Tuning of Essential Oil Properties by Enzymatic Treatment: Towards Sustainable Processes for the Generation of New Fragrance Ingredients
Abstract
:1. Introduction
2. Discussion
2.1. Pre-Treatment
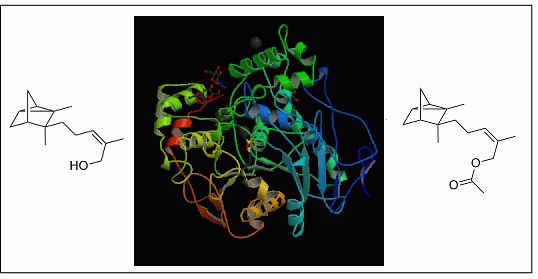
2.2. Post-Treatment
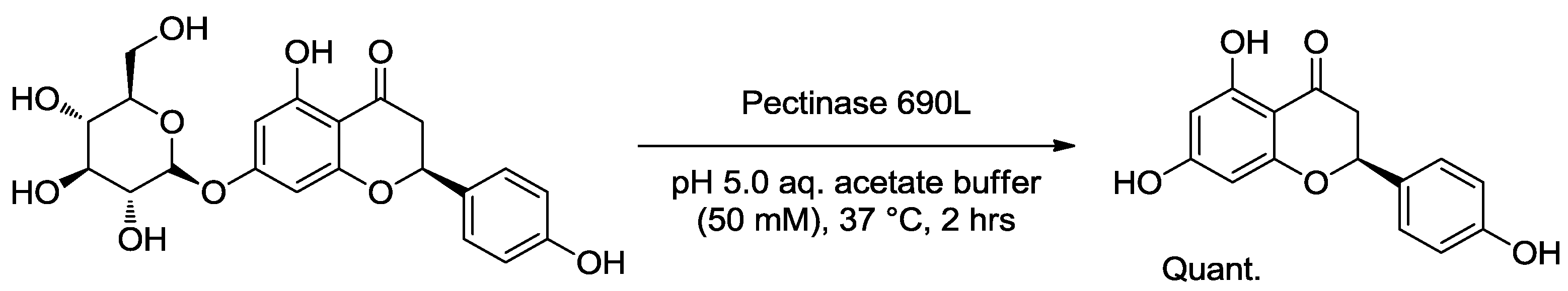
2.2.1. Tuning of Biological Properties
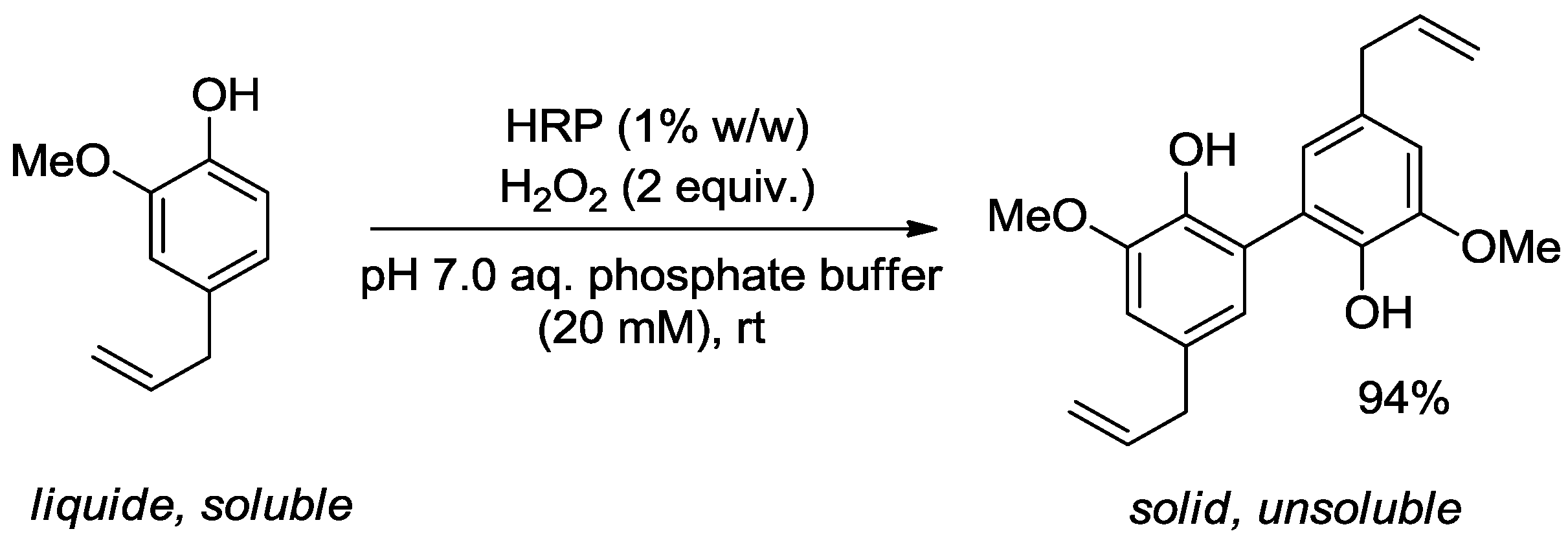
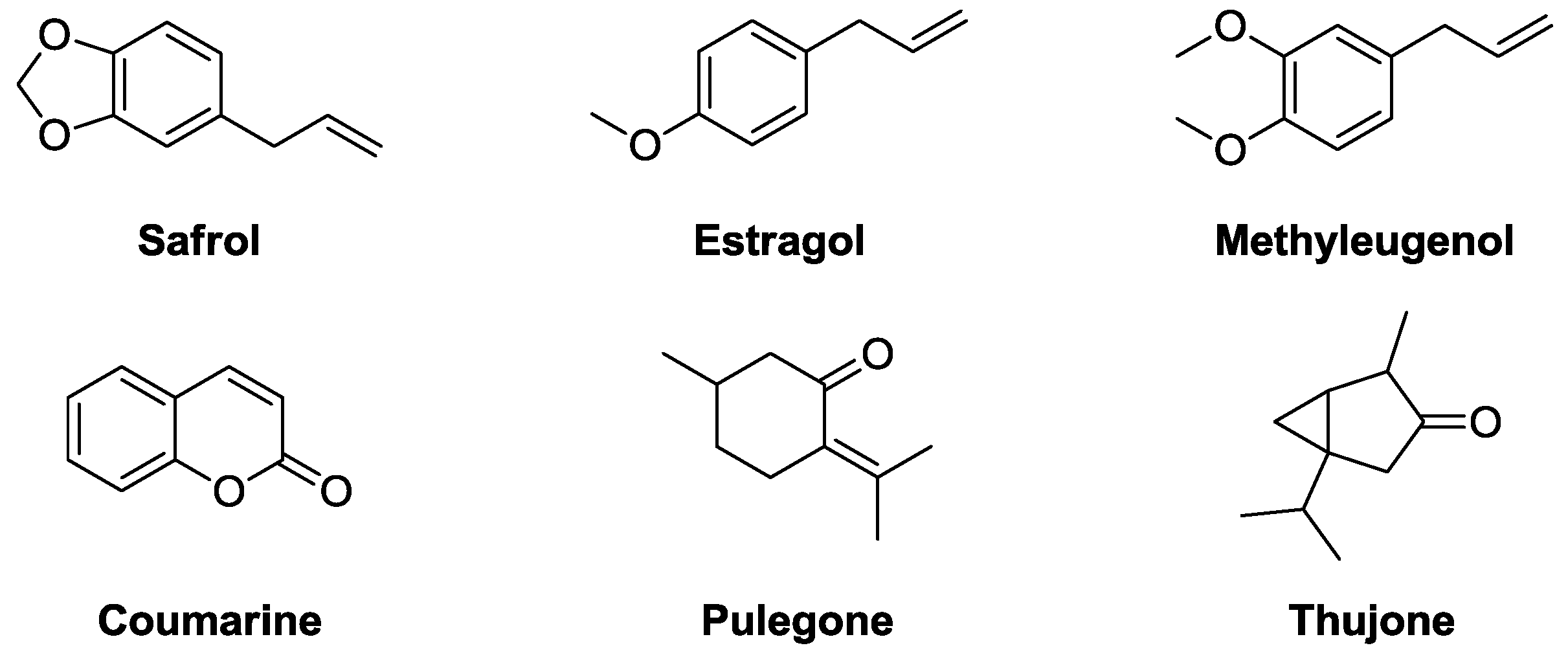
2.2.2. Tuning of Olfactory Properties

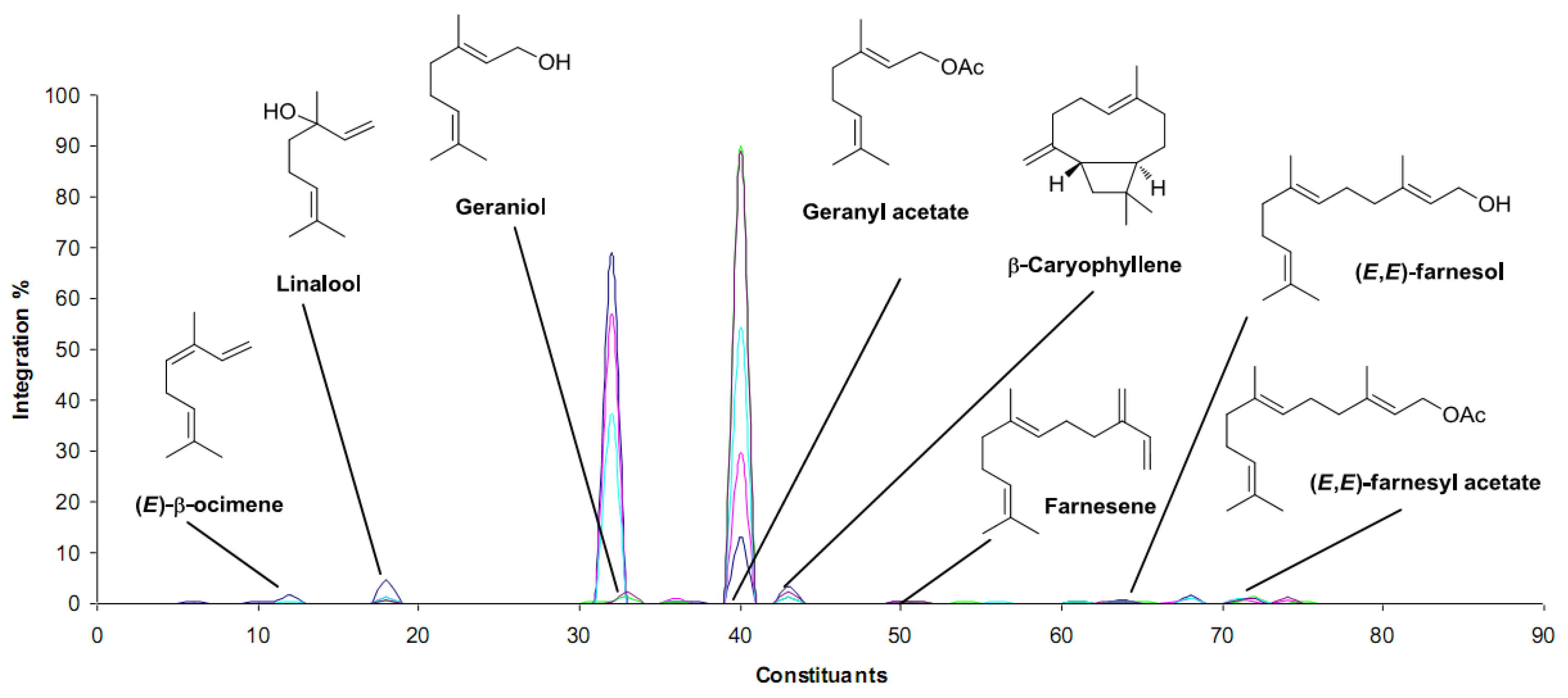
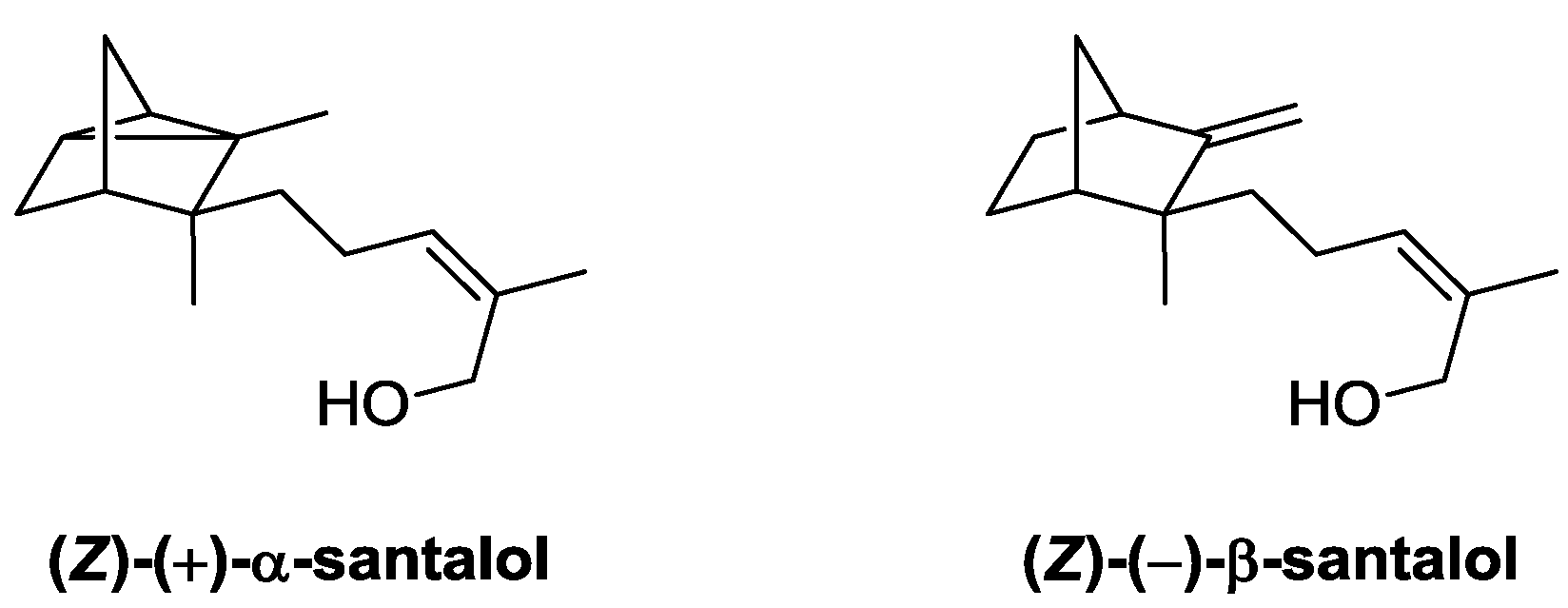
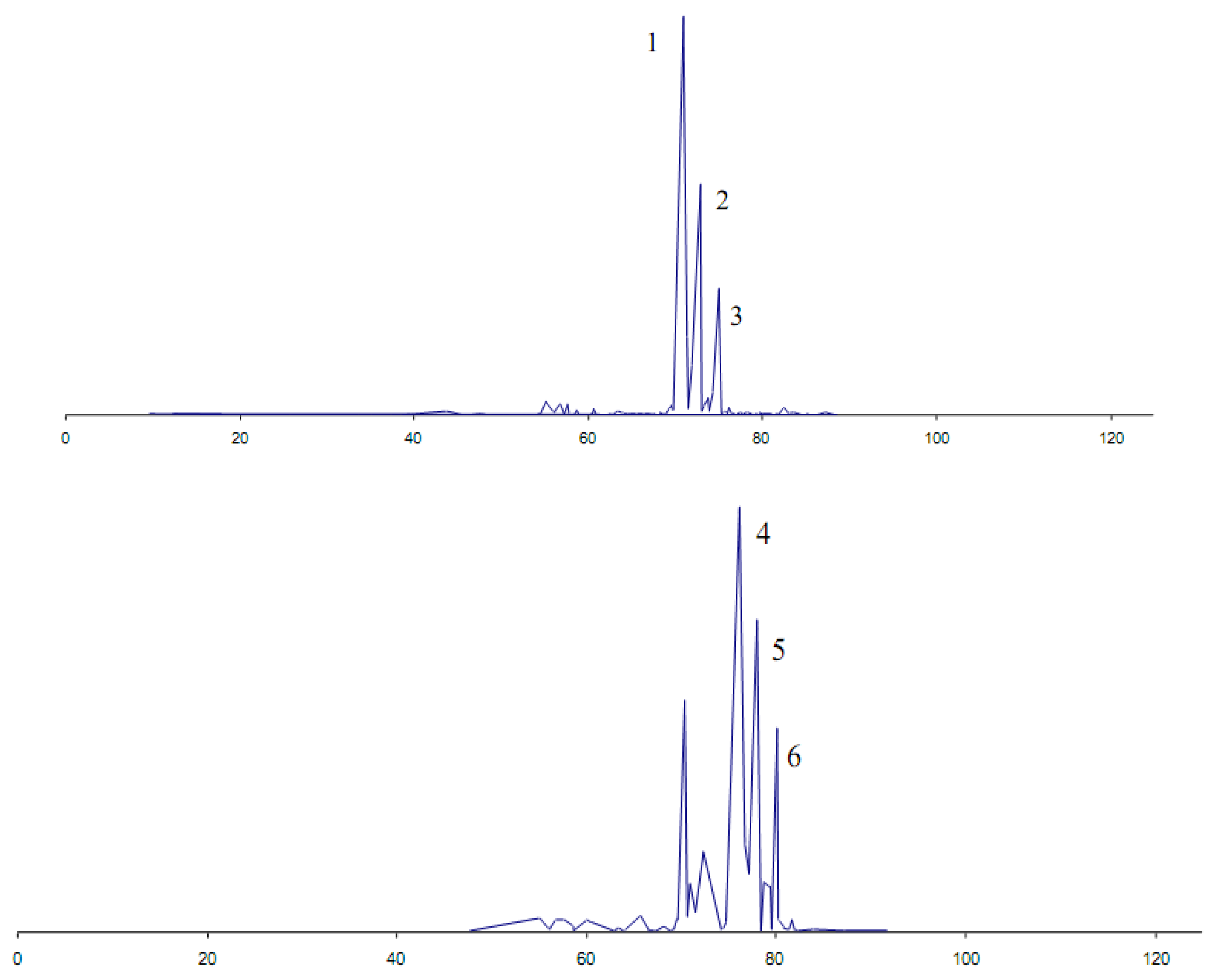
3. Conclusions
| Enzymes | Essential oils/extracts | Effects | Properties modified | Ref. |
|---|---|---|---|---|
| Pre-treatment | ||||
| Cellulase, pectinase, protease, viscozyme | Cumin (Cuminum cyminum L.) | Yield improved | None | [18] |
| Cellulase, pectinase, protease, viscozyme | Celery (Apium graveolens L.) | Yield improved | None | [21] |
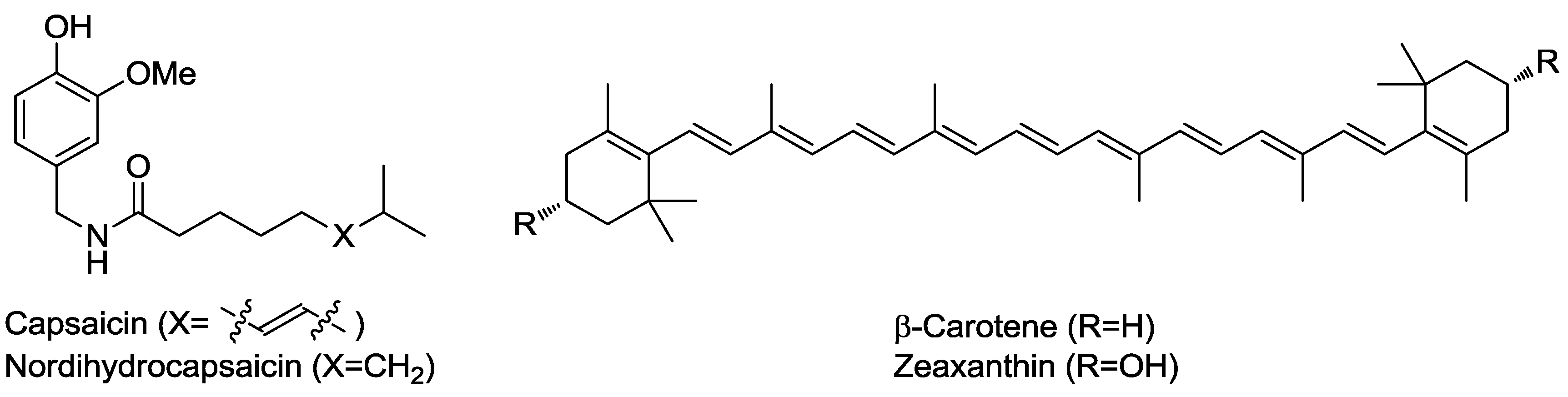
| Hydrolytic enzymes (extract) | Chili guajillo (Capsicum annuum L.) | Carotenoids/capsaicinoids recovery | Organoleptic | [19,20] |
| Cellulase, β-glucanase, pectinase, xylanase | Black pepper | Yield improved, caryophyllene content increased | Organoleptic | [22] |
| Cellulase, β-glucanase, pectinase, xylanase | Cardamom | Yield improved, α-terpenyl acetate content increased | Organoleptic | [22] |
| Cellulase, β-glucanase, pectinase, xylanase | Thymus (Thymus capitatus) | Yield improved, carvacrol content increased | Anti-microbial | [23] |
| Cellulase, β-glucanase, pectinase, xylanase | Rosemary (Rosmarinus officinalis) | Yield improved, 1,8-cineole content decreased | Anti-microbial | [23] |
| Extracts (β-glucosidase, α-rhamnosidase) | Bergamot (Citrus bergamia Risso) | Flavonoids content increased | Anti-oxidant | [24] |
| β-Glucosidase | Sweet olive (Osmanthus fragrans Lour) | Yield improved, nonanal, dihydro-β-ionol, and (E)-β-ionone contents increased | Organoleptic | [26] |
| β-Glucosidase | Arabian pea (Psoralea bituminosa) | Hex-3-en-1-ol and oct-1-en-3-ol contents increased | Organoleptic | [27] |
| β-Glucosidase and α-amylglucosidase | Clove (Syzygium aromaticum (L.) merr. and perry (Myrtaceae)) | Eugenol, isoeugenol, farnesol, and nerolidol contents increased | Organoleptic | [28] |
| β-Glucosidase and pectinol C | Hyssop (Hyssopus officinalis L.) | Terpenyl alcohols, and phenolics contents increased | Organoleptic | [29] |
| Post-treatment | ||||
| Horseradish peroxidase | Rose (Rosa sp.) | Eugenol content decreased | Toxicity/allergenicity | [34] |
| Candida rugosa lipase | Palmarosa (Cymbopogon martinii) | Terpenyl alcohols contents decreased, terpenyl esters contents increased | Organoleptic | [35] |
| Candida antartica lipase B | Rose (Rosa sp.) | Terpenyl alcohols contents decreased, terpenyl esters contents increased | Organoleptic | [36] |
| Candida rugosa lipase | Sandalwood (Santalum austrocaledonicum) | α- and β-santol contents decreased in favor of their esters | Organoleptic | [38] |
Acknowledgments
Conflicts of Interest
References
- Kasper, S.; Gastpar, M.; Müller, W.E.; Volz, H.-P.; Möller, H.-J.; Dienel, A.; Schläfke, S. Silexan, an orally administered Lavandula oil preparation, is effective in the treatment of ‘subsyndromal’ anxiety disorder: A randomized, double-blind, placebo controlled trial. Int. Clin. Psychopharm. 2010, 25, 277–287. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Frag. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Kunicka-Styczyńska, A.; Sikora, M.; Kalemba, D. Antimicrobial activity of lavender, tea tree and lemon oils in cosmetic preservative systems. J. Appl. Microbiol. 2009, 107, 1903–1911. [Google Scholar] [CrossRef]
- Dweck, A.C. Natural preservatives - An update. In Formulating Natural Cosmetics; Dweck, A.C., Ed.; Allured Publishing Corporation: Carol Stream, 2010; pp. 107–130. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods: A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Johnson, J.D.; Abdo, K.M. Methyleugenol in the diet: Toxic and pathological aspects. Rev. Food Nutr. Toxic. 2005, 3, 1–60. [Google Scholar]
- Tsujimura, K.; Asamoto, M.; Suzuki, S.; Hokaiwado, N.; Ogawa, K.; Shirai, T. Prediction of carcinogenic potential by a toxicogenomic approach using rat hepatoma cells. Cancer Sci. 2006, 97, 1002–1010. [Google Scholar] [CrossRef]
- Smith, R.L.; Adams, T.B.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Rogers, A.E.; et al. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances - methyl eugenol and estragole. Food Chem. Toxicol. 2002, 40, 851–870. [Google Scholar] [CrossRef]
- Faber, K. Biotransformations in Organic Chemistry, 5th ed.; Springer-Verlag: Berlin, Germany, 2004; p. 454. [Google Scholar]
- Faber, K.; Kroutil, W. New enzymes for biotransformations. Curr. Opin. Chem. Biol. 2005, 9, 181–187. [Google Scholar]
- Groussin, A.-L.; Antoniotti, S. Valuable chemicals by the enzymatic modification of molecules of natural origin: Terpenoids, steroids, phenolics and related compounds. Bioresource Technol. 2012, 115, 237–243. [Google Scholar] [CrossRef]
- Serra, S.; Fuganti, C.; Brenna, E. Biocatalytic preparation of natural flavours and fragrances. Trends Biotechnol. 2005, 23, 193–198. [Google Scholar]
- Schrader, J.; Etschmann, M.M.W.; Sell, D.; Hilmer, J.M.; Rabenhorst, J. Applied biocatalysis for the synthesis of natural flavour compounds - current industrial processes and future prospects. Biotechnol. Lett. 2004, 26, 463–472. [Google Scholar] [CrossRef]
- Akoh, C.; Jennings, B.; Lillard, D. Enzymatic modification of evening primrose oil: Incorporation of polyunsaturated fatty acids. J. Am. Oil Chem. Soc. 1996, 73, 1059–1062. [Google Scholar] [CrossRef]
- Jennings, B.H.; Akoh, C.C. Lipase-catalyzed modification of rice bran oil to incorporate capric acid. J. Agric. Food Chem. 2000, 48, 4439–4443. [Google Scholar]
- Panyam, D.; Kilara, A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci. Tech. 1996, 7, 120–125. [Google Scholar] [CrossRef]
- Babu, K.G.D.; Singh, B.; Joshi, V.P.; Singh, V. Essential oil composition of Damask rose (Rosa damascena Mill.) distilled under different pressures and temperatures. Flavour Frag. J. 2002, 17, 136–140. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Srinivas, P.; Purnima, K.T.; Krishnamurthy, N. Enzyme-assisted extraction of volatiles from cumin (Cuminum cyminum L.) seeds. Food Chem. 2011, 127, 1856–1861. [Google Scholar]
- Santamaria, R.I.; Reyes-Duarte, M.D.; Barzana, E.; Fernando, D.; Gama, F.M.; Mota, M.; Lopez-Munguia, A. Selective enzyme-mediated extraction of capsaicinoids and carotenoids from chili guajillo puya (Capsicum annuum L.) using ethanol as solvent. J. Agric. Food Chem. 2000, 48, 3063–3067. [Google Scholar] [CrossRef]
- Salgado-Roman, M.; Botello-Àlvarez, E.; Rico-Martìnez, R.; Jiménez-Islas, H.; Cardenas-Manrìquez, M.; Navarrete-Bolaños, J.L. Enzymatic treatment to improve extraction of capsaicinoids and carotenoids from chili (Capsicum annuum) fruits. J. Agric. Food Chem. 2008, 56, 10012–10018. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Srinivas, P.; Krishnamurthy, N. Effect of enzymes on extraction of volatiles from celery seeds. Food Chem. 2010, 120, 230–234. [Google Scholar] [CrossRef]
- Chandran, J.; Amma, K.P.P.; Menon, N.; Purushothaman, J.; Nisha, P. Effect of enzyme assisted extraction on quality and yield of volatile oil from black pepper and cardamom. Food Sci. Biotechnol. 2012, 21, 1611–1617. [Google Scholar]
- Hosni, K.; Hassen, I.; Chaâbane, H.; Jemli, M.; Dallali, S.; Sebei, H.; Casabianca, H. Enzyme-assisted extraction of essential oils from thyme (Thymus capitatus L.) and rosemary (Rosmarinus officinalis L.): Impact on yield, chemical composition and antimicrobial activity. Ind. Crop. Prod. 2013, 47, 291–299. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.N.; Kirby, A.R.; lo Curto, R.B.; Bisignano, G.; Waldron, K.W.; Faulds, C.B. Enzymatic hydrolysis of flavonoids and pectic oligosaccharides from bergamot (Citrus bergamia Risso) peel. J. Agric. Food Chem. 2006, 54, 8307–8313. [Google Scholar] [CrossRef]
- Yao, W.-R.; Zhang, Y.-Z.; Chen, Y.; Yang, Z.-P. Aroma enhancement and characterization of the absolute osmanthus fragrans lour. J. Essent. Oil Res. 2010, 22, 97–102. [Google Scholar] [CrossRef]
- Zhiping, Y.; Weirong, Y.; He, Q. Studies on vapor phase extraction of rose oil enhanced by β-glucosidase. Flavour Frag. J. 2006, 21, 776–782. [Google Scholar] [CrossRef]
- Bertoli, A.; Menichini, F.; Noccioli, C.; Morelli, I.; Pistelli, L. Volatile constituents of different organs of Psoralea bituminosa L. Flavour Frag. J. 2004, 19, 166–171. [Google Scholar] [CrossRef]
- Menon, A.N.; Narayanan, C.S. Glycosidically bound volatiles of clove Syzygium aromaticum (L.) merr. et perry (Myrtaceae). Flavour Frag. J. 1992, 7, 155–157. [Google Scholar] [CrossRef]
- Schulz, G.; Stahl-Biskup, E. Essential oils and glycosidic bound volatiles from leaves, stems, flowers and roots of Hyssopus officinalis L. (Lamiaceae). Flavour Frag. J. 1991, 6, 69–73. [Google Scholar] [CrossRef]
- Johansen, J.D.; Bernard, G.; Gimenez-Arnau, E.; Lepoittevin, J.-P.; Bruze, M.; Andersen, K.E. Comparison of elicitation potential of chloroatranol and atranol - 2 allergens in oak moss absolute. Contact Dermatitis 2006, 54, 192–195. [Google Scholar] [CrossRef]
- Standards IFRA. Available online: http://www.ifraorg.org/en-us/standards (accessed on 6 May 2014).
- Moulin, C.; Petit, A.; Baccou, J.C. Selective laser photolysis of organic molecules in complex matrixes. J. Photochem. Photobiol. A 1995, 85, 165–172. [Google Scholar] [CrossRef]
- Pertsovich, S.I. A Method to Selectively Remove Safrole from Nutmeg Oil. WO2007/117174, 18 October 2007. [Google Scholar]
- Bouhlel, C.; Dolhem, G.A.; Fernandez, X.; Antoniotti, S. Model study of the enzymatic modification of natural extracts: Peroxidase-based removal of eugenol from rose essential oil. J. Agric. Food Chem. 2012, 60, 1052–1058. [Google Scholar] [CrossRef]
- Ramilijaona, J.; Raynaud, E.; Bouhlel, C.; Sarrazin, E.; Fernandez, X.; Antoniotti., S. Enzymatic modification of palmarosa essential oil: Chemical analysis and olfactory evaluation of acylated products. Chem. Biodiv. 2013, 10, 2291–2301. [Google Scholar] [CrossRef]
- Antoniotti, S.; Fernandez, X.; Duñach, E. Reaction design for evaluation of the substrate range of hydrolases. Biocatal. Biotransfor. 2008, 26, 228–234. [Google Scholar] [CrossRef]
- Baldovini, N.; Delasalle, C.; Joulain, D. Phytochemistry of the heartwood from fragrant Santalum species: A review. Flavour Frag. J. 2011, 26, 7–26. [Google Scholar] [CrossRef]
- Raynaud, E.; Sarrazin, E.; Fernandez, X.; Antoniotti, S. University Nice Sophia Antipolis: Nice, France, Unpublished work; 2009.
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniotti, S. Tuning of Essential Oil Properties by Enzymatic Treatment: Towards Sustainable Processes for the Generation of New Fragrance Ingredients. Molecules 2014, 19, 9203-9214. https://doi.org/10.3390/molecules19079203
Antoniotti S. Tuning of Essential Oil Properties by Enzymatic Treatment: Towards Sustainable Processes for the Generation of New Fragrance Ingredients. Molecules. 2014; 19(7):9203-9214. https://doi.org/10.3390/molecules19079203
Chicago/Turabian StyleAntoniotti, Sylvain. 2014. "Tuning of Essential Oil Properties by Enzymatic Treatment: Towards Sustainable Processes for the Generation of New Fragrance Ingredients" Molecules 19, no. 7: 9203-9214. https://doi.org/10.3390/molecules19079203
APA StyleAntoniotti, S. (2014). Tuning of Essential Oil Properties by Enzymatic Treatment: Towards Sustainable Processes for the Generation of New Fragrance Ingredients. Molecules, 19(7), 9203-9214. https://doi.org/10.3390/molecules19079203