Immobilization of Brassica oleracea Chlorophyllase 1 (BoCLH1) and Candida rugosa Lipase (CRL) in Magnetic Alginate Beads: An Enzymatic Evaluation in the Corresponding Proteins
Abstract
:1. Introduction
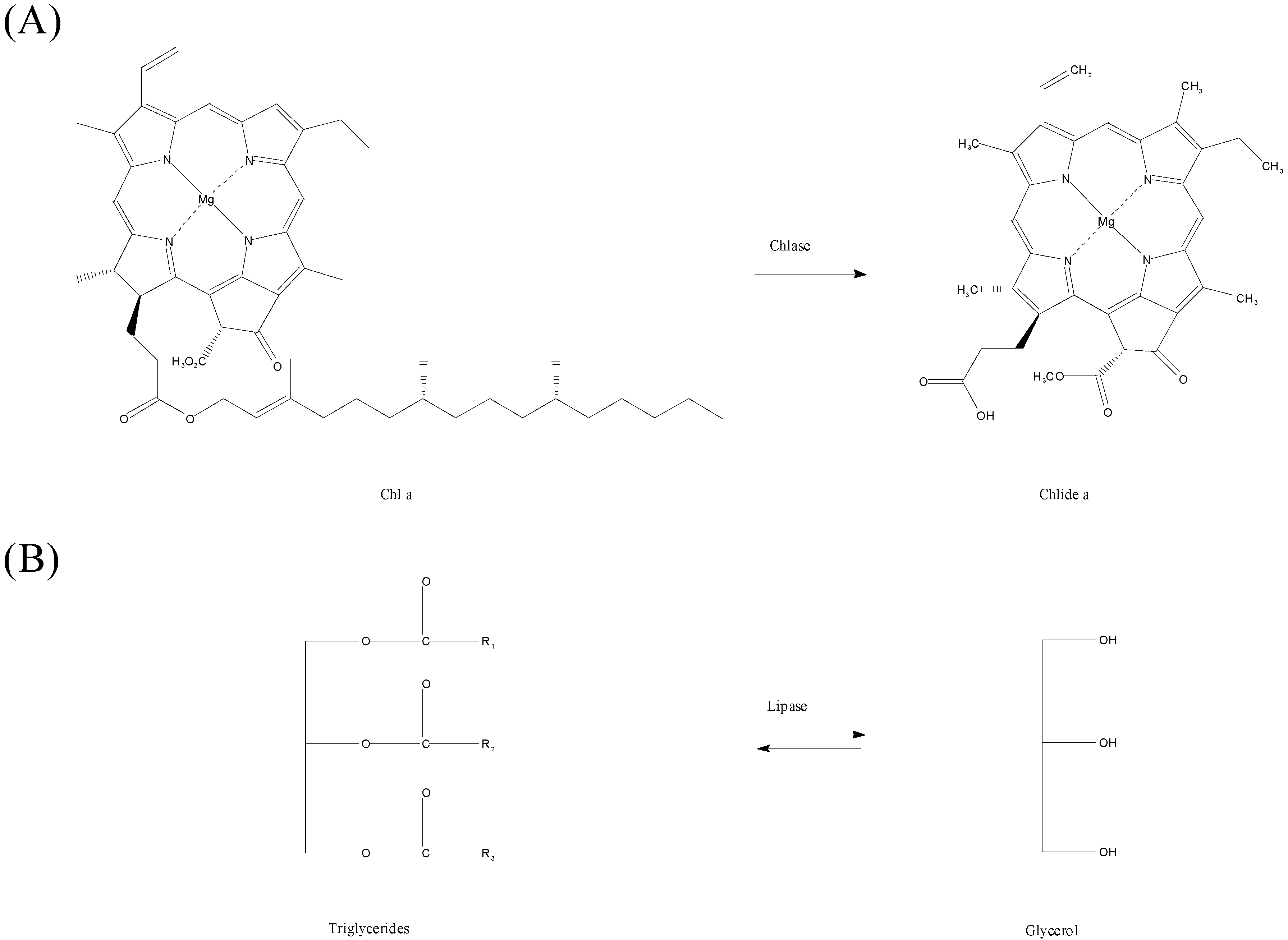
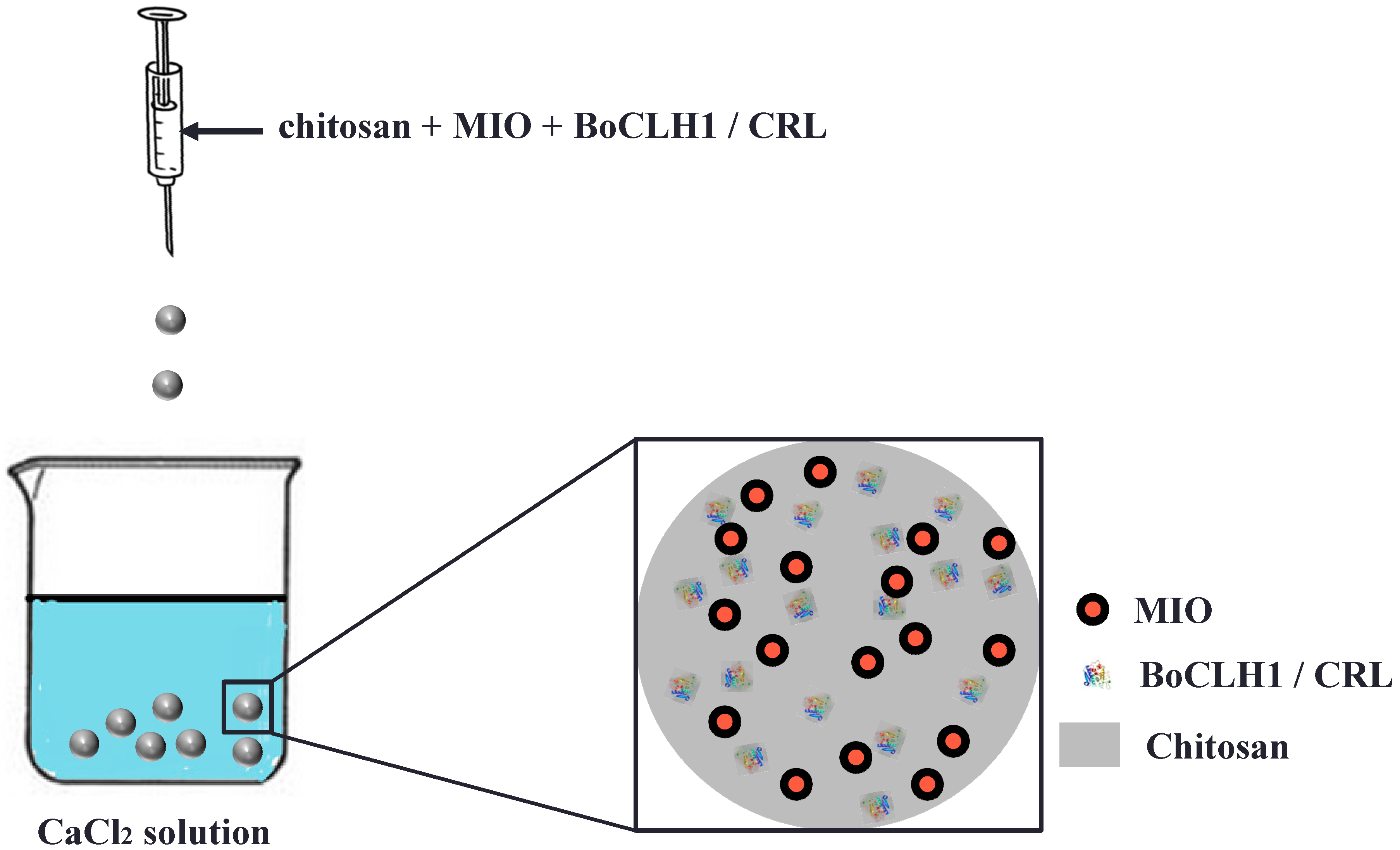
2. Results and Discussion
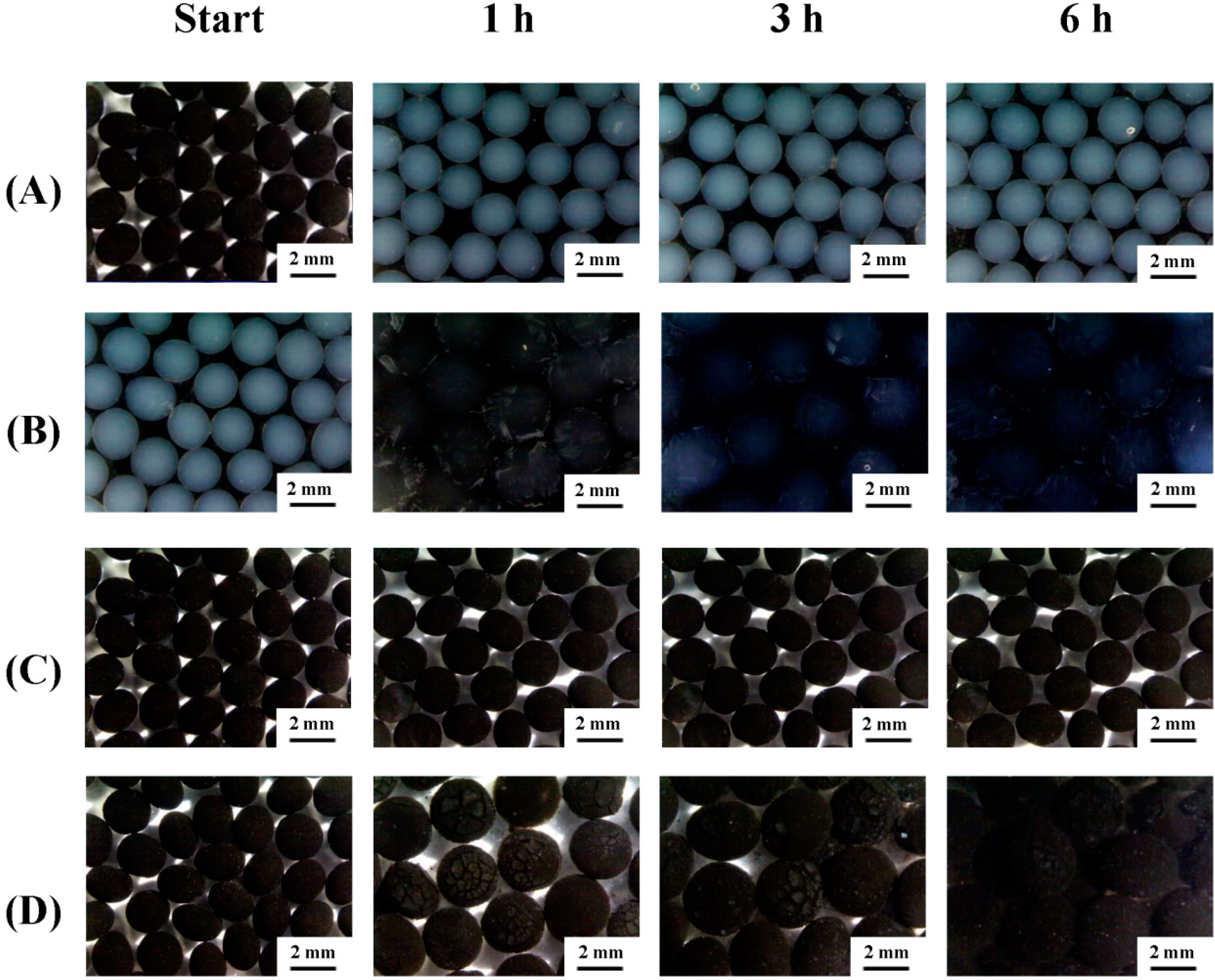
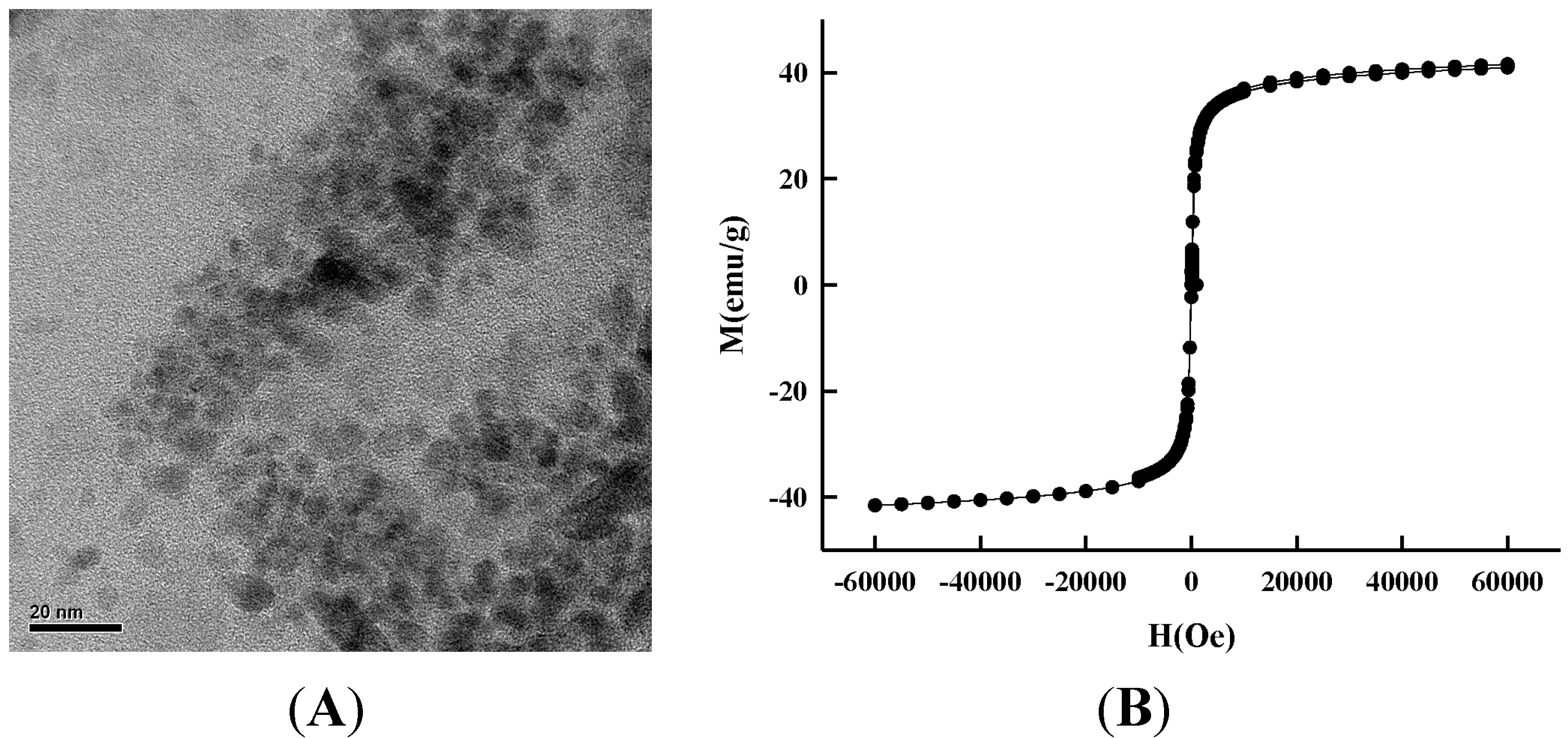
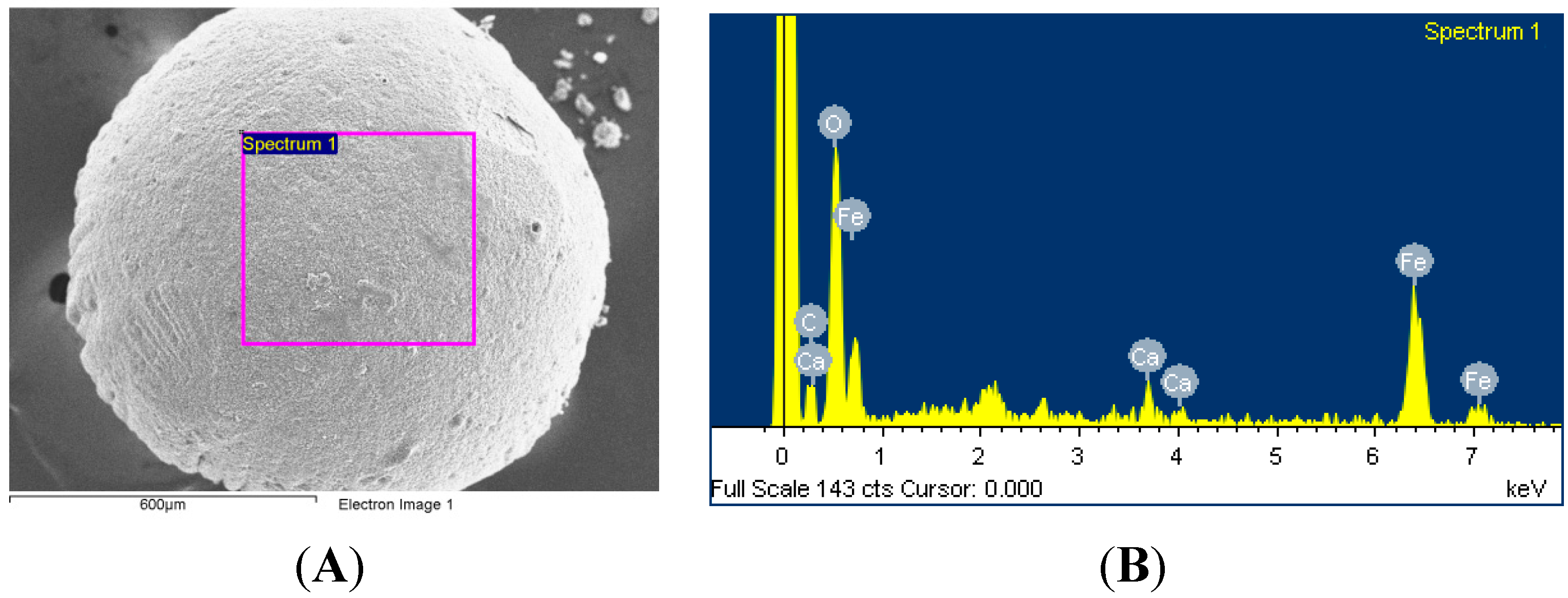
2.1. Morphology
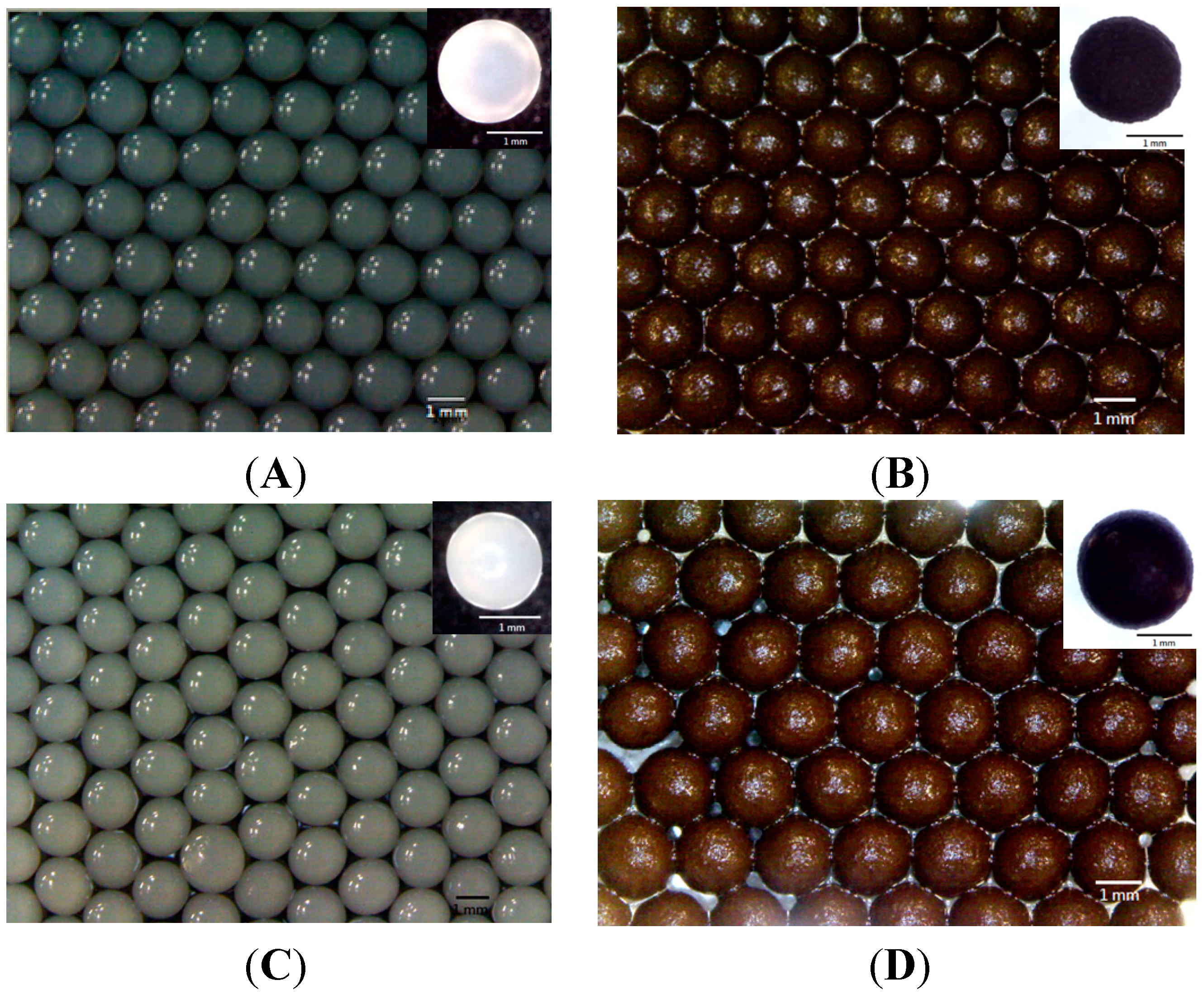
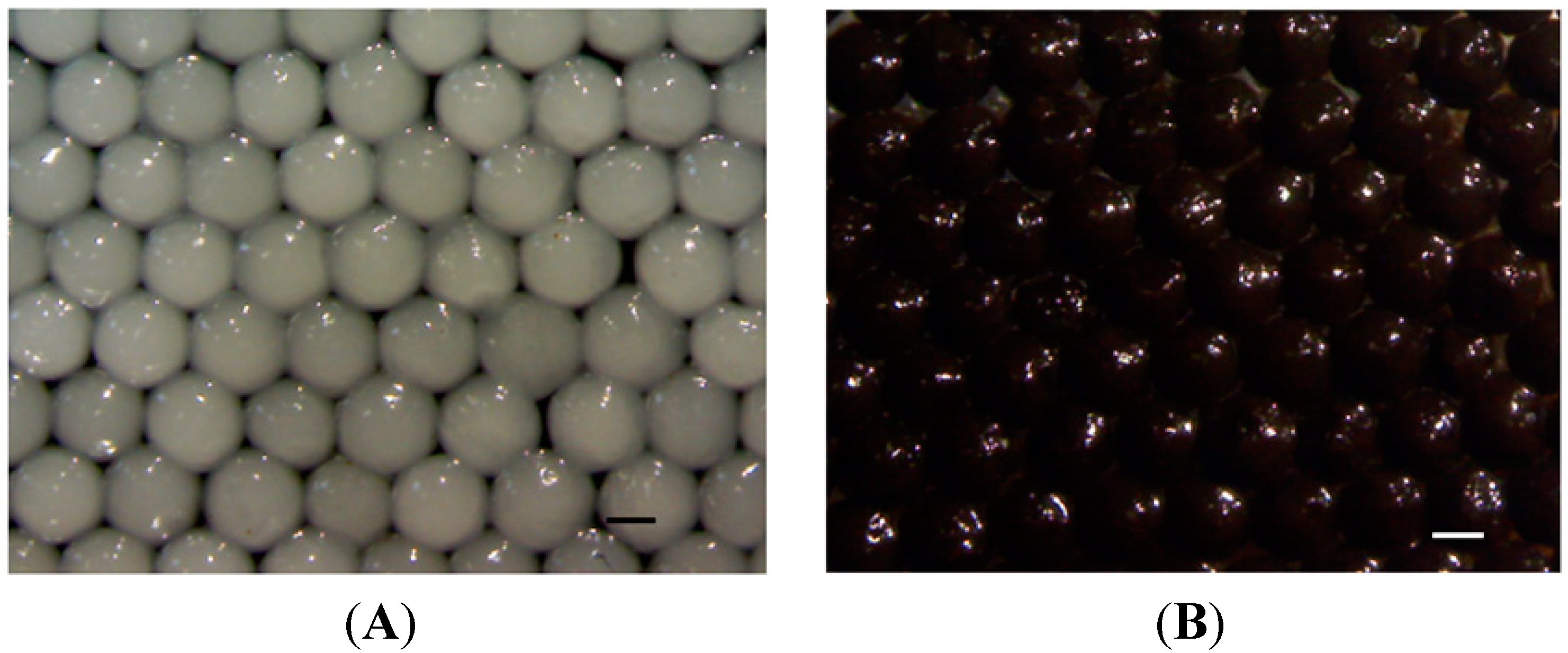
2.2. pH Sensitive Reactions of the Prepared Alginate Composite Beads
2.3. The Enzyme Entrapped and Immobilization Yield
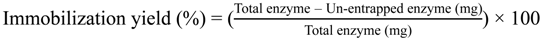
| Samples | Enzyme Entrapped (mg/g gel) | Immobilization Yield (%) |
|---|---|---|
| Free BoCLH1 | 0 | ND |
| BoCLH1 in alginate | 10.45 | 70% |
| BoCLH1 in MIO NPs-alginate | 9.26 | 62% |
| Free CRL | 0 | ND |
| CRL in alginate | 6.32 | 63.2% |
| CEL in MIO NPs-alginate | 5.93 | 59.3% |
2.4. The Immobilized Enzymes Catalytic Activity and Specific Activity
| Samples | Enzymatic Activity (U/g gel) | Specific Activity (U/mg protein) |
|---|---|---|
| Free BoCLH1 | ND | 3.68 ± 0.027 (100%) a |
| BoCLH1 in alginate | 2.96 ± 0.264 | 8.52 ± 0.758 (231.5%) |
| BoCLH1 in MIO NPs-alginate | 3.36 ± 0.469 | 10.9 ± 1.521 (296.2%) |
| Free CRL | ND | 60.78 ± 1.311 (100%) |
| CRL in alginate | 94.83 ± 7.929 | 15.01 ± 1.255 (24.7%) |
| CRL in MIO NPs-alginate | 11.81 ± 0.618 | 1.99 ± 0.104 (3.3%) |
2.5. pH Effects of Immobilized BoCLH1 and CRL Activity
 ), BoCLH1 in alginate (□) and BoCLH1 in MIO NPs-alginate (■) indicated, respectively. (B) The pH activity of free CRL (
), BoCLH1 in alginate (□) and BoCLH1 in MIO NPs-alginate (■) indicated, respectively. (B) The pH activity of free CRL (  ), CEL in alginate (△) and CRL in MIO NPs-alginate (▲) indicated, respectively. The Chlase and esterase assay using Chl a and p-nitrophenol butyrate as substrate respectively. Relative activity (%) was triplicate measured according to the standard Chlase and esterase assay.
), CEL in alginate (△) and CRL in MIO NPs-alginate (▲) indicated, respectively. The Chlase and esterase assay using Chl a and p-nitrophenol butyrate as substrate respectively. Relative activity (%) was triplicate measured according to the standard Chlase and esterase assay.
 ), BoCLH1 in alginate (□) and BoCLH1 in MIO NPs-alginate (■) indicated, respectively. (B) The pH activity of free CRL (
), BoCLH1 in alginate (□) and BoCLH1 in MIO NPs-alginate (■) indicated, respectively. (B) The pH activity of free CRL (  ), CEL in alginate (△) and CRL in MIO NPs-alginate (▲) indicated, respectively. The Chlase and esterase assay using Chl a and p-nitrophenol butyrate as substrate respectively. Relative activity (%) was triplicate measured according to the standard Chlase and esterase assay.
), CEL in alginate (△) and CRL in MIO NPs-alginate (▲) indicated, respectively. The Chlase and esterase assay using Chl a and p-nitrophenol butyrate as substrate respectively. Relative activity (%) was triplicate measured according to the standard Chlase and esterase assay.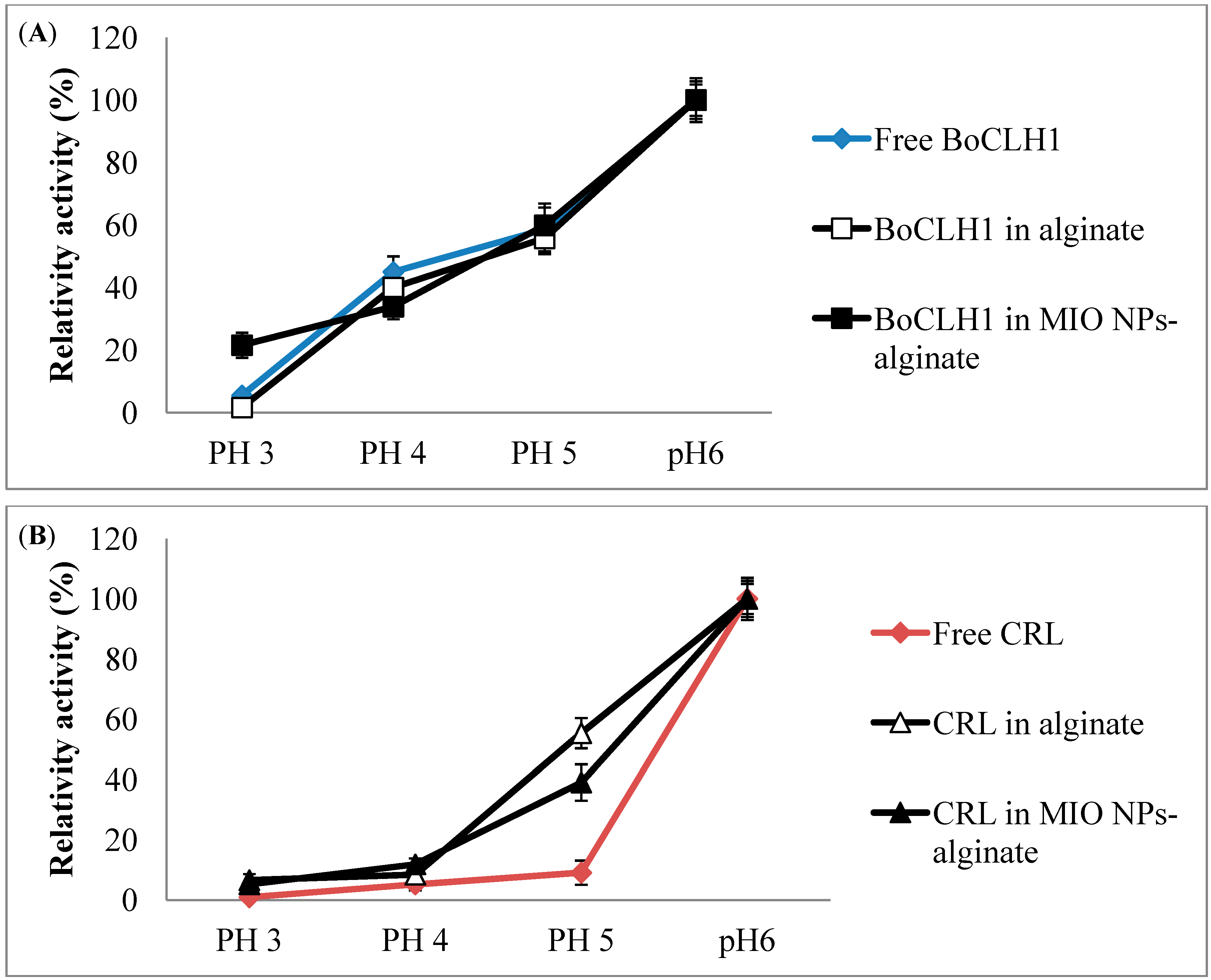
2.6. Repeated Use of Immobilized Enzymes
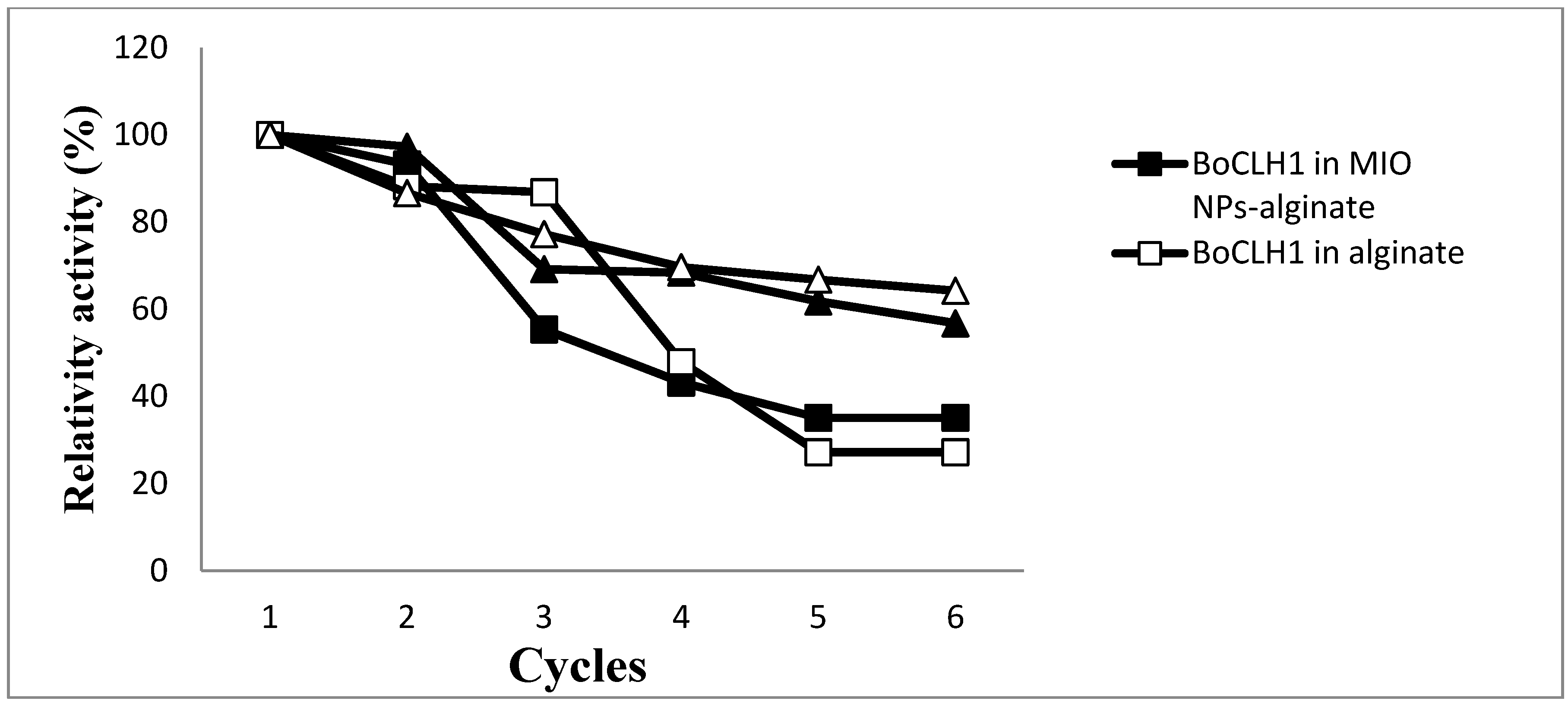
3. Experimental Section
3.1. Materials
3.2. Enzyme Preparation
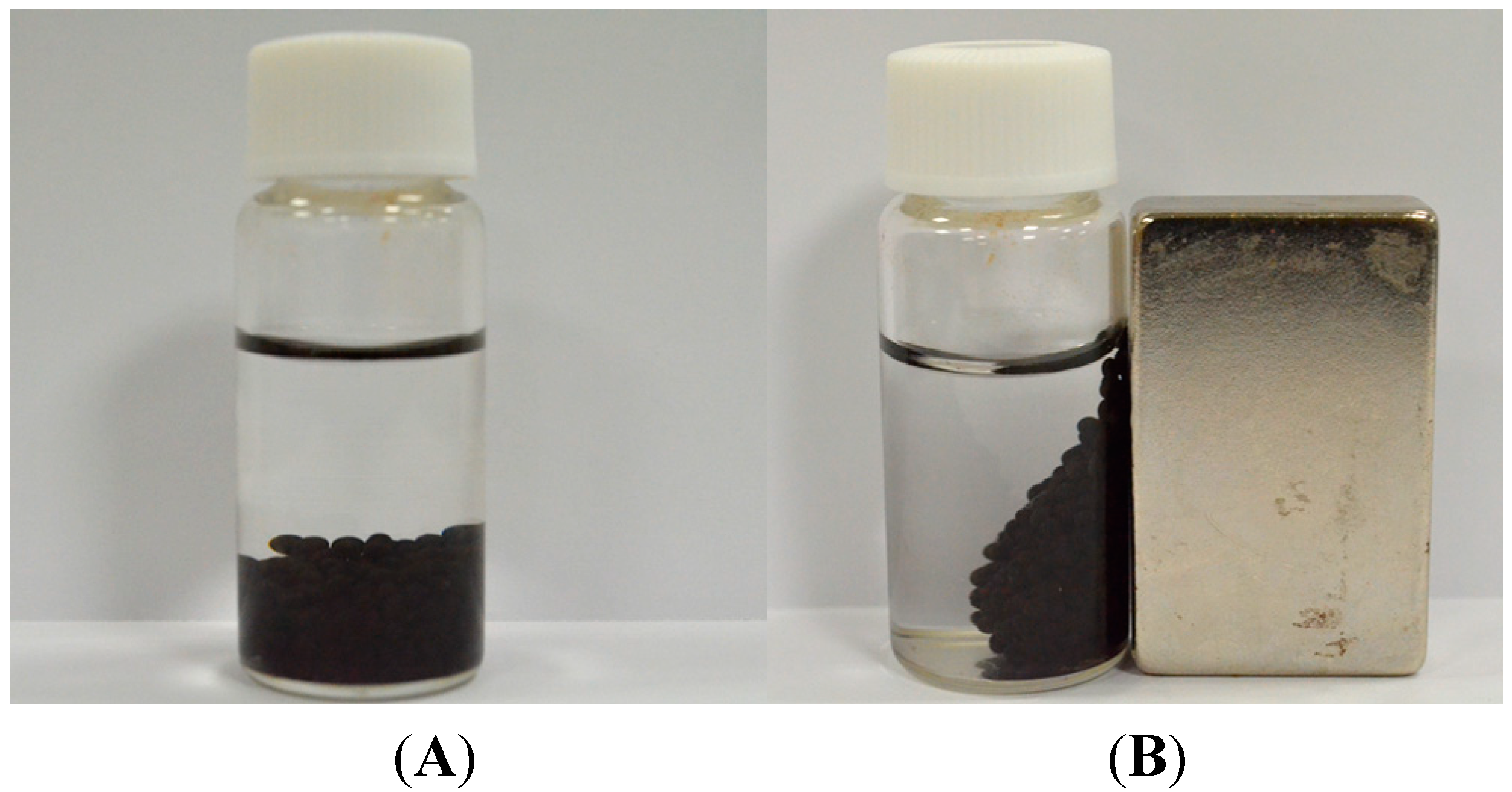
3.3. Synthesis of Magnetic Iron Oxide Nanoparticles
3.4. Synthesis of MIO NPs-Alginate Beads
3.5. Preparation of Enzyme Encapsulated in Magnetic Alginate Composite Beads
3.6. Chlorophyllase Assay of the Immobilized BoCLH1
3.7. Esterase Assay of the Immobilized CRL
3.8. Operational Stability
3.9. The pH Effects of Immobilized BoCLH1 and CRL Activity
3.10. Instruments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fang, Y.; Huang, X.J.; Chen, P.C.; Xu, Z.K. Polymer materials for enzyme immobilization and their application in bioreactors. BMB Rep. 2011, 44, 87–95. [Google Scholar] [CrossRef]
- Mateo, C.; Grazu, V.; Palomo, J.M.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protocols 2007, 2, 1022–1033. [Google Scholar] [CrossRef]
- Klibanov, A.M. Immobilized enzymes and cells as practical catalysts. Science 1983, 219, 722–727. [Google Scholar]
- Zhang, D.H.; Yuwen, L.X.; Peng, L.J. Parameters Affecting the Performance of Immobilized Enzyme. J. Chem. 2013, 2013, 7. [Google Scholar]
- Datta, S.; Christena, L.R.; Rajaram, Y. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar]
- Boscolo, B.; Trotta, F.; Ghibaudi, E. High catalytic performances of Pseudomonas fluorescens lipase adsorbed on a new type of cyclodextrin-based nanosponges. J. Mol. Catal. B Enzym. 2010, 62, 155–161. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, Y.; Zhou, L.; Gao, J. Immobilization of Candida antarctica lipase B by adsorption in organic medium. New Biotechnol. 2010, 27, 53–58. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sezgin, M. Enhancement of the activity and enantioselectivity of lipase by sol-gel encapsulation immobilization onto beta-cyclodextrin-based polymer. Appl. Biochem. Biotechnol. 2012, 166, 1927–1940. [Google Scholar] [CrossRef]
- Akdemir, Z.S.; Demir, S.; Kahraman, M.V.; Apohan, N.K. Preparation and characterization of UV-curable polymeric support for covalent immobilization of xylanase enzyme. J. Mol. Catal. B Enzym. 2011, 68, 104–108. [Google Scholar] [CrossRef]
- Edupuganti, S.R.; Edupuganti, O.P.; O’Kennedy, R.; Defrancq, E.; Boullanger, S. Use of T-2 toxin-immobilized amine-activated beads as an efficient affinity purification matrix for the isolation of specific IgY. J. Chromatogr. B 2013, 923–924, 98–101. [Google Scholar] [CrossRef]
- Hu, B.; Pan, J.; Yu, H.L.; Liu, J.W.; Xu, J.H. Immobilization of Serratia marcescens lipase onto amino-functionalized magnetic nanoparticles for repeated use in enzymatic synthesis of Diltiazem intermediate. Process Biochem. 2009, 44, 1019–1024. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Jeya, M.; Lee, J.K. Enhanced activity and stability of L-arabinose isomerase by immobilization on aminopropyl glass. Appl. Microbiol. Biotechnol. 2011, 89, 1435–1442. [Google Scholar] [CrossRef]
- Anwar, A.; Qader, S.A.U.; Raiz, A.; Iqbal, S.; Azhar, A. Calcium alginate: A support material for immobilization of proteases from newly isolated strain of Bacillus subtilis KIBGE-HAS. World Appl. Sci. J. 2009, 7, 1281–1286. [Google Scholar]
- Zhang, S.; Shang, W.; Yang, X.; Zhang, S.; Zhang, X.; Chen, J. Immobilization of lipase using alginate hydrogel beads and enzymatic evaluation in hydrolysis of p-Nitrophenol butyrate. Bull. Korean Chem. Soc. 2013, 34, 2741–2746. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Papain entrapment in alginate beads for stability improvement and site-specific delivery: Physicochemical characterization and factorial optimization using neural network modeling. AAPS PharmSciTech 2005, 6, E209–E222. [Google Scholar]
- Vu, T.K.; Le, V.V.M. Biochemical studies on the immobilization of the enzyme invertase (EC.3.2.1.26) in alginate gel and its kinetics. ASEAN Food J. 2008, 15, 73–78. [Google Scholar]
- Won, K.; Kim, S.; Kim, K.-J.; Park, H.W.; Moon, S.-J. Optimization of lipase entrapment in Ca-alginate gel beads. Process Biochem. 2005, 40, 2149–2154. [Google Scholar]
- Jordan, J.; Theegala, C. Probing the limitations for recycling cellulase enzymes immobilized on iron oxide (Fe3O4) nanoparticles. Biomass Conv. Bioref. 2014, 4, 25–33. [Google Scholar] [CrossRef]
- Solanki, K.; Gupta, M.N. Simultaneous purification and immobilization of Candida rugosa lipase on superparamagnetic Fe3O4 nanoparticles for catalyzing transesterification reactions. New J. Chem. 2011, 35, 2551–2556. [Google Scholar] [CrossRef]
- Wu, X.C.; Zhang, Y.; Wu, C.Y.; Wu, H.X. Preparation and characterization of magnetic Fe3O4/CRGO nanocomposites for enzyme immobilization. T. Nonferr. Metal. Soc. 2012, 22, s162–s168. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Suzuki, T.; Yamada, T.; Shimada, H.; Masuda, T.; Ohta, H.; Takamiya, K. Chlorophyllase as a serine hydrolase: Identification of a putative catalytic triad. Plant Cell Physiol. 2003, 44, 96–101. [Google Scholar]
- Hortensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta 2011, 1807, 977–988. [Google Scholar]
- Karboune, S.; Neufeld, R.; Kermasha, S. Immobilization and biocatalysis of chlorophyllase in selected organic solvent systems. J. Biotechnol. 2005, 120, 273–283. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Yang, C.M.; Chen, C.M.; Chao, P.Y.; Hu, S.P. Effects of chlorophyll-related compounds on hydrogen peroxide induced DNA damage within human lymphocytes. J. Agric. Food Chem. 2005, 53, 2746–2750. [Google Scholar]
- Hsu, C.Y.; Chen, Y.H.; Chao, P.Y.; Chen, C.M.; Hsieh, L.L.; Hu, S.P. Naturallyoccurring chlorophyll derivatives inhibit aflatoxin B1-DNA adduct formation in hepatoma cells. Mutat. Res. 2008, 657, 98–104. [Google Scholar] [CrossRef]
- Guo, H.; Pan, X.; Mao, R.; Zhang, X.; Wang, L.; Lu, X.; Chang, J.; Guo, J.T.; Passic, S.; Krebs, F.C.; et al. Alkylated porphyrins have broad antiviral activity against hepadnaviruses, flaviviruses, filoviruses, and arenaviruses. Antimicrob. Agents Chemother. 2011, 55, 478–486. [Google Scholar] [CrossRef]
- Komiya, T.; Kyohkon, M.; Ohwaki, S.; Eto, J.; Katsuzaki, H.; Imai, K.; Kataoka, T.; Yoshioka, K.; Ishii, Y.; Hibasami, H. Phytol induces programmed cell death in human lymphoid leukemia Molt 4B cells. Int. J. Mol. Med. 1999, 4, 377–380. [Google Scholar]
- Saikia, D.; Parihar, S.; Chanda, D.; Ojha, S.; Kumar, J.K; Chanotiya, C.S.; Shanker, K.; Negi, A.S. Antitubercular potential of some semisynthetic analogues of phytol. Bioorg. Med. Chem. Lett. 2010, 20, 508–512. [Google Scholar] [CrossRef]
- Aachoui, Y.; Schulte, M.L.; Fitch, R.W.; Ghosh, S.K. Synthetic adjuvants for vaccine formulations: Evaluation of new phytol derivatives in induction and persistence of specific immune response. Cell. Immunol. 2011, 271, 308–318. [Google Scholar] [CrossRef]
- Wang, Y.J.; Sheu, J.Y.; Wang, F.F.; Shaw, J.F. The lipase catalyzed oil hydrolysis in the absence of added emulsifier. Biotechnol Bioeng. 1988, 31, 628–633. [Google Scholar] [CrossRef]
- Klibanov, A.M. Asymmetric transformations catalyzed in organic solvents. Acc. Chem. Res. 1990, 23, 114–120. [Google Scholar] [CrossRef]
- Shaw, J.F.; Chang, R.C.; Wang, F.F.; Wang, Y.J. Lipolytic activities of a lipase immobilized on six selected supporting materials. Biotechnol. Bioeng. 1990, 35, 132–137. [Google Scholar] [CrossRef]
- Valivety, R.H.; Halling, P.J.; Peilow, A.D.; Macrae, A.R. Relationship between water activity and catalytic activity of lipases in organic media. Effects of supports, loading and enzyme preparation. Eur. J. Biochem. 1994, 222, 461–466. [Google Scholar] [CrossRef]
- Gandhi, N.N. Applications of lipase. J. Am. Oil Chem. Soc. 1997, 74, 621–634. [Google Scholar]
- Benjamin, S.; Pandey, A. Candida rugosa lipases: Molecular biology and versatility in biotechnology. Yeast 1998, 14, 1069–1087. [Google Scholar] [CrossRef]
- Svendsen, A. Lipase protein engineering. Biochim. Biophys. Acta 2000, 1543, 223–238. [Google Scholar] [CrossRef]
- Lee, G.C.; Chepyshko, H.; Chen, H.H.; Chu, C.C.; Chou, Y.F.; Akoh, C.C.; Shaw, J.F. Genes and biochemical characterization of three novel chlorophyllase isozymes from Brassica oleracea. J. Agric. Food Chem. 2010, 58, 8651–8657. [Google Scholar] [CrossRef]
- Lee, L.C.; Yen, C.C.; Malmis, C.C.; Chen, L.F.; Chen, J.C.; Lee, G.C.; Shaw, J.F. Characterization of codon-optimized recombinant Candida rugosa lipase 5 (LIP5). J. Agric. Food Chem. 2011, 59, 10693–10698. [Google Scholar] [CrossRef]
- Kouassi, G.K.; Irudayaraj, J.; McCarty, G. Examination of Cholesterol oxidase attachment to magnetic nanoparticles. J. Nanobiotechnolog. 2005, 3, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Tsai, M.G.; Chi, M.C.; Wang, T.F.; Lin, L.L. Covalent immobilization of Bacillus licheniformis γ-glutamyl transpeptidase on aldehyde-functionalized magnetic nanoparticles. Int. J. Mol. Sci. 2013, 14, 4613–4628. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, C.Y.; Huang, K.S; Kung, C.P.; Chang, Y.C.; Shaw, J.F. Microfluidic one-step synthesis of Fe3O4-chitosan composite particles and their applications. Int. J. Phytorem. 2014, 463, 155–160. [Google Scholar]
- Cotar, A.I.; Grumezescu, A.M.; Huang, K.S.; Chifiriuc, C.M.; Radulescu, R. Magnetite nanoparticles influence the efficacy of antibiotics against biofilm embedded Staphylococcus aureus cells. Biointerface Res. Appl. Chem. 2013, 3, 559–565. [Google Scholar]
- Voicu, G.; Andronescu, E.; Grumezescu, A.M.; Huang, K.S.; Ficai, A.; Yang, C.H.; Bleotu, C.; Chifiriuc, M.C. Antitumor activity of magnetite nanoparticles: Influence of hydrocarbonated chain of saturated aliphatic monocarboxylic acids. Curr. Org. Chem. 2013, 17, 831–840. [Google Scholar]
- Wang, C.Y.; Yang C.H., Huang, K.S.; Chang F.R., Wang, A.H.J.; Chen, C.H. Electrostatic droplets assisted in-situ synthesis of superparamagnetic chitosan microparticles for magnetic-responsive controlled drug release and copper ion removal. J. Mater. Chem. B 2013, 1, 2205–2212. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, C.-H.; Yen, C.-C.; Jheng, J.-J.; Wang, C.-Y.; Chen, S.-S.; Huang, P.-Y.; Huang, K.-S.; Shaw, J.-F. Immobilization of Brassica oleracea Chlorophyllase 1 (BoCLH1) and Candida rugosa Lipase (CRL) in Magnetic Alginate Beads: An Enzymatic Evaluation in the Corresponding Proteins. Molecules 2014, 19, 11800-11815. https://doi.org/10.3390/molecules190811800
Yang C-H, Yen C-C, Jheng J-J, Wang C-Y, Chen S-S, Huang P-Y, Huang K-S, Shaw J-F. Immobilization of Brassica oleracea Chlorophyllase 1 (BoCLH1) and Candida rugosa Lipase (CRL) in Magnetic Alginate Beads: An Enzymatic Evaluation in the Corresponding Proteins. Molecules. 2014; 19(8):11800-11815. https://doi.org/10.3390/molecules190811800
Chicago/Turabian StyleYang, Chih-Hui, Chih-Chung Yen, Jen-Jyun Jheng, Chih-Yu Wang, Sheau-Shyang Chen, Pei-Yu Huang, Keng-Shiang Huang, and Jei-Fu Shaw. 2014. "Immobilization of Brassica oleracea Chlorophyllase 1 (BoCLH1) and Candida rugosa Lipase (CRL) in Magnetic Alginate Beads: An Enzymatic Evaluation in the Corresponding Proteins" Molecules 19, no. 8: 11800-11815. https://doi.org/10.3390/molecules190811800
APA StyleYang, C.-H., Yen, C.-C., Jheng, J.-J., Wang, C.-Y., Chen, S.-S., Huang, P.-Y., Huang, K.-S., & Shaw, J.-F. (2014). Immobilization of Brassica oleracea Chlorophyllase 1 (BoCLH1) and Candida rugosa Lipase (CRL) in Magnetic Alginate Beads: An Enzymatic Evaluation in the Corresponding Proteins. Molecules, 19(8), 11800-11815. https://doi.org/10.3390/molecules190811800






