237(30) | 50.51 (50.63) | 4.76 (4.64) | 17.28 (17.72) |
| 14 | 90 | white crystals | 46.82 (34.83) | 4.77 (3.69) | 19.48 (11.08) | |||
| 16 | 42 | white powder | 198–200 | |||||
| 17 | 40 | white crystals | 215–216 | 3129, 1616, 1573, 1543 | 48.25 (48.40) | 4.07 (4.06) | 18.37 (18.82) | |
| Compound | 7 | 13 | 14 |
|---|---|---|---|
| Unit Cell Information | |||
| Cell axes [Å] a | 15.8405(4) | 10.0640(20) | 7.4895(7) |
| B | 4.5179(1) | 8.9376(18) | 10.3023(9) |
| C | 18.8892(6) | 13.4110(30) | 11.2194(10) |
| Cell angles [deg] α | 90.00 | 90.00 | 110.270(1) |
| Β | 126.546(2) | 107.480(30) | 95.492(2) |
| Γ | 90.00 | 90.00 | 104.655(1) |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P 21/c | P 21/c | P-1 |
| Molecular Formula | C10H7N3S3 | C10H11N3S2 | C11H16N3OS2I |
| Density [g cm−1] | 1.62 | 1.37 | 1.71 |
| Formula weight | 265.4 | 237.3 | 397.3 |
| No. Form. Units Z | 4 | 4 | 2 |
| Reflection Data | |||
| No. Meas. | 10175 | 10627 | 7445 |
| No. Uniq. | 2173 | 2023 | 2706 |
| No. Obs. | 1971 | 1896 | 2301 |
| Current Refinement | |||
| No. Refln. | 2173 | 2023 | 2706 |
| No. Param. | 147 | 136 | 166 |
| Delta-rho [eÅ−3] max, min | 0.361, −0.518 | 0.392, −0.332 | 1.012, −1.134 |
| R_all, R_obs | 0.045, 0.042 | 0.045, 0.043 | 0.079, 0.065 |
| wR2_all, wR2_obs | 0.117, 0.113 | 0.122, 0.120 | 0.130, 0.125 |
3.2. Procedures to Obtain 2-Aminobenzimidazole Sulfur Derivatives
3.2.1. 1,3-Dimethyl-1,3-dihydrobenzimidazol-2-ylideneammonium Iodide (6)
3.2.2. 2-Methylthio-4H-[1,3,5]thiadiazino[3,4-a]benzimidazole-4-thione (7)
3.2.3. 2-Aminobenzimidazole-1-carbodithioic Acid Methyl Ester (8)
3.2.4. 1-Methyl-3-(methylthiocarbonothioyl)-1H-benzo[d]imidazol-2(3H)-iminium Iodide (9)
3.2.5. 2-Imino-3-methyl-2,3-dihydrobenzimidazol-1-carbodithioic Acid Methyl Ester (10)
3.2.6. N-(1-Methyl-3-(methylthiocarbonothioyl)-1H-benzo[d]imidazol-2(3H)-ylidene)methanaminium Iodide (11)
3.2.7. (E)-3-Methyl-2-methylimino-2,3-dihydrobenzimidazole-1-carbodithioic Acid Methyl Ester (12)
3.2.8. Dimethyl 1H-benzo[d]imidazol-2-ylcarbonimidodithioate (13)
3.2.9. 2-(Bis(methyltio)methyleneamino)-1-methyl-1H-benzo[d]imidazol-3-ium Iodide (14)
3.2.10. Dimethyl 1-methyl-1H-benzo[d]imidazol-2-ylcarbonodithioimidate (15)
3.2.11. (1,3-Dihydrobenzimidazol-2-ylidene)-dithiocarbamic Acid methyl Ester (16) and 2-(bis(methyl- thio)methyleneamino)-1H-benzo[d]imidazol-3-ium Iodide (17)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andrade-López, N.; Ariza-Castolo, A.; Contreras, R.; Vázquez-Olmos, A.; Barba-Behrens, N.; Tlahuext, H. Versatile behaviour of 2-guanidinobenzimidazole nitrogen atoms toward protonation, coordination and methylation. Heteroat. Chem. 1997, 8, 397–410. [Google Scholar] [CrossRef]
- Andrade-López, N.; Cartas-Rosado, R.; García-Báez, E.; Contreras, R.; Tlahuext, H. Boron heterocycles derived from 2-guanidinobenzimidazole. Heteroat. Chem. 1998, 9, 399–409. [Google Scholar]
- Fialon, M.-P.; Andrade-López, N.; Barba-Behrens, N.; Contreras, R. Organometallic tin complexes derived from 2-guanidinebenzimidazole. Heteroat. Chem. 1998, 9, 637–641. [Google Scholar]
- Fialon, M.-P.; García-Baez, E.; Andrade-López, N.; Osorio-Monreal, G.; Canseco-Melchor, V; Velázquez-Montes, I.; Barba-Behrens, N.; Contreras, R. Boron and transition metal compounds derived from 2-uroylbenzimidazole. Heteroat. Chem. 1999, 10, 577–584. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar]
- Chaudhary, P.; Sharma, K.P.; Sharma, A.; Varshney, J. Recent advances in pharmacological activity of benzothiazole derivatives. Int. J. Curr. Pharm. Res. 2010, 2, 5–11. [Google Scholar]
- Téllez, F.; Cruz, A.; López-Sandoval, H.; Ramos-García, I; Gayosso, M.; Castillo-Sierra, R.N.; Paz-Michel, B.; Nöth, H.; Flores-Parra, A.; Contreras, R. Dithiocarbamates, thiocarbamic esters, dithiocarboimidates, guanidines, thioureas, isothioureas, and tetraaza-thiapentalene derived from 2-aminobenzothiazole. Eur. J. Org. Chem. 2004. [Google Scholar] [CrossRef]
- Merchán, F.; Garín, J.; Meléndez, E. A facile synthesis of dimethyl N-(2-benzothiazolyl)-dithiocarbonimidates and methyl N-(2-benzothiazolyl)-dithiocarbamates. Synthesis 1982. [Google Scholar] [CrossRef]
- Merchán, F.; Garín, J.; Meléndez, E.; Tejero, T. 2-(2-Benzimidazolylamino)-benzothiazoles and 2-(2-Imidazolidinylidenamino)-benzothiazoles. Synthesis 1982. [Google Scholar] [CrossRef]
- Garín, J.; Meléndez, E.; Merchan, F.L.; Ortíz, D.; Tejero, T. Facile synthesis of 8-aryl-aminoand 8-hetarylaminopurines and their 1- and 3-deaza analogs. Synthesis 1985. [Google Scholar] [CrossRef]
- Merchan, F.L.; Garín, J.; Meléndez, E.; Tejero, T. A facile synthesis of 2-(2-benzothiazolyl-amino)-1,3-heterazoles. Synthesis 1987. [Google Scholar] [CrossRef]
- Cruz, A.; Gayosso, M.; Contreras, R. Syntheses of optically active 2-(2-benzothiazoly-limino)-heterazolidines. Heteroat. Chem. 2001, 12, 586–593. [Google Scholar]
- Cruz, A.; Padilla-Martínez, I.I.; García-Báez, E.V. A synthetic method to access symmetric and non-symmetric 2-(N,N'-disubstituted)guanidinebenzothiazoles. Molecules 2012, 17, 10178–10191. [Google Scholar]
- Cruz, A.; Padilla-Martínez, I.I.; García-Báez, E.V.; Juárez-Juárez, M. S-Methyl-(-N-aryl and -N-alkyl)isothioureas derived from 2-aminobenzothiazole. ARKIVOC 2007, 2008, 200–209. [Google Scholar]
- Esparza-Ruiz, A.; Peña-Hueso, A.; Mijangos, E.; Osorio-Monreal, G.; Nöth, H.; Flores-Parra, A.; Contreras, R.; Barba-Beherens, N. Cobalt(II), Nikel(II) and Zinc(II) coordination compounds derived from aromatic amines. Polyhedron 2011, 30, 2090–2098. [Google Scholar]
- Peña-Hueso, A.; Téllez, F.; Vieto-Peña, R.; Esquivel, R.O.; Esparza-Ruiz, A.; Ramos-García, I.; Contreras, R.; Barba-Behrens, N.; Flores-Parra, A. Synthesis and structure of dithiocarbonimidates derived from aromatic heterocycles: Role of weak interactions in the preferred conformation. J. Mol. Struct. 2010, 984, 409–415. [Google Scholar]
- Bruker. APEX II, SAINT, SADABS and SHELXTL; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SHELXS97 and SHELXL97; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Spek, A.L. PLATON, Version of March 2002; University of Utrecht: Heidelberglaan, The Netherlands, 2002.
- Farrugia, L.J. WinGX suite for small molecule single crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar]
- Sample Availability: Samples of the compounds 7–17 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cruz, A.; Padilla-Martínez, I.I.; García-Báez, E.V.; Guerrero-Muñoz, G. Synthesis and Structure of Sulfur Derivatives from 2-Aminobenzimidazole. Molecules 2014, 19, 13878-13893. https://doi.org/10.3390/molecules190913878
Cruz A, Padilla-Martínez II, García-Báez EV, Guerrero-Muñoz G. Synthesis and Structure of Sulfur Derivatives from 2-Aminobenzimidazole. Molecules. 2014; 19(9):13878-13893. https://doi.org/10.3390/molecules190913878
Chicago/Turabian StyleCruz, Alejandro, Itzia I. Padilla-Martínez, Efrén V. García-Báez, and Gerardo Guerrero-Muñoz. 2014. "Synthesis and Structure of Sulfur Derivatives from 2-Aminobenzimidazole" Molecules 19, no. 9: 13878-13893. https://doi.org/10.3390/molecules190913878
APA StyleCruz, A., Padilla-Martínez, I. I., García-Báez, E. V., & Guerrero-Muñoz, G. (2014). Synthesis and Structure of Sulfur Derivatives from 2-Aminobenzimidazole. Molecules, 19(9), 13878-13893. https://doi.org/10.3390/molecules190913878