Evaluation of Styrene-Divinylbenzene Beads as a Support to Immobilize Lipases
Abstract
:1. Introduction
2. Results and Discussion
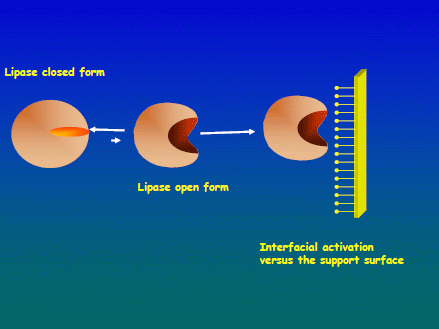
2.1. Immobilization Courses of the Different Lipases on Both Supports

| Lipase/support | Octyl-Sepharose® | MCI GEL® CHP20P |
|---|---|---|
| RML | 11 ± 2 | 310 ± 20 |
| TLL | 20 ± 2 | 90 ± 8 |
| LU | 30 ± 3 | 180 ± 15 |
2.2. Effect of pH on Immobilized Enzyme Activities Versus pNPB
| Biocatalyst | pH | |||||
|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | 10 | |
| TLL-OS | 26 | 33 | 34 | 35 | 55 | 38 |
| TLL-MCI | 1.38 | 1.39 | 1.41 | 3.72 | 4.66 | 4.44 |
| RML-OS | 9.7 | 11.25 | 12.1 | 10.2 | 6.4 | 1.3 |
| RML-MCI | 0.45 | 0.45 | 0.50 | 0.47 | 0.33 | 0.02 |
| LU-OS | 10.5 | 13 | 11.5 | 16.5 | 16.8 | 28 |
| LU-MCI | 0.16 | 0.50 | 0.42 | 0.37 | 0.36 | 0.45 |
2.3. Thermal Stability of MCI GEL® CHP20P and octyl-Sepharose® Lipase Preparations
| Biocatalyst | pH | ||
|---|---|---|---|
| 5 | 7 | 9 | |
| TLL-OS * | >90% after 10h | >90% after 10h | >90% after 10h |
| TLL- MCI | 23 | 20 | 23 |
| RML-OS | 1800 | 750 | 26 |
| RML-MCI | 180 | 105 | 30 |
| LU-OS ** | 510 | 900 | 30 |
| LU-MCI ** | 30 | 45 | 24 |
2.4. Stability of MCI GEL® CHP20P and octyl-Sepharose® Lipase Preparations in Presence of acetonitrile

2.5. Enzyme Activity Versus other Substrates
| Biocatalyst/substrate | pH | ||
|---|---|---|---|
| 5 | 7 | 8.5 | |
| TLL-OS/EH | 480 | 165 | 17 |
| TLL- MCI/EH | 125 | 25 | 20 |
| TLL-OS/MP | 0.12 | 0.21 | 0.09 |
| TLL- MCI/MP | 0.03 | 0.06 | 0.03 |
| TLL-OS/MM | 4.8 | 6 | 3.1 |
| TLL- MCI/MM | 10 | 16 | 17.5 |
| RML-OS/EH | 671 | 435 | 0.13 |
| RML-MCI/EH | 333 | 13 | 0.2 |
| RML-OS/MP | 0.02 | 0.03 | <10−3 |
| RML-MCI/MP | 0.07 | 2.1 | 2.24 |
| RML-OS/MM | 4.5 | 6.2 | 4.5 |
| RML-MCI/MM | 15 | 105 | 142 |
| LU-OS/EH | 0.04 | 0.66 | 0.1 |
| LU-MCI/EH | 32 | 30 | 29 |
| LU-OS/MP | <0.01 | <0.01 | <0.001 |
| LU-MCI/MP | 4.41 | 4.5 | 10 |
| LU-OS/MM | 0.55 | 1.35 | 11 |
| LU-MCI/MM | 5.8 | 55 | 47 |
| Biocatalyst/Substrate | pH | ||
|---|---|---|---|
| 5 | 7 | 8.5 | |
| IM-TLL/EH | 1133 | 186 | 150 |
| TLL- MCI/EH | 6325 | 1249 | 1010 |
| IM-TLL/MP | 15 | 29 | 9 |
| TLL- MCI/MP | 14 | 31.5 | 13.5 |
| IM-TLL /MM | 6.25 | 8.5 | 3.2 |
| TLL- MCI/MM | 510 | 800 | 870 |
| IM-RML/EH | 985 | 78 | 17 |
| RML-MCI/EH | 16650 | 642 | 10.2 |
| IM-RML/MP | 7820 | 2310 | 7900 |
| RML-MCI/MP | 3.7 | 105 | 112 |
| IM-RML/MM | 75 | 490 | 590 |
| RML-MCI/MM | 712 | 5250 | 7100 |
3. Experimental
3.1. Materials
3.2. Standard Determination of Enzyme Activity
3.3. Immobilization of Lipases
3.4. Determination of the Loading Capacity of the Different Supports
3.5. Thermal Inactivation of Different Lipase Immobilized Preparations
3.6. Inactivation of Different Enzyme Preparations in the Presence of Acetonitrile
3.7. Hydrolysis of Ethyl Hexanoate
3.8. Hydrolysis of Methyl Phenylacetate
3.9. Hydrolysis of Methyl Mandelate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jaeger, K.E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Sharma, R.; Chisti, Y.; Banerjee, U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef]
- Pandey, A.; Benjamin, S.; Soccol, C.R.; Nigam, P.; Krieger, N.; Soccol, V.T. The realm of microbial lipases in biotechnology. Biotechnol. Appl. Biochem. 1999, 29, 119–131. [Google Scholar]
- Reetz, M.T. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 2002, 6, 145–150. [Google Scholar] [CrossRef]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing lipases for biocatalysis: A survey of chemical, physical and molecular biological approaches. J. Mol. Catal. B 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Lozano, P.; Garcia-Verdugo, E.; Luis, S.V.; Pucheault, M.; Vaultier, M. (bio) catalytic continuous flow processes in scco2 and/or ils: Towards sustainable (bio)catalytic synthetic platforms. Curr. Org. Synth. 2011, 8, 810–823. [Google Scholar]
- Lozano, P. Enzymes in neoteric solvents: From one-phase to multiphase systems. Green Chem. 2010, 12, 555–569. [Google Scholar] [CrossRef]
- Ghanem, A. Trends in lipase-catalyzed asymmetric access to enantiomerically pure/enriched compounds. Tetrahedron 2007, 63, 1721–1754. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Methods to increase enantioselectivity of lipases and esterases. Curr. Opin. Biotechnol. 2002, 13, 543–547. [Google Scholar]
- Yahya, A.R.M.; Anderson, W.A.; Moo-Young, M. Ester synthesis in lipase-catalyzed reactions. Enzyme Microb. Technol. 1998, 23, 438–450. [Google Scholar] [CrossRef]
- Santaniello, E.; Ferraboschi, P.; Grisenti, P. Lipase-catalyzed transesterification in organic solvents: Applications to the preparation of enantiomerically pure compounds. Enzyme Microb. Technol. 1993, 15, 367–382. [Google Scholar] [CrossRef]
- Bajaj, A.; Lohan, P.; Jha, P.N.; Mehrotra, R. Biodiesel production through lipase catalyzed transesterification: An overview. J. Mol. Catal. B 2010, 62, 9–14. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Karanth, N.G. Lipases and lipase-catalyzed esterification reactions in nonaqueous media. Catal. Rev. Sci. Eng. 2002, 44, 499–591. [Google Scholar] [CrossRef]
- Carboni-Oerlemans, C.; Domínguez de María, P.; Tuin, B.; Bargeman, G.; van der Meer, A.; van Gemert, R. Hydrolase-catalysed synthesis of peroxycarboxylic acids: Biocatalytic promiscuity for practical applications. J. Biotechnol. 2006, 126, 140–151. [Google Scholar] [CrossRef]
- Humble, M.S.; Berglund, P. Biocatalytic promiscuity. Eur. J. Org. Chem. 2011, 2011, 3391–3401. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process. Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization-aqueous and non-aqueous environment. Process. Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Verma, M.L.; Barrow, C.J.; Puri, M. Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Appl. Microbiol. Biotechnol. 2013, 97, 23–39. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. Lipases: Interfacial enzymes with attractive applications. Angew. Chem. Int. Ed. 1998, 37, 1609–1633. [Google Scholar]
- Verger, R. Interfacial activation of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Reis, P.; Holmberg, K.; Watzke, H.; Leser, M.E.; Miller, R. Lipases at interfaces: A review. Adv. Colloid Interface Sci. 2009, 147–148, 237–250. [Google Scholar] [CrossRef]
- Derewenda, Z.S.; Derewenda, U.; Dodson, G.G. The crystal and molecular structure of the rhizomucor miehei triacylglyceride lipase at 1.9 å resolution. J. Mol. Biol. 1992, 227, 818–839. [Google Scholar] [CrossRef]
- Uppenberg, J.; Patkar, S.; Bergfors, T.; Jones, T.A. Crystallization and preliminary X-ray studies of lipase b from Candida antarctica. J. Mol. Biol. 1994, 235, 790–792. [Google Scholar] [CrossRef]
- Carrasco-López, C.; Godoy, C.; de las Rivas, B.; Fernández-Lorente, G.; Palomo, J.M.; Guisán, J.M.; Fernández-Lafuente, R.; Martínez-Ripoll, M.; Hermoso, J.A. Activation of bacterial thermo alkalophilic lipases is spurred by dramatic structural rearrangements. J. Biol. Chem. 2009, 284, 4365–4372. [Google Scholar] [CrossRef]
- Palomo, J.M.; Peñas, M.M.; Fernández-Lorente, G.; Mateo, C.; Pisabarro, A.G.; Fernández-Lafuente, R.; Ramírez, L.; Guisán, J.M. Solid-phase handling of hydrophobins: Immobilized hydrophobins as a new tool to study lipases. Biomacromolecules 2003, 4, 204–210. [Google Scholar] [CrossRef]
- Palomo, J.M.; Ortiz, C.; Fernández-Lorente, G.; Fuentes, M.; Guisán, J.M.; Fernández-Lafuente, R. Lipase-lipase interactions as a new tool to immobilize and modulate the lipase properties. Enzyme Microb. Technol. 2005, 36, 447–454. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Self-assembly of pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Cabrera, Z.; Fernandez-Lorente, G.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Novozym 435 displays very different selectivity compared to lipase from Candida antarctica B adsorbed on other hydrophobic supports. J. Mol. Catal. B 2009, 57, 171–176. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process. Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Hernandez, K.; Garcia-Galan, C.; Fernandez-Lafuente, R. Simple and efficient immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads. Enzyme Microb. Technol. 2011, 49, 72–78. [Google Scholar] [CrossRef]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One biocatalyst—many applications: The use of Candida antarctica B-lipase in organic synthesis. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Friedrich, J.L.R.; Peña, F.P.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Effect of immobilization protocol on optimal conditions of ethyl butyrate synthesis catalyzed by lipase B from Candida antarctica. J. Chem. Technol. Biotechnol. 2013, 88, 1089–1095. [Google Scholar] [CrossRef]
- Poppe, J.K.; Garcia-Galan, C.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Optimization of synthesis of fatty acid methyl esters catalyzed by lipase B from Candida antarctica immobilized in hydrophobic supports. J. Mol. Catal. B 2013, 94, 51–56. [Google Scholar] [CrossRef]
- Izquierdo, D.F.; Barbosa, O.; Burguete, M.I.; Lozano, P.; Luis, S.V.; Fernandez-Lafuente, R.; Garcia-Verdugo, E. Tuning lipase B from Candida antarctica c-c bond promiscuous activity by immobilization on poly-styrene-divinylbenzene beads. RSC Adv. 2014, 4, 6219–6225. [Google Scholar] [CrossRef]
- Graebin, N.G.; Martins, A.B.; Lorenzoni, A.S.G.; Garcia-Galan, C.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Immobilization of lipase B from Candida antarctica on porous styrene–divinylbenzene beads improves butyl acetate synthesis. Biotechnol. Prog. 2012, 28, 406–412. [Google Scholar] [CrossRef]
- Martins, A.B.; Friedrich, J.L.R.; Cavalheiro, J.C.; Garcia-Galan, C.; Barbosa, O.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Improved production of butyl butyrate with lipase from Thermomyces lanuginosus immobilized on styrene-divinylbenzene beads. Bioresour. Technol. 2013, 134, 417–422. [Google Scholar] [CrossRef]
- De Abreu, L.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Volpato, G.; Ayub, M.A.Z. Efficient purification-immobilization of an organic solvent-tolerant lipase from Staphylococcus warneri EX17 on porous styrene-divinylbenzene beads. J. Mol. Catal. B 2014, 99, 51–55. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as an industrial biocatalyst in chemical process. J. Mol. Catal. B 2010, 64, 1–22. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as a biocatalyst in fats and oils modification. J. Mol. Catal. B 2010, 66, 15–32. [Google Scholar] [CrossRef]
- De Maria, L.; Vind, J.; Oxenbøll, K.M.; Svendsen, A.; Patkar, S. Phospholipases and their industrial applications. Appl. Microbiol. Biotechnol. 2007, 74, 290–300. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar]
- Palomo, J.M.; Ortiz, C.; Fuentes, M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Use of immobilized lipases for lipase purification via specific lipase-lipase interactions. J. Chromatogr. A 2004, 1038, 267–273. [Google Scholar] [CrossRef]
- Palomo, J.M.; Fuentes, M.; Fernández-Lorente, G.; Mateo, C.; Guisan, J.M.; Fernández-Lafuente, R. General trend of lipase to self-assemble giving bimolecular aggregates greatly modifies the enzyme functionality. Biomacromolecules 2003, 4, 1–6. [Google Scholar]
- Pavlidis, I.V.; Vorhaben, T.; Tsoufis, T.; Rudolf, P.; Bornscheuer, U.T.; Gournis, D.; Stamatis, H. Development of effective nanobiocatalytic systems through the immobilization of hydrolases on functionalized carbon-based nanomaterials. Bioresour. Technol. 2012, 115, 164–171. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernández-Lorente, G.; Pedroche, J.; Fernández-Lafuente, R.; Guisan, J.M.; Tam, A.; Daminati, M. Epoxy sepabeads: A novel epoxy support for stabilization of industrial enzymes via very intense multipoint covalent attachment. Biotechnol. Prog. 2002, 18, 629–634. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Séverac, E.; Galy, O.; Turon, F.; Pantel, C.A.; Condoret, J.S.; Monsan, P.; Marty, A. Selection of CALB immobilization method to be used in continuous oil transesterification: Analysis of the economical impact. Enzyme Microb. Technol. 2011, 48, 61–70. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the biocatalyst are not available.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Garcia-Galan, C.; Barbosa, O.; Hernandez, K.; Santos, J.C.S.d.; Rodrigues, R.C.; Fernandez-Lafuente, R. Evaluation of Styrene-Divinylbenzene Beads as a Support to Immobilize Lipases. Molecules 2014, 19, 7629-7645. https://doi.org/10.3390/molecules19067629
Garcia-Galan C, Barbosa O, Hernandez K, Santos JCSd, Rodrigues RC, Fernandez-Lafuente R. Evaluation of Styrene-Divinylbenzene Beads as a Support to Immobilize Lipases. Molecules. 2014; 19(6):7629-7645. https://doi.org/10.3390/molecules19067629
Chicago/Turabian StyleGarcia-Galan, Cristina, Oveimar Barbosa, Karel Hernandez, Jose C. S. dos Santos, Rafael C. Rodrigues, and Roberto Fernandez-Lafuente. 2014. "Evaluation of Styrene-Divinylbenzene Beads as a Support to Immobilize Lipases" Molecules 19, no. 6: 7629-7645. https://doi.org/10.3390/molecules19067629
APA StyleGarcia-Galan, C., Barbosa, O., Hernandez, K., Santos, J. C. S. d., Rodrigues, R. C., & Fernandez-Lafuente, R. (2014). Evaluation of Styrene-Divinylbenzene Beads as a Support to Immobilize Lipases. Molecules, 19(6), 7629-7645. https://doi.org/10.3390/molecules19067629