Structure and Absolute Configuration of 20β-Hydroxyprednisolone, a Biotransformed Product of Predinisolone by the Marine Endophytic Fungus Penicilium lapidosum
Abstract
:1. Introduction
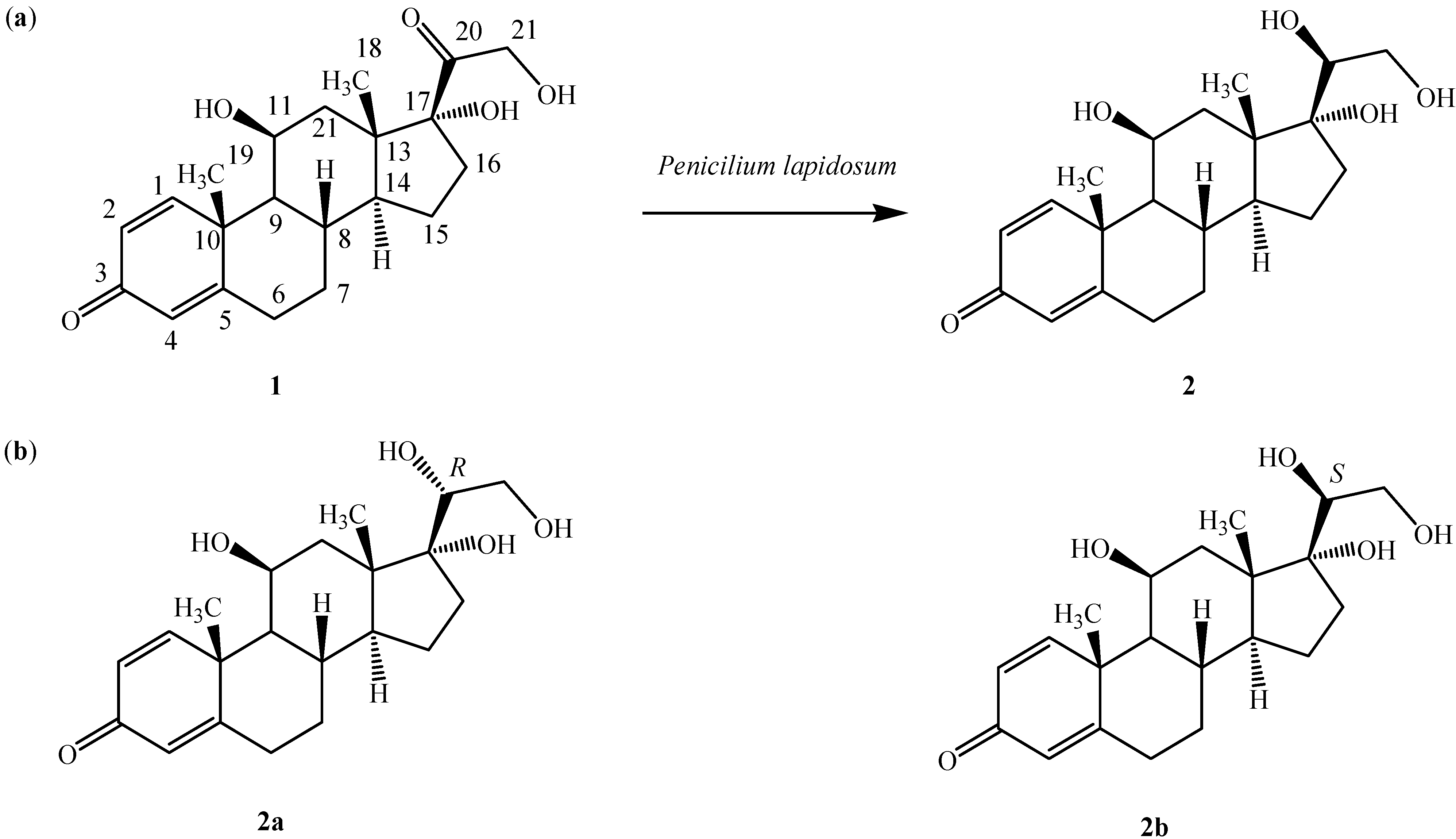
2. Results and Discussion
2.1. Conformational Study
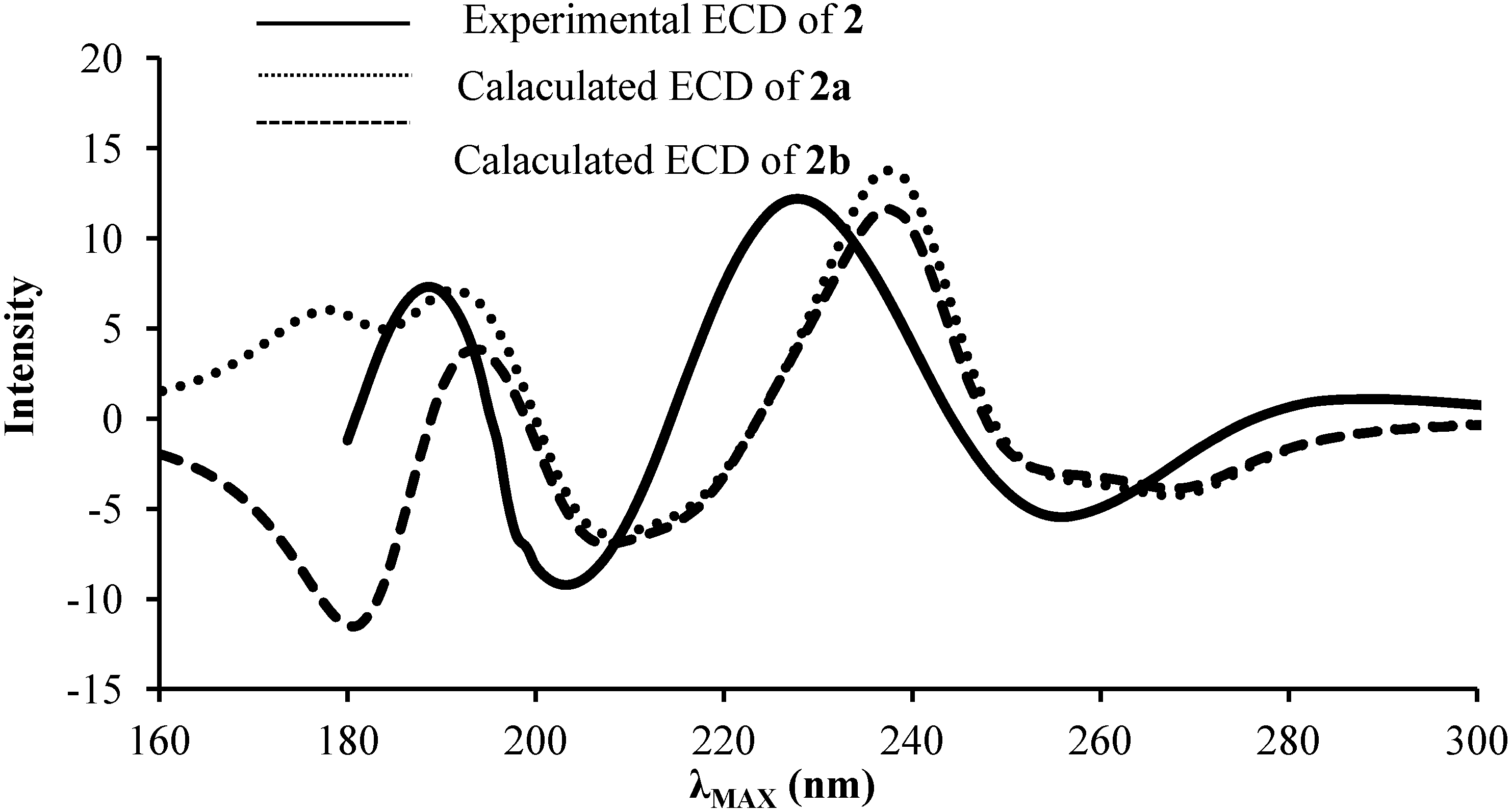
2.2. ECD and UV/Vis Spectra Prediction
| Gas | PCM | Experimental | ||||||
|---|---|---|---|---|---|---|---|---|
| λMAX | EMAX | f | λMAX | EMAX | f | λMAX | EMAX | |
| 2a | 231 | 5.37 | 0.12 | 239 | 5.19 | 0.25 | 240 | 4.18 |
| 2b | 231 | 5.36 | 0.11 | 239 | 5.19 | 0.25 | ||
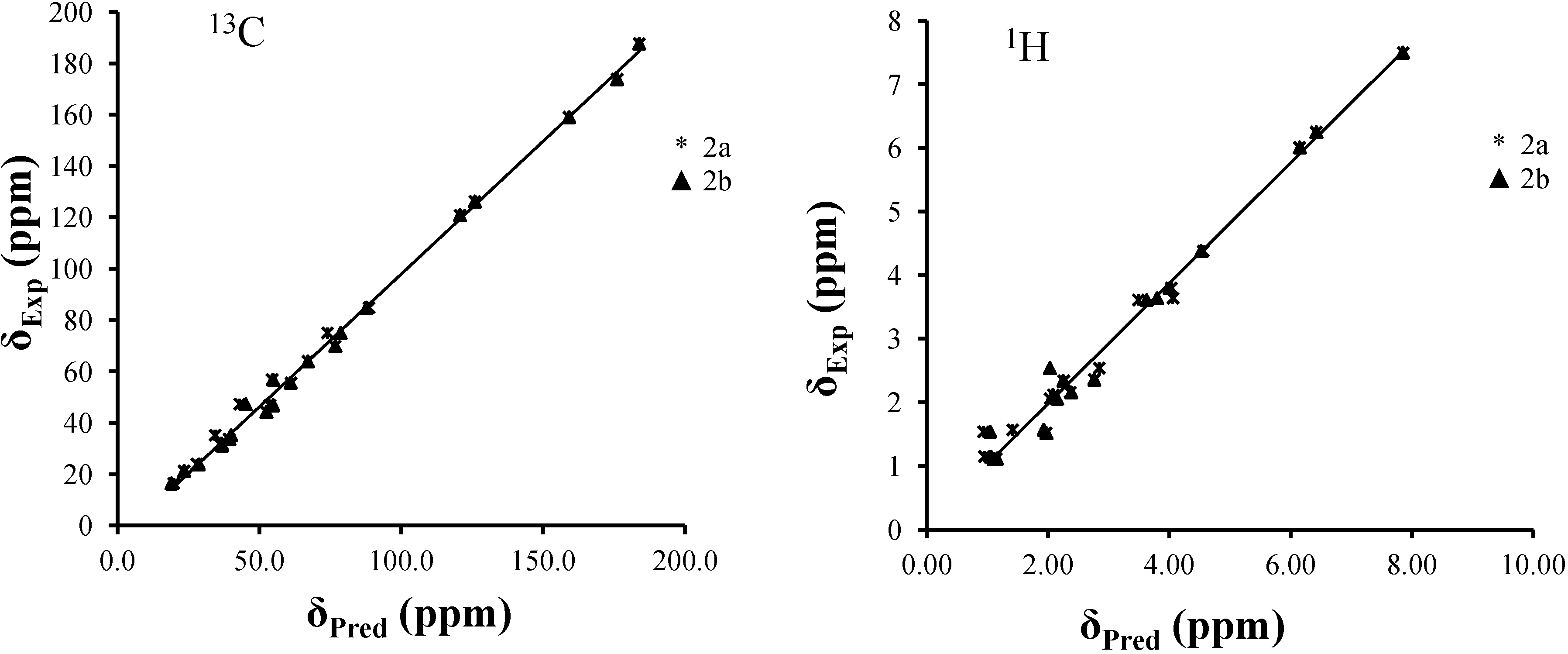
2.3. NMR Spectra Prediction
| 2a | 2b | Experimental | |||
|---|---|---|---|---|---|
| Gas | PCM | Gas | PCM | ||
| H1 | 6.85 | 7.45 | 7.01 | 7.52 | 7.5 |
| H2 | 6.24 | 6.11 | 6.33 | 6.17 | 6.25 |
| H4 | 5.97 | 5.86 | 6.06 | 5.91 | 6.01 |
| H6a | 2.10 | 2.23 | 2.08 | 2.22 | 2.34 |
| H6b | 2.49 | 2.70 | 2.47 | 2.70 | 2.36 |
| H7a | 1.15 | 1.19 | 1.13 | 1.18 | 1.12 |
| H7b | 1.96 | 2.07 | 1.92 | 2.05 | 2.12 |
| H8 | 2.28 | 2.33 | 2.28 | 2.34 | 2.16 |
| H9 | 1.08 | 1.14 | 1.08 | 1.13 | 1.11 |
| H11 | 4.45 | 4.37 | 4.49 | 4.37 | 4.38 |
| H12a | 1.78 | 1.44 | 2.12 | 1.91 | 1.57 |
| H12b | 2.03 | 2.02 | 2.49 | 2.12 | 2.06 |
| H14 | 2.02 | 1.85 | 2.00 | 1.85 | 1.77 |
| H15a | 1.48 | 1.52 | 1.45 | 1.51 | 1.51 |
| H15b | 1.64 | 1.74 | 1.61 | 1.74 | 1.8 |
| H16a | 0.59 | 0.99 | 0.71 | 1.07 | 1.54 |
| H16b | 3.16 | 2.77 | 1.95 | 2.01 | 2.54 |
| H18 | 1.11 | 1.01 | 1.37 | 1.09 | 1.15 |
| H19 | 2.15 | 1.95 | 2.13 | 1.95 | 1.52 |
| H20 | 4.07 | 3.88 | 3.97 | 3.87 | 3.8 |
| H21a | 3.28 | 3.38 | 3.54 | 3.52 | 3.61 |
| H21b | 4.02 | 3.91 | 3.64 | 3.68 | 3.64 |
| 2a | 2b | Experimental | |||
|---|---|---|---|---|---|
| Gas | PCM | Gas | PCM | ||
| C1 | 154.2 | 159.2 | 154.4 | 159.2 | 159.11 |
| C2 | 130.6 | 125.1 | 130.1 | 124.8 | 126.28 |
| C3 | 185.2 | 184.6 | 185.3 | 184.7 | 187.76 |
| C4 | 124.1 | 119.7 | 123.9 | 119.4 | 120.94 |
| C5 | 172.2 | 176.6 | 172.0 | 176.7 | 173.88 |
| C6 | 32.6 | 33.2 | 31.8 | 32.3 | 31.9 |
| C7 | 35.3 | 36.0 | 34.5 | 35.2 | 33.7 |
| C8 | 32.2 | 33.2 | 31.5 | 32.6 | 31.2 |
| C9 | 60.9 | 58.5 | 59.8 | 57.6 | 55.6 |
| C10 | 46.5 | 49.6 | 45.5 | 48.8 | 44.2 |
| C11 | 75.0 | 74.4 | 74.5 | 73.9 | 69.9 |
| C12 | 36.9 | 39.9 | 37.6 | 41.2 | 47.3 |
| C13 | 51.4 | 50.8 | 52.1 | 51.2 | 46.9 |
| C14 | 52.3 | 51.6 | 51.8 | 51.1 | 56.9 |
| C15 | 23.4 | 24.3 | 23.2 | 24.1 | 23.9 |
| C16 | 32.0 | 31.0 | 37.3 | 35.9 | 35.2 |
| C17 | 88.2 | 86.7 | 86.4 | 85.3 | 84.94 |
| C18 | 14.9 | 16.0 | 12.9 | 14.1 | 16.42 |
| C19 | 20.9 | 19.7 | 20.0 | 18.7 | 21.3 |
| C20 | 72.3 | 71.6 | 76.9 | 75.8 | 75.02 |
| C21 | 65.3 | 64.6 | 64.7 | 63.9 | 63.98 |
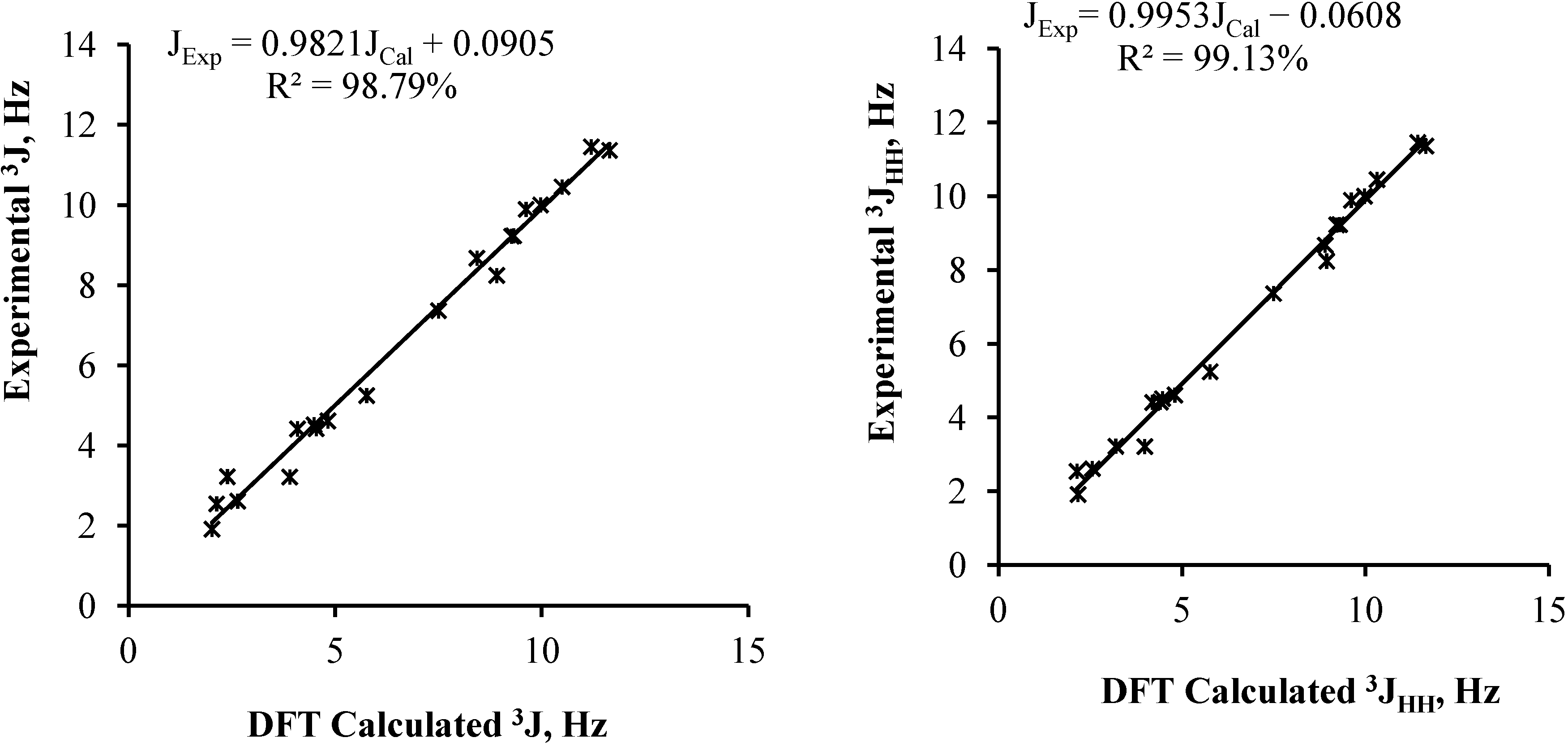
| 2a | 2b | Experimental | |
|---|---|---|---|
| H1-H2 | 9.97 | 9.97 | 10.0 |
| H6-H7 | 5.76 | 5.76 | 5.24 |
| 11.64 | 11.64 | 11.36 | |
| 2.13 | 2.13 | 2.54 | |
| 4.82 | 4.80 | 4.61 | |
| H7-H8 | 4.49 | 4.46 | 4.51 |
| 9.62 | 9.61 | 9.89 | |
| H8-H9 | 9.32 | 9.29 | 9.23 |
| H9-H11 | 4.09 | 4.19 | 4.41 |
| H11-H12 | 2.64 | 2.55 | 2.61 |
| 3.90 | 3.98 | 3.21 | |
| H8-H14 | 8.91 | 8.94 | 8.24 |
| H14-H15 | 10.49 | 10.31 | 10.45 |
| 7.50 | 7.49 | 7.36 | |
| H15-H16 | 4.54 | 4.40 | 4.42 |
| 11.20 | 11.42 | 11.45 | |
| 9.27 | 9.21 | 9.22 | |
| 2.02 | 2.16 | 1.91 | |
| H20-H21 | −0.82 | 3.19 | 3.22 |
| −1.84 | 8.90 | 8.67 |
3. Experimental Section
3.1. General Information
3.2. Isolation and Identification of Marine Endophytic Fungus
3.3. Culture Conditions and Media Preparation
3.4. Preliminary Screening Experiments
3.5. Preparative Fermentation
3.6. Isolation of the Metabolites
3.7. Characterization of the Transformed Product 20β-Hydroxyprednisolone (2)
3.8. Theoretical Details
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- NIAID Global Health Research Plan for HIV/AIDS, Malaria, and Tuberculosis; National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services: Washington, DC, USA, 2001.
- Siegel, M.R.; Latch, G.; Johnson, M.C. Acremonium fungal endophytes of tall fescue and perennial ryegrass: Significance and control. Plant Dis. 1985, 69, 179–181. [Google Scholar]
- Shrestha, K.; Strobel, G.A.; Shrivastava, S.P.; Gewali, M.B. Evidence for paclitaxel from three new endophytic fungi of Himalayan yew of Nepal. Planta Med. 2001, 67, 374–376. [Google Scholar]
- Sultan, S.; Sun, L.; Blunt, J.W.; Cole, A.L.; Munro, M.H.; Ramasamy, K.; Weber, J.F.F. Evolving trends in the dereplication of natural product extracts. 3: Further lasiodiplodins from Lasiodiplodia theobromae, an endophyte from Mapania kurzii. Tetrahedron Lett. 2014, 55, 453–455. [Google Scholar] [CrossRef]
- Sultan, S.; Shah, S.A.A.; Sun, L.; Ramasami, K.; Cole, A.; Blunt, J.; Munro, M.; Weber, J.F.F. Bioactive Fungal metabolites of 9PR2 isolated from roots of Callophyllum ferrugineum. Int. J. Pharm. Pharm. Sci. 2011, 3, 7–9. [Google Scholar]
- Rajesh, P.; Ravishankar Rai, V. Hydrolytic enzymes and quorum sensing inhibitors from endophytic fungi of Ventilago madraspatana Gaertn. Biocatal. Agric. Biotechnol. 2013, 2, 120–124. [Google Scholar]
- Devi, N.N.; Prabakaran, J.J.; Wahab, F. Phytochemical analysis and enzyme analysis of endophytic fungi from Centella asiatica. Asian. Pac. J. Trop. Biomed. 2012, 2, S1280–S1284. [Google Scholar] [CrossRef]
- Sultan, S.; Choudhary, M.I.; Khan, S.N.; Fatima, U.; Atif, M.; Ali, R.A.; Rahman, A.U.; Fatmi, M.Q. Fungal transformation of cedryl acetate and α-glucosidase inhibition assay, quantum mechanical calculations and molecular docking studies of its metabolites. Eur. J. Med. Chem. 2013, 62, 764–770. [Google Scholar]
- Casañola-Martín, G.M.; Marrero-Ponce, Y.; Khan, M.T.H.; Ather, A.; Sultan, S.; Torrens, F.; Rotondo, R. TOMOCOMD-CARDD descriptors-based virtual screening of tyrosinase inhibitors: Evaluation of different classification model combinations using bond-based linear indices. Bioorg. Med. Chem. 2007, 15, 1483–1503. [Google Scholar]
- Choudhary, M.I.; Sultan, S.; Khan, M.T.H.; Rahman, A.U. Microbial transformation of 17α-ethynyl-and 17α-ethylsteroids, and tyrosinase inhibitory activity of transformed products. Steroids 2005, 70, 798–802. [Google Scholar]
- Choudhary, M.I.; Sultan, S.; Hassan Khan, M.T.; Yasin, A.; Shaheen, F.; Atta-Ur-Rahman. Biotransformation of (+)-androst-4-ene-3,17-dione. Nat. Prod. Res. 2004, 18, 529–535. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Atta-Ur-Rahman; Choudhary, M.I.; Sultan, S. Microbial transformation of (+)-adrenosterone. Nat. Prod. Lett. 2002, 16, 345–349. [Google Scholar] [CrossRef]
- Shah, S.; Sultan, S.; Adnan, H. Solid Phase Microbial Transformation Of Cortexolone And Prolyl Endopeptidase Inhibiotory Activity Of The Transformed Products. Int. J. Pharm. Pharm. Sci. 2011, 3, 1–6. [Google Scholar]
- Choudhary, M.I.; Sultan, S.; Jalil, S.; Anjum, S.; Rahman, A.A.; Fun, H.K. Microbial transformation of mesterolone. Chem. Biodivers. 2005, 2, 392–400. [Google Scholar]
- Choudhary, M.I.; Sultan, S.; Yaqoob, M.; Musharraf, S.G.; Yasin, A.; Shaheen, F.; Atta-Ur-Rahman. Microbial transformation of cortisol and prolyl endopeptidase inhibitory activity of its transformed products. Nat. Prod. Res. 2003, 17, 389–395. [Google Scholar]
- Shah, S.A.A.; Sultan, S.; Zaimi bin Mohd Noor, M. Biotransformation of tissue-specific hormone tibolone with fungal culture Trichothecium roseum. J. Mol. Struct. 2013, 1042, 118–122. [Google Scholar]
- DellaGreca, M.; Previtera, L.; Fiorentino, A.; Pinto, G.; Pollio, A. Prednisolone biotransformation by the green alga T76 Scenedesmus quadricauda. Tetrahedron 1997, 53, 8273–8280. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Yazdi, M.T.; Amini, M.; Shafiee, A. Prednisolone bio-transformation in the culture of filamentous fungus Acremonium strictum. Biotechnology 2008, 7, 343–346. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Wu, M.; Zhang, R.; Xie, L.; Wang, H. Transformation of prednisolone to a 20β-hydroxy prednisolone compound by Streptomyces roseochromogenes TS79. Appl. Microbiol. Biotechnol. 2011, 92, 727–735. [Google Scholar]
- Restaino, O.F.; Marseglia, M.; de Castro, C.; Diana, P.; Forni, P.; Parrilli, M.; de Rosa, M.; Schiraldi, C. Biotechnological transformation of hydrocortisone to 16α-hydroxy hydrocortisone by Streptomyces roseochromogenes. Appl. Microbiol. Biotechnol. 2014, 98, 1291–1299. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar]
- Petrini, O.; Dreyfuss, M. Endophytische pilze in epiphytischen Araceae, Bromeliaceae und Orchidaceae. In Sydowia: Annales mycologici; Verlag Ferdinand Berger & Söhne Gesellschaft m.b.H.: Horn, Austria, 1981. [Google Scholar]
- Martin, K.J.; Rygiewicz, P.T. Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol. 2005, 5, 28. [Google Scholar]
- Buchan, A.; Newell, S.; Moreta, J.; Moran, M. Analysis of internal transcribed spacer (ITS) regions of rRNA genes in fungal communities in a southeastern US salt marsh. Microb. Ecol. 2002, 43, 329–340. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Furche, F.; Ahlrichs, R. Adiabatic time-dependent density functional methods for excited state properties. J. Chem. Phys. 2002, 117, 7433–7447. [Google Scholar]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107:1–094107:15. [Google Scholar]
- Gauss, J. Effects of electron correlation in the calculation of nuclear magnetic resonance chemical shifts. J. Chem. Phys. 1993, 99, 3629–3643. [Google Scholar]
- Jacquemin, D.; Wathelet, V.; Perpete, E.A.; Adamo, C. Extensive TD-DFT benchmark: Singlet-excited states of organic molecules. J. Chem. Theory Comput. 2009, 5, 2420–2435. [Google Scholar]
- Liu, X.; Cole, J.M.; Low, K.S. Solvent effects on the UV-Vis absorption and emission of optoelectronic Coumarins: A comparison of three empirical solvatochromic models. J. Phys. Chem. C 2013, 117, 14731–14741. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02 ed; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sultan, S.; Noor, M.Z.b.M.; Anouar, E.H.; Shah, S.A.A.; Salim, F.; Rahim, R.; Trabolsy, Z.B.K.A.; Weber, J.-F.F. Structure and Absolute Configuration of 20β-Hydroxyprednisolone, a Biotransformed Product of Predinisolone by the Marine Endophytic Fungus Penicilium lapidosum. Molecules 2014, 19, 13775-13787. https://doi.org/10.3390/molecules190913775
Sultan S, Noor MZbM, Anouar EH, Shah SAA, Salim F, Rahim R, Trabolsy ZBKA, Weber J-FF. Structure and Absolute Configuration of 20β-Hydroxyprednisolone, a Biotransformed Product of Predinisolone by the Marine Endophytic Fungus Penicilium lapidosum. Molecules. 2014; 19(9):13775-13787. https://doi.org/10.3390/molecules190913775
Chicago/Turabian StyleSultan, Sadia, Muhammad Zaimi bin Mohd Noor, El Hassane Anouar, Syed Adnan Ali Shah, Fatimah Salim, Rohani Rahim, Zuhra Bashir Khalifa Al Trabolsy, and Jean-Frédéric Faizal Weber. 2014. "Structure and Absolute Configuration of 20β-Hydroxyprednisolone, a Biotransformed Product of Predinisolone by the Marine Endophytic Fungus Penicilium lapidosum" Molecules 19, no. 9: 13775-13787. https://doi.org/10.3390/molecules190913775
APA StyleSultan, S., Noor, M. Z. b. M., Anouar, E. H., Shah, S. A. A., Salim, F., Rahim, R., Trabolsy, Z. B. K. A., & Weber, J.-F. F. (2014). Structure and Absolute Configuration of 20β-Hydroxyprednisolone, a Biotransformed Product of Predinisolone by the Marine Endophytic Fungus Penicilium lapidosum. Molecules, 19(9), 13775-13787. https://doi.org/10.3390/molecules190913775





