Synthesis, Molecular Docking and Biological Evaluation of Glycyrrhizin Analogs as Anticancer Agents Targeting EGFR
Abstract
:1. Introduction
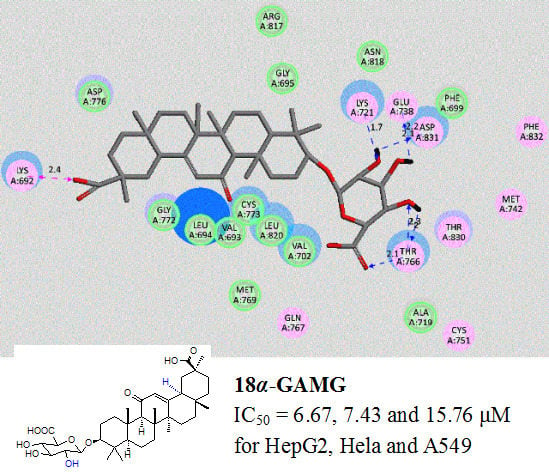
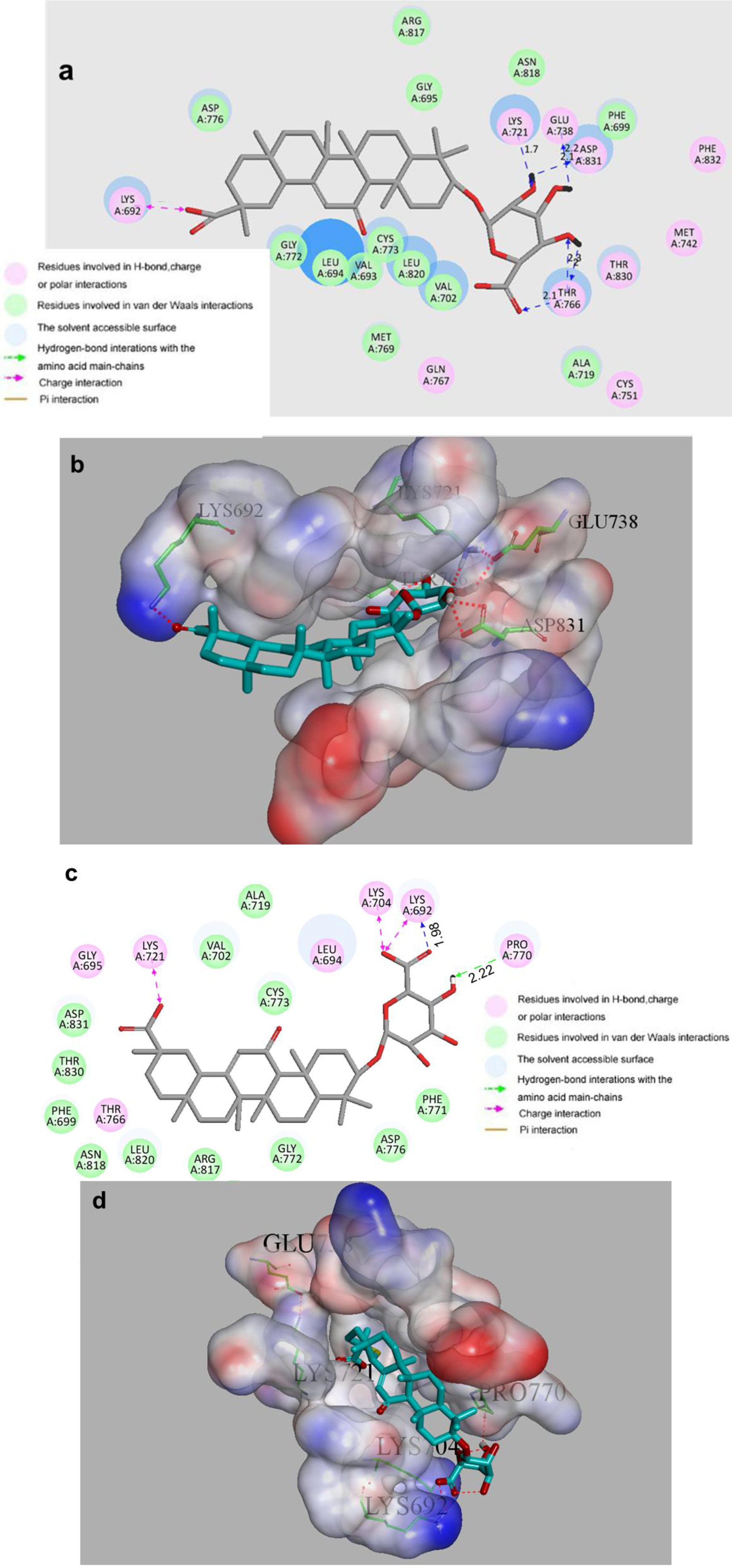
2. Results and Discussion
| Compd. | IC50, (μM) a | ||
|---|---|---|---|
| HepG2 b | HeLa b | A549 b | |
| 18α-GAMG | 6.67 | 7.43 | 15.76 |
| 18β-GAMG | 33.60 | 8.39 | 21.55 |
| 18α-GA | 54.24 | 15.13 | 41.57 |
| 18β-GA | 63.59 | 18.93 | 51.92 |
| Erlotinib | 0.12 | 0.20 | 0.13 |
| Compd. | EGFR (IC50, μM) a | Compd. | EGFR (IC50, μM) a |
|---|---|---|---|
| 18α-GAMG | 0.028 | 18β-GA | 0.092 |
| 18β-GAMG | 0.069 | Erlotinib | 0.030 |
| 18α-GA | 0.081 |
| Compd. | -EDOCKER_INTERACTION_ENERG ΔG (kcal/mol) |
|---|---|
| 18α-GAMG | 72.0274 |
| 18β-GAMG | 66.9106 |
| 18α-GA | 58.7009 |
| 18β-GA | 58.6731 |
| Erlotinib | 44.3732 |
| Models | Groups | Animal number (End, n) | Body weight (g) | Tumor weight (g) | Inhibition rate (%) | |
|---|---|---|---|---|---|---|
| Beginning | End | |||||
| Control | 10 | 19.80 ± 1.32 | 23.97 ± 2.23 | 1.91 ± 0.29 | ||
| 18α-GAMG | 9 | 19.40 ± 1.07 | 25.25 ± 1.80 | 1.15 ± 0.50 ** | 39.8 | |
| S180 | 18β-GAMG | 10 | 19.80 ± 1.39 | 25.02 ± 2.58 | 1.25 ± 0.19 ** | 34.6 |
| 18α-GA | 10 | 20.00 ± 1.49 | 25.20 ± 1.11 | 1.29 ± 0.47 ** | 32.5 | |
| 18β-GA | 9 | 19.70 ± 1.25 | 24.48 ± 2.37 | 1.33 ± 0.67 ** | 30.4 | |
| Control | 10 | 19.4 ± 1.35 | 25.05 ± 1.89 | 1.95 ± 0.22 | ||
| 18α-GAMG | 10 | 19.6 ± 1.51 | 27.02 ± 2.10 | 0.98 ± 0.43 ** | 49.7 | |
| HepG2 | 18β-GAMG | 10 | 19.00 ± 0.94 | 27.8 ± 1.57 | 1.20 ± 0.35 ** | 38.4 |
| 18α-GA | 10 | 20.1 ± 1.45 | 26.08 ± 1.26 | 1.22 ± 0.46 ** | 37.4 | |
| 18β-GA | 10 | 19.4 ± 1.08 | 26.94 ± 2.05 | 1.26 ± 0.65 ** | 35.4 | |
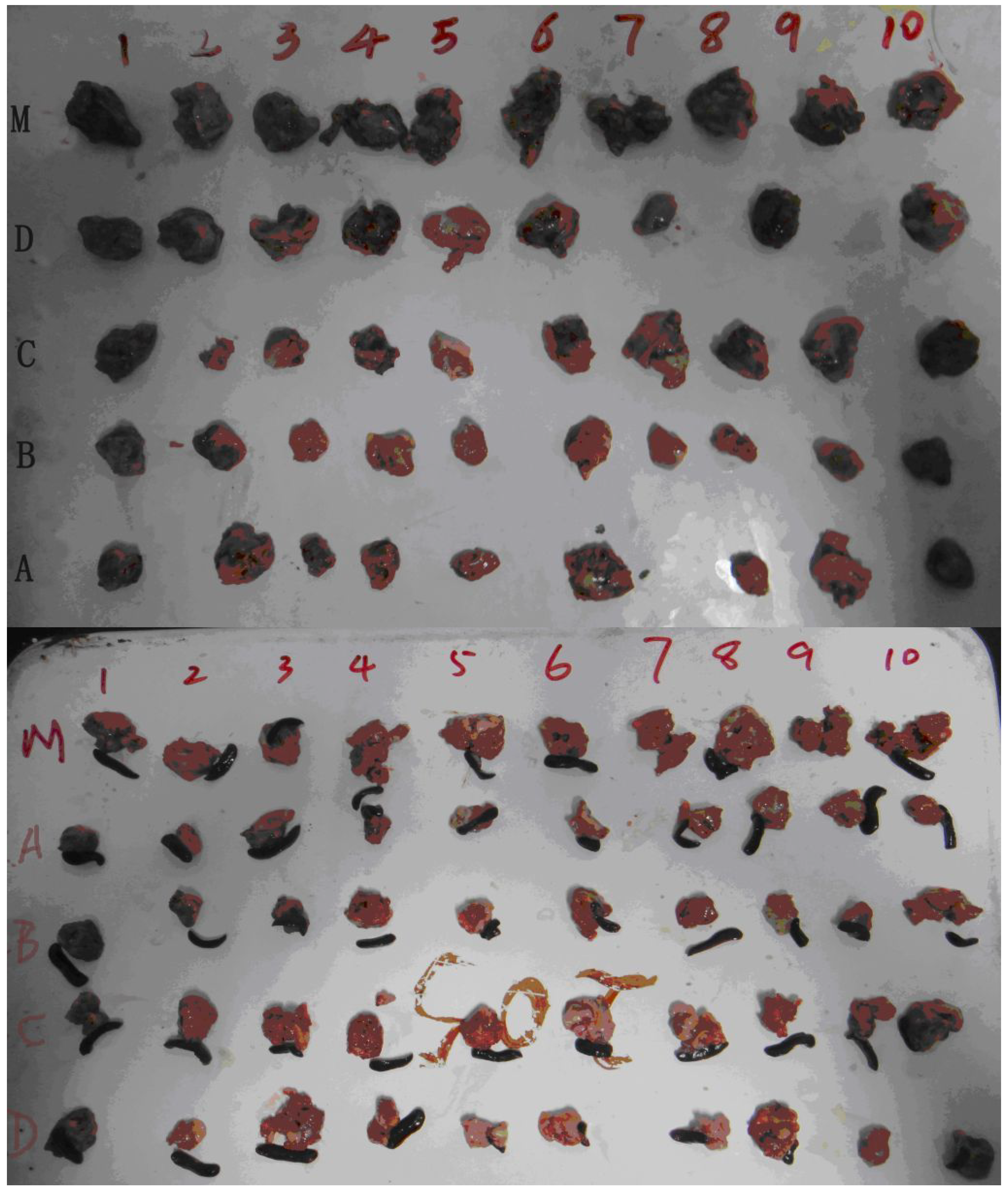
| Groups | Animal number (n) | Body weight (g) | Survival time a (d) | Survival rate (%) |
|---|---|---|---|---|
| Control | 10 | 19.0 ± 1.25 | 16.40 ± 2.07 | |
| 18α-GAMG | 10 | 19.5 ± 1.27 | 23.85 ± 5.41* | 45.4 |
| 18β-GAMG | 10 | 19.0 ± 0.82 | 21.05 ± 4.65* | 28.4 |
| 18α-GA | 10 | 19.4 ± 0.97 | 19.75 ± 3.08* | 20.4 |
| 18β-GA | 10 | 19.4 ± 1.27 | 19.40 ± 3.77* | 18.3 |
3. Experimental Section
3.1. Synthesis of Glycyrrhizin Analogs
3.1.1. General Methods
3.1.2. Preparation of 18β-GAMG from 18β-GA via Biotransformation
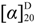 = +52 (c = 1.0, MeOH); 13C-NMR (75 MHz, DMSO-d6): Table S1. TOF-HRMS: m/z [M + Na]+ calcd for C42H62NaO16: 845.3930; found: 845.3935.
= +52 (c = 1.0, MeOH); 13C-NMR (75 MHz, DMSO-d6): Table S1. TOF-HRMS: m/z [M + Na]+ calcd for C42H62NaO16: 845.3930; found: 845.3935.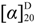 = +91 (c = 1.0, MeOH); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 0.76 (s, 3H, 24-CH3), 0.77 (s, 3H, 28-CH3), 0.99 (s, 3H, 23-CH3), 1.06 (s, 2 × 3H, 25-CH3, 26-CH3), 1.10 (s, 3H, 29-CH3), 1.34 (s, 3H, 27-CH3), 2.34 (s, 1H, 9-H), 3.01 (m, 1H, 4'-H), 3.08 (dd, 1H, J1= 4.8 Hz, J2= 11.2 Hz, 3-H), 3.15 (t, 1H, J= 9.0 Hz, 3'-H), 3.30 (m, 1H, overlapped, 2'-H), 3.58 (d, 1H, J= 9.7 Hz, 5'-H), 4.25 (d, 1H, J= 7.8 Hz, 1'-H), 5.40 (s, 1H, 12-H); 13C-NMR (125 MHz, DMSO-d6): Table S1. TOF-HRMS: m/z [M + Na]+ calcd for C36H54NaO10: 669.3609; found: 669.3608.
= +91 (c = 1.0, MeOH); 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 0.76 (s, 3H, 24-CH3), 0.77 (s, 3H, 28-CH3), 0.99 (s, 3H, 23-CH3), 1.06 (s, 2 × 3H, 25-CH3, 26-CH3), 1.10 (s, 3H, 29-CH3), 1.34 (s, 3H, 27-CH3), 2.34 (s, 1H, 9-H), 3.01 (m, 1H, 4'-H), 3.08 (dd, 1H, J1= 4.8 Hz, J2= 11.2 Hz, 3-H), 3.15 (t, 1H, J= 9.0 Hz, 3'-H), 3.30 (m, 1H, overlapped, 2'-H), 3.58 (d, 1H, J= 9.7 Hz, 5'-H), 4.25 (d, 1H, J= 7.8 Hz, 1'-H), 5.40 (s, 1H, 12-H); 13C-NMR (125 MHz, DMSO-d6): Table S1. TOF-HRMS: m/z [M + Na]+ calcd for C36H54NaO10: 669.3609; found: 669.3608. 3.1.3. General Procedure of Alkaline Isomerization of the 18β-isomer to the 18α-isomer
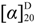 = +20 (c = 1.0, MeOH); 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 0.65 (s, 3H, 28-CH3), 0.73 (s, 3H, 24-CH3), 0.95 (s, 3H, 23-CH3), 1.04 (s, 3H, 26-CH3), 1.10 (s, 3H, 25-CH3), 1.16 (s, 3H, 29-CH3), 1.33 (s, 3H, 27-CH3), 4.31 (d, 1H, J= 7.3 Hz, 1'-H), 4.49 (d, 1H, J= 7.6 Hz, 1''-H), 5.33 (s, 1H, 12-H); 13C-NMR (75 MHz, DMSO-d6): Table S1. TOF-HRMS: m/z [M + Na]+ calcd for C42H62NaO16: 845.3930; found: 845.3938.
= +20 (c = 1.0, MeOH); 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 0.65 (s, 3H, 28-CH3), 0.73 (s, 3H, 24-CH3), 0.95 (s, 3H, 23-CH3), 1.04 (s, 3H, 26-CH3), 1.10 (s, 3H, 25-CH3), 1.16 (s, 3H, 29-CH3), 1.33 (s, 3H, 27-CH3), 4.31 (d, 1H, J= 7.3 Hz, 1'-H), 4.49 (d, 1H, J= 7.6 Hz, 1''-H), 5.33 (s, 1H, 12-H); 13C-NMR (75 MHz, DMSO-d6): Table S1. TOF-HRMS: m/z [M + Na]+ calcd for C42H62NaO16: 845.3930; found: 845.3938.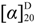 = +24 (c = 1.0, MeOH); 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 0.65 (s, 3H, 28-CH3), 0.77 (s, 3H, 24-CH3), 0.92 (s, 3H, 23-CH3), 0.98 (s, 3H, 25-CH3), 1.04 (s, 3H, 26-CH3), 1.16 (s, 3H, 29-CH3), 1.33 (s, 3H, 27-CH3), 2.27 (overlapped, 9-H), 3.01 (t, 1H,J= 8.4 Hz, 4'-H), 3.07 (dd, 1H, J1= 6.5 Hz, J2= 9.7 Hz, 3-H), 3.15 (t, 1H, J= 9.0 Hz, 3'-H), 3.30 (t, 1H, J= 9.8 Hz, 2'-H), 3.58 (d, 1H, J= 9.7 Hz, 5'-H), 4.24 (d, 1H, J= 7.8 Hz, 1'-H), 5.33 (s, 1H, 12-H); 13C-NMR (75 MHz, DMSO-d6): Table S1. TOF-HRMS: m/z [M + Na]+ calcd for C36H54NaO10: 669.3609; found: 669.3600.
= +24 (c = 1.0, MeOH); 1H-NMR (300 MHz, DMSO-d6) δ (ppm): 0.65 (s, 3H, 28-CH3), 0.77 (s, 3H, 24-CH3), 0.92 (s, 3H, 23-CH3), 0.98 (s, 3H, 25-CH3), 1.04 (s, 3H, 26-CH3), 1.16 (s, 3H, 29-CH3), 1.33 (s, 3H, 27-CH3), 2.27 (overlapped, 9-H), 3.01 (t, 1H,J= 8.4 Hz, 4'-H), 3.07 (dd, 1H, J1= 6.5 Hz, J2= 9.7 Hz, 3-H), 3.15 (t, 1H, J= 9.0 Hz, 3'-H), 3.30 (t, 1H, J= 9.8 Hz, 2'-H), 3.58 (d, 1H, J= 9.7 Hz, 5'-H), 4.24 (d, 1H, J= 7.8 Hz, 1'-H), 5.33 (s, 1H, 12-H); 13C-NMR (75 MHz, DMSO-d6): Table S1. TOF-HRMS: m/z [M + Na]+ calcd for C36H54NaO10: 669.3609; found: 669.3600.3.2. Biological Assay of in Vitro Anticancer Activities
3.3. General Procedure for Preparation, Purification of EGFR, and Inhibitory Assay
3.4. Evaluation of the in Vivo Antitumor Activities
3.4.1. Animals and Cell Lines
3.4.2. In Vivo Tumor Xenograft Model



4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Citri, A.; Yarden, Y. EGF-ERBB signaling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef]
- Mendelsohn, J. Targeting the epidermal growth factor receptor for cancer therapy. J. Clin. Oncol. 2002, 20, 1S–13S. [Google Scholar]
- Zandi, R.; Larsen, A.B.; Andersen, P.; Stockhausen, M.T.; Poulsen, H.S. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007, 19, 2013–2023. [Google Scholar] [CrossRef]
- Berasain, C.; Castillo, J.; Prieto, J.; Avila, M.A. New molecular targets for hepatocellular carcinoma: The ErbB1 signaling system. Liver Int. 2007, 27, 174–185. [Google Scholar] [CrossRef]
- Berasain, C.; Ujue, L.M.; Urtasun, R.; Goñi, S.; Elizalde, M.; Garcia-Irigoyen, O.; Azcona, M.; Prieto, J.; Avila, M.A. Epidermal growth factor receptor (EGFR) crosstalks in liver cancer. Cancers 2011, 3, 2444–2461. [Google Scholar] [CrossRef]
- Natarajan, A.; Wagner, B.; Sibilia, M. The EGF receptor is required for efficient liver regeneration. Proc. Natl. Acad. Sci. USA 2007, 104, 17081–17086. [Google Scholar] [CrossRef]
- Mitchell, C.; Nivison, M.; Jackson, L.F.; Fox, R.; Lee, D.C.; Campbell, J.S.; Fausto, N. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J. Biol. Chem. 2005, 280, 2562–2568. [Google Scholar]
- Hopfner, M.; Sutter, A.P.; Huether, A.; Schuppan, D.; Zeitz, M.; Scherubl, H. Targeting the epidermal growth factor receptor by gefitinib for treatment of hepatocellular carcinoma. J. Hepatol. 2004, 41, 1008–1016. [Google Scholar] [CrossRef]
- Huether, A.; Hopfner, M.; Sutter, A.P.; Schuppan, D.; Scherubl, H. Erlotinib induces cell cycle arrest and apoptosis in hepatocellular cancer cells and enhances chemosensitivity towards cytostatics. J. Hepatol. 2005, 43, 661–669. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981−2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Baltina, L.A. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr. Med. Chem. 2003, 10, 155–171. [Google Scholar]
- Morgan, A.G.; McAdam, W.A. Glycyrrhiza glabra. (Monograph). Altern. Med. Rev. 2005, 10, 230–237. [Google Scholar]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar]
- Van Rossum, T.G.; Vulto, A.G.; de Man, R.A.; Brouwer, J.T.; Schalm, S.W. Glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol. Ther. 1998, 12, 199–205. [Google Scholar] [CrossRef]
- Arase, Y.; Ikeda, K.; Murashima, N.; Chayama, K.; Tsubota, A.; Koida, I.; Suzuki, Y.; Saitoh, S.; Kobayashi, M.; Kumada, H. The long-term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer 1997, 79, 1494–1500. [Google Scholar]
- Chayama, K. Management of chronic hepatitis C and prevention of hepatocellular carcinoma. J. Gastroenterol. 2002, 37, 69–73. [Google Scholar] [CrossRef]
- Van Rossum, T.G.; Vulto, A.G.; Hop, W.C.; Schalm, S.W. Glycyrrhizin-induced reduction of ALT in European patients with chronic hepatitis C. Am. J. Gastroenterol. 2001, 96, 2432–2437. [Google Scholar] [CrossRef]
- Thirugnanam, S.; Xu, L.; Ramaswamy, K.; Gnanasekar, M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol. Rep. 2008, 20, 1387–1392. [Google Scholar]
- Tripathi, M.; Singh, B.K.; Kakkar, P. Glycyrrhizic acid modulates t-BHP induced apoptosis in primary rat hepatocytes. Food Chem. Toxicol. 2009, 47, 339–347. [Google Scholar] [CrossRef]
- Kim, K.J.; Choi, J.S.; Kim, K.W.; Jeong, J.W. The anti-angiogenic activities of glycyrrhizic acid in tumor progression. Phytother. Res. 2013, 27, 841–846. [Google Scholar] [CrossRef]
- Feng, S.; Li, C.; Xu, X.; Wang, X. Screening strains for directed biosynthesis of β-d-mono-glucuronide-glycyrrhizin and kinetics of enzyme production. J. Mol. Catal. B: Enzym. 2006, 43, 63–67. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, Y.; Yu, H.S.; Huang, H.Z.; Kang, L.P.; Cao, M.; Cui, J.M.; Yu, L.Y.; Song, X.B.; Ma, B.P. Preparation of glycyrrhetinic acid monoglucuronide by selective hydrolysis of glycyrrhizic acid via biotransformation. Chin. Herb. Med. 2012, 4, 324–328. [Google Scholar]
- Mizutani, K.; Kambara, T.; Masuda, H.; Tamura, Y.; Ikeda, T.; Tanak, O.; Tokuda, H.; Nishino, H.; Kozuka, M.; Konoshima, T.; et al. Glycyrrhetic acid monoglucuronide (MGGR): Biological activities. Int. Congr. Ser. 1998, 1157, 225–235. [Google Scholar]
- Park, H.Y.; Park, S.H.; Yoon, H.K.; Han, M.J.; Kim, D.H. Anti-allergic activity of 18α-glycyrrhetinic acid-3-O-α-d-glucuronide. Arch. Pharm. Res. 2004, 27, 57–60. [Google Scholar] [CrossRef]
- Baltina, L.A.; Stolyarova, O.V.; Baltina, L.A.; Kondratenko, R.M.; Plyasunova, O.A.; Pokrovskii, A.G. Synthesis and antiviral activity of 18α-glycyrrhizic acid and its esters. Pharm. Chem. J. 2010, 44, 299–302. [Google Scholar] [CrossRef]
- Shetty, A.V.; Thirugnanam, S.; Dakshinamoorthy, G.; Samykutty, A.; Zheng, G.; Chen, A.; Bosland, M.C.; Kajdacsy-Balla, A.; Gnanasekar, M. 18α-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. Int. J. Oncol. 2011, 39, 635–640. [Google Scholar]
- Qu, Y.; Chen, W.H.; Zong, L.; Xu, M.Y.; Lu, L.G. 18α-Glycyrrhizin induces apoptosis and suppresses activation of rat hepatic stellate cells. Med. Sci. Monit. 2012, 18, BR24–32. [Google Scholar]
- Kimura, M.; Inoue, H.; Hirabayashi, K.; Natsume, H.; Ogihara, M. Glycyrrhizin and some analogues induce growth of primary cultured adult rat hepatocytes via epidermal growth factor receptors. Eur. J. Pharmacol. 2001, 431, 151–161. [Google Scholar]
- Tsou, H.R.; Mamuya, N.; Johnson, B.D.; Reich, M.F.; Gruber, B.C.; Ye, F.; Nilakantan, R.; Shen, R.; Discafani, C.; DeBlanc, R.; et al. 6-Substituted-4-(3-bromophenylamino)quinazolines as putative irreversible inhibitors of the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor (HER-2) tyrosine kinases with enhanced antitumor activity. J. Med. Chem. 2001, 44, 2719–2734. [Google Scholar] [CrossRef]
- Li, W.; Sha, Y.; Chen, L.X.; Qiu, F.; Wu, L.J. NMR data assignments of two 18-epimers of diammonium glycyrrhizinate. J. Shenyang Pharm. Univ. 2005, 7, 273–278. [Google Scholar]
- Mollica, L.; Marchis, F.D.; Spitaleri, A.; Dallacosta, C.; Pennacchini, D.; Zamai, M.; Agresti, A.; Trisciuoglio, L.; Musco, G.; Bianchi, M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007, 14, 431–441. [Google Scholar] [CrossRef]
- Du, D.; Yan, J.; Ren, J.H.; Lv, H.; Li, Y.; Xu, S.; Wang, Y.D.; Ma, S.G.; Qu, J.; Tang, W.B.; et al. Synthesis, biological evaluation, and molecular modeling of glycyrrhizin derivatives as potent high-mobility group box-1 inhibitors with anti-heart-failure activity in vivo. J. Med. Chem. 2013, 56, 97–108. [Google Scholar]
- Huggins, J.D.; Sherman, W.; Tidor, B. Rational approaches to improving selectivity in drug design. J. Med. Chem. 2012, 55, 1424–1444. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the four compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, Y.-A.; Tang, W.-J.; Zhang, X.; Yuan, J.-W.; Liu, X.-H.; Zhu, H.-L. Synthesis, Molecular Docking and Biological Evaluation of Glycyrrhizin Analogs as Anticancer Agents Targeting EGFR. Molecules 2014, 19, 6368-6381. https://doi.org/10.3390/molecules19056368
Yang Y-A, Tang W-J, Zhang X, Yuan J-W, Liu X-H, Zhu H-L. Synthesis, Molecular Docking and Biological Evaluation of Glycyrrhizin Analogs as Anticancer Agents Targeting EGFR. Molecules. 2014; 19(5):6368-6381. https://doi.org/10.3390/molecules19056368
Chicago/Turabian StyleYang, Yong-An, Wen-Jian Tang, Xin Zhang, Ji-Wen Yuan, Xin-Hua Liu, and Hai-Liang Zhu. 2014. "Synthesis, Molecular Docking and Biological Evaluation of Glycyrrhizin Analogs as Anticancer Agents Targeting EGFR" Molecules 19, no. 5: 6368-6381. https://doi.org/10.3390/molecules19056368
APA StyleYang, Y.-A., Tang, W.-J., Zhang, X., Yuan, J.-W., Liu, X.-H., & Zhu, H.-L. (2014). Synthesis, Molecular Docking and Biological Evaluation of Glycyrrhizin Analogs as Anticancer Agents Targeting EGFR. Molecules, 19(5), 6368-6381. https://doi.org/10.3390/molecules19056368