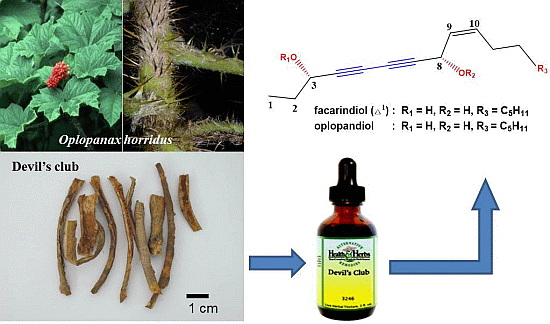
Anticancer Activities of Polyynes from the Root Bark of Oplopanax horridus and Their Acetylated Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
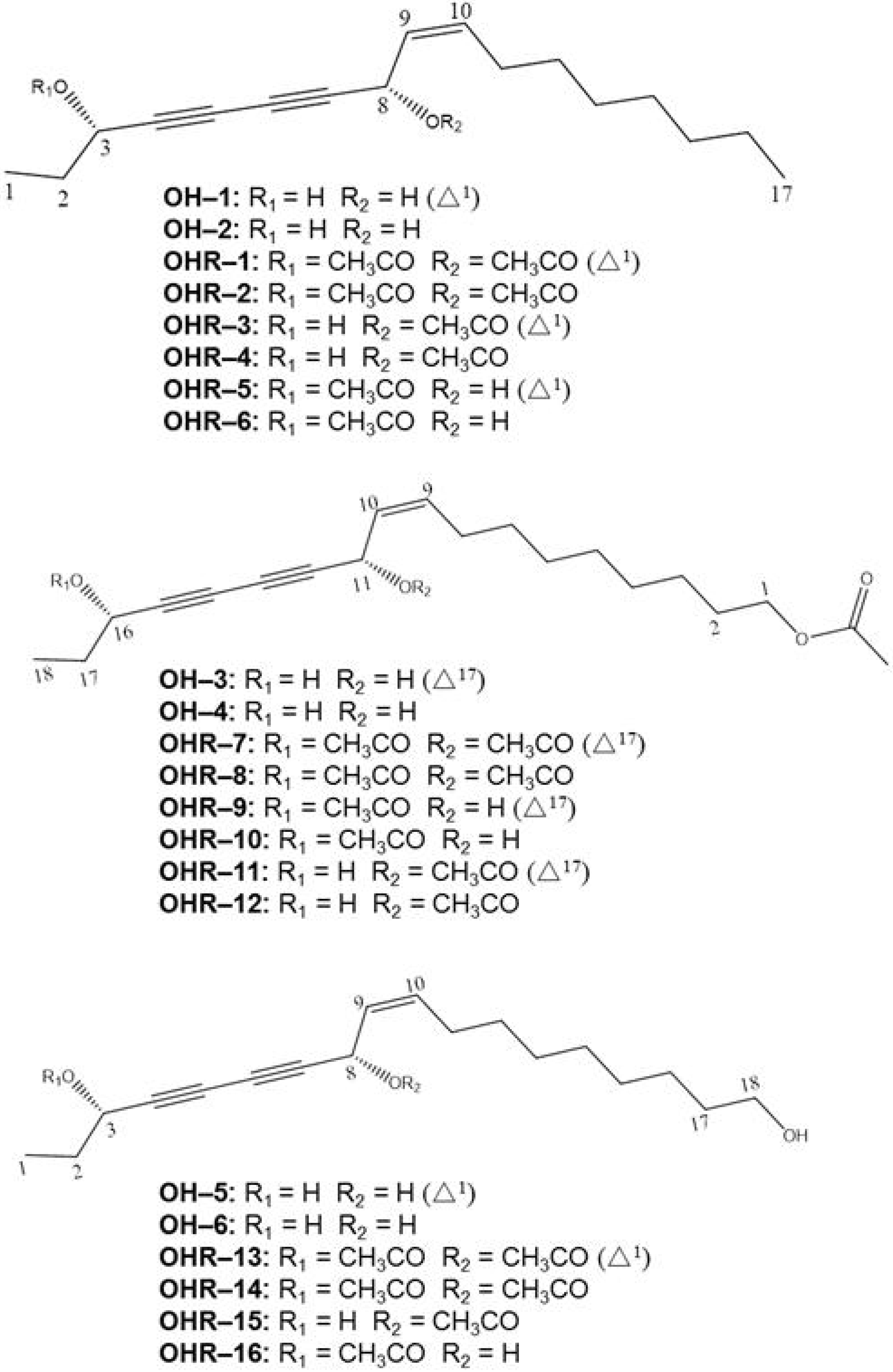
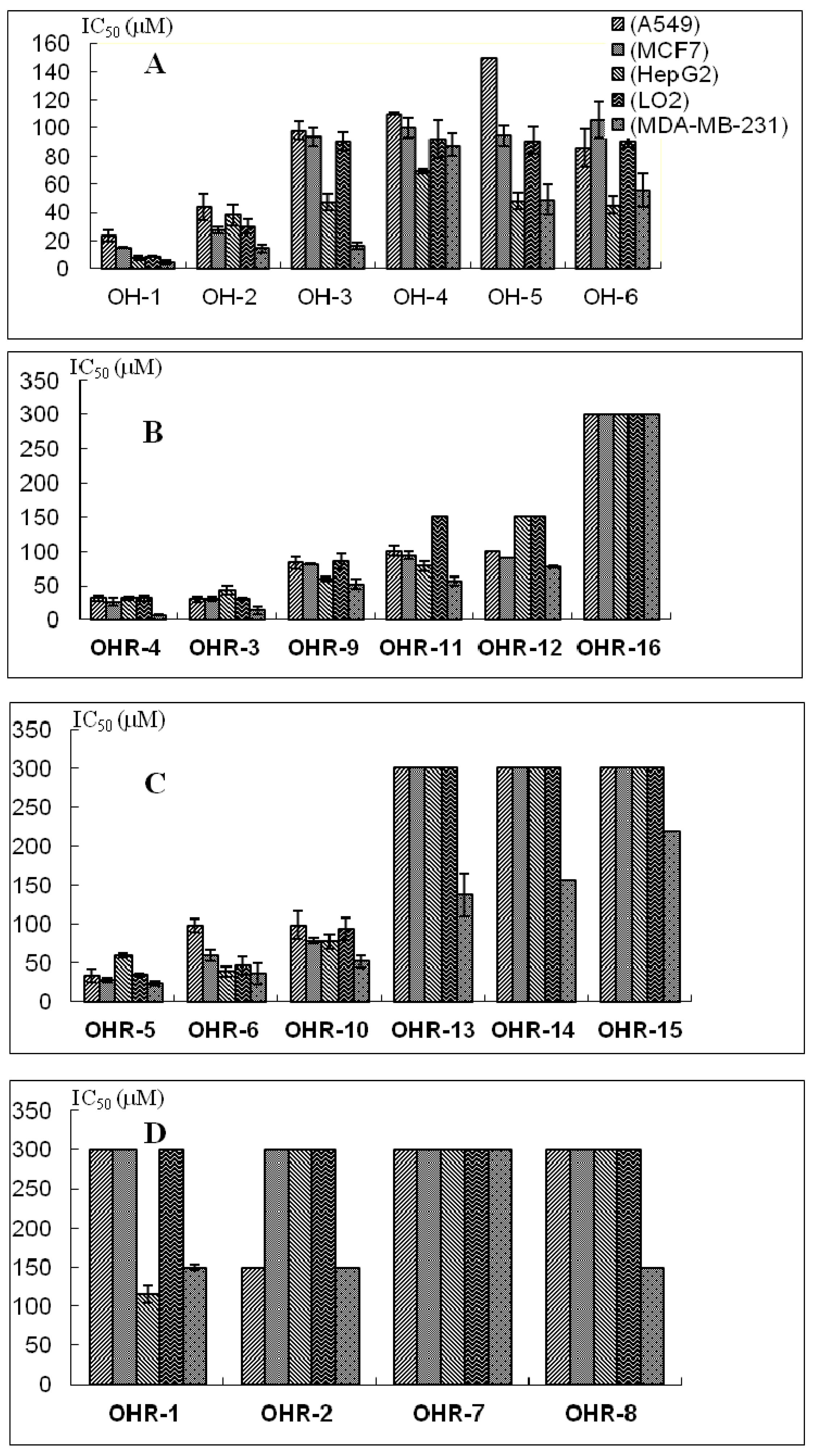
2.2. Effects of the 22 Polyynes on Proliferation of Selected Cancer Cells
| Polyynes | IC50 (μM) | ||||
|---|---|---|---|---|---|
| A549 | MCF-7 | HepG2 | LO2 | MDA-MB-231 | |
| OH-1 | 23.5 ± 4.1 | 15.3 ± 0.3 | 7.7 ± 1.3 | 8.7 ± 0.5 | 4.7 ± 1.4 |
| OH-2 | 44.1 ± 9.4 | 27.5 ± 2.2 | 38.4 ± 7.5 | 30.2 ± 4.8 | 14.7 ± 2.7 |
| OH-3 | 98.0 ± 6.3 | 93.6 ± 6.1 | 47.2 ± 5.9 | 90.3 ± 6.4 | 16.5 ± 2.2 |
| OH-4 | 109.9 ± 0.8 | 100.0 ± 7.0 | 69.1 ± 1.8 | 91.9 ± 13.4 | 87.8 ± 7.9 |
| OH-5 | 150-300 | 94.4 ± 7.3 | 48.3 ± 5.9 | 91.0 ± 9.5 | 49.0 ± 10.9 |
| OH-6 | 85.7 ± 13.2 | 106 ± 13.2 | 45.3 ± 6.3 | 90.8 ± 4.0 | 55.7 ± 11.8 |
| OHR-1 | >300 | >300 | 114.9 ± 17.1 | >300 | 13.9 ± 3.4 |
| OHR-2 | 24.7 ± 5.6 | 36.9 ± 4.8 | 6.6 ± 1.5 | 32.1 ± 9.0 | 4.8 ± 0.5 |
| OHR-3 | 31.4 ± 4.3 | 25.8 ± 5.8 | 31.5 ± 2.1 | 31.5 ± 4.0 | 6.6 ± 0.8 |
| OHR-4 | 33.3 ± 8.5 | 28.0 ± 2.9 | 59.5 ± 3.1 | 34.3 ± 1.5 | 23.4 ± 3.2 |
| OHR-5 | 30.1 ± 4.4 | 30.6 ± 3.1 | 43.7 ± 6.6 | 31.1 ± 1.8 | 13.9 ± 5.8 |
| OHR-6 | 97.5 ± 8.2 | 59.6 ± 7.5 | 38.5 ± 6.2 | 46.1 ± 11.7 | 36.2 ± 13.4 |
| OHR-7 | >300 | >300 | >300 | >300 | >300 |
| OHR-8 | >300 | >300 | 100–300 | >300 | 19.6 ± 6.9 |
| OHR-9 | 83.1 ± 8.4 | 81.3 ± 1.0 | 59.9 ± 3.0 | 86.2 ± 11.3 | 52.0 ± 7.5 |
| OHR-10 | 100.9 ± 7.3 | 94.3 ± 6.1 | 78.4 ± 6.5 | 100–300 | 56.7 ± 6.9 |
| OHR-11 | 98.2 ± 17.5 | 78.1 ± 3.4 | 77.0 ± 9.5 | 93.1 ± 14.0 | 51.5 ± 8.4 |
| OHR-12 | 100–300 | 90.7 ± 0.3 | 100–300 | 100–300 | 77.7 ± 1.9 |
| OHR-13 | >300 | >300 | >300 | >300 | 137.0 ± 28.4 |
| OHR-14 | >300 | >300 | 150–300 | >300 | 155.5 ± 35.5 |
| OHR-15 | >300 | >300 | >300 | >300 | 100–300 |
| OHR-16 | >300 | >300 | >300 | >300 | >300 |
2.3. Anti-Proliferative Activity and Possible Structure-Activity Relationships
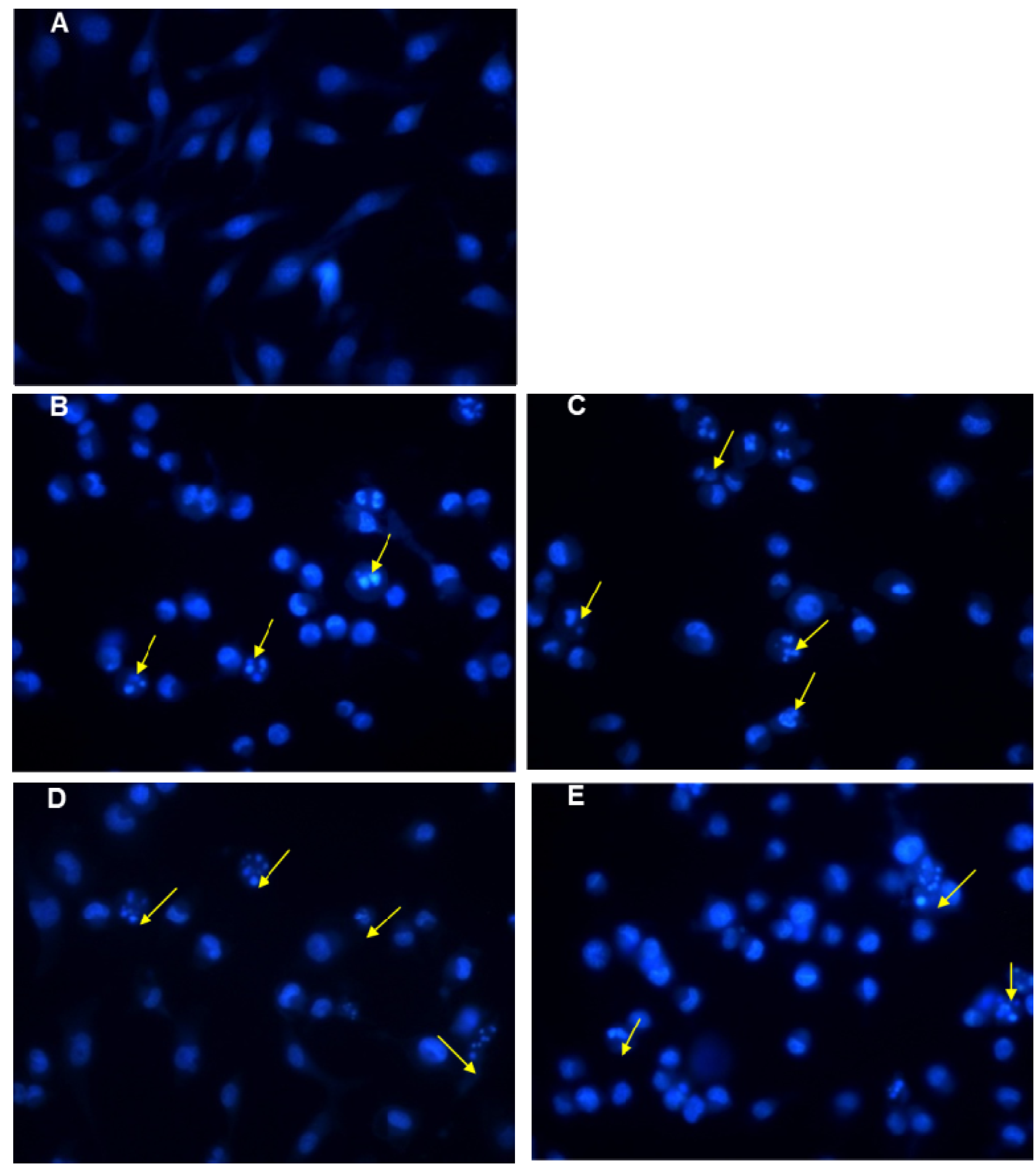
2.4. Apoptosis and Cell Cycle Assays
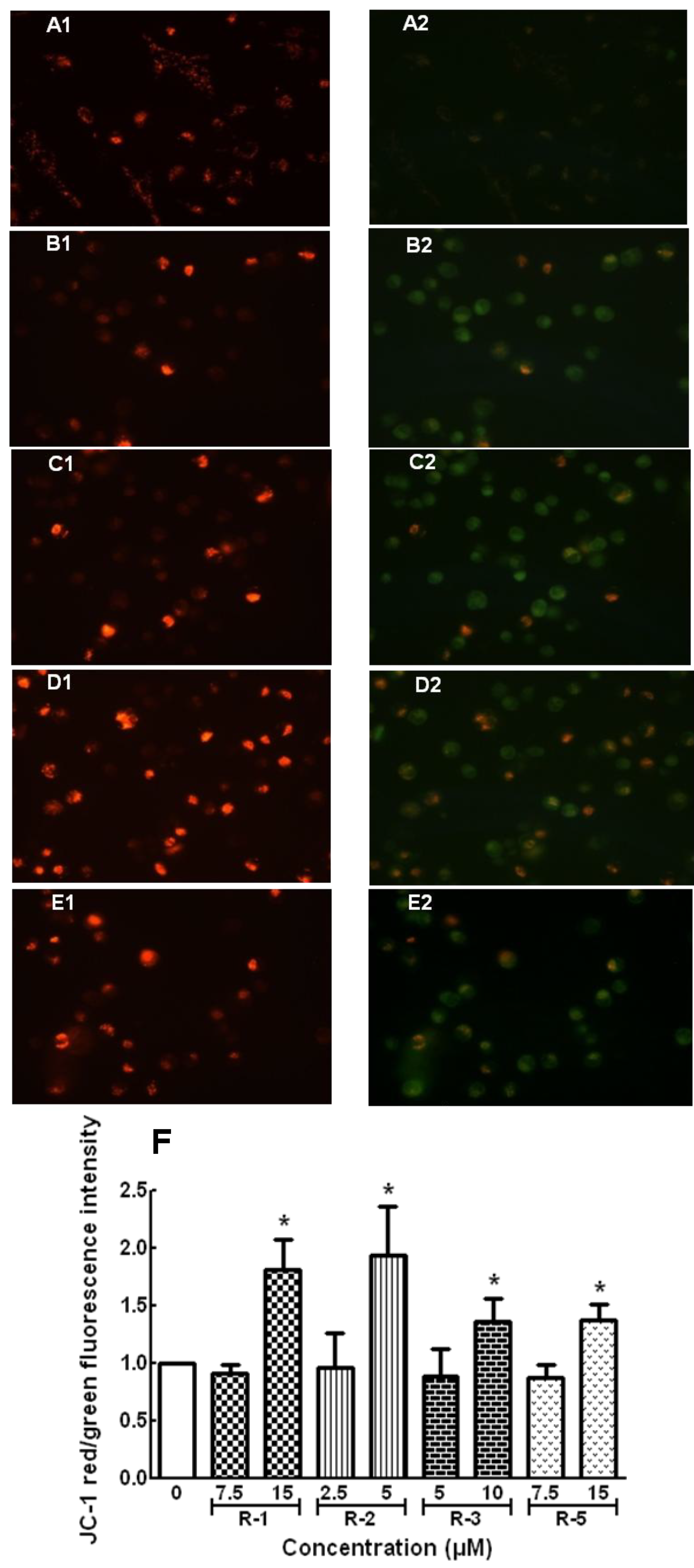
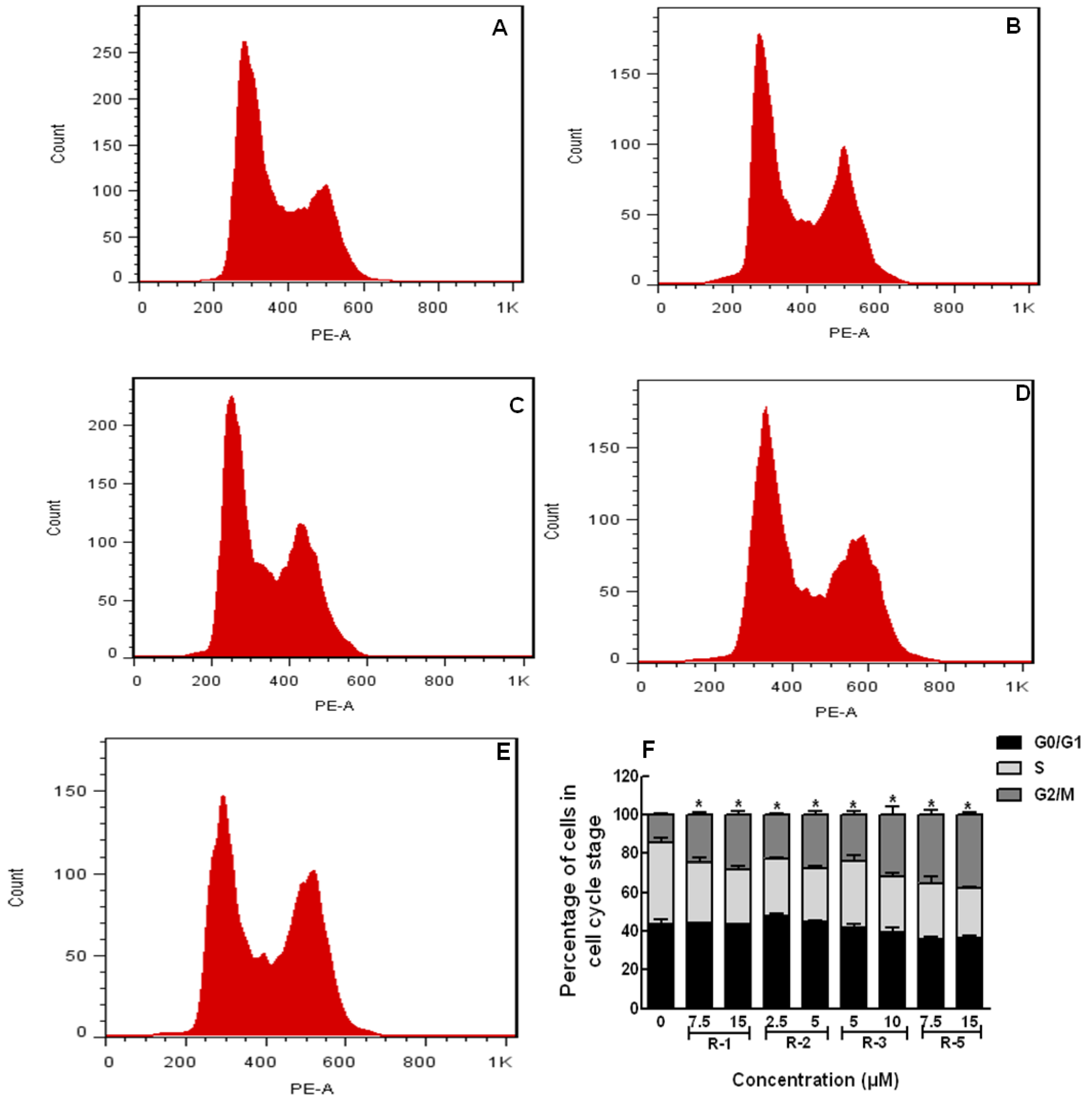
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Chemicals
3.4. Extraction and Isolation
3.5. Synthesis of Acetylated Polyynes
3.6. Chemical Characteristics of Compounds OH–1~OH-6 and OHR–1~OHR-16
 + 213.5° (c = 0.36, CHCl3); UV (CHCl3) λmax (log ξ): 217 (0.81), 243 (1.39), 260 (1.75) and 286 (2.11) nm; IR (KBr) νmax: 3345, 3020, 2932, 2850, 2251, 1466, 1021, 938 and 875 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 2.10 (2H, q, 7.0, H-11), 4.94 (1H, brd, 5.0 Hz, H-3), 5.20 (1H, d, 8.5 Hz, H-8), 5.25 (1H, d, 10.0 Hz, H-1a), 5.46 (1H, ddt, 11.5, 7.5, 1.0 Hz, H-10), 5.50 (1H, ddt, 11.5, 5.0, 1.5 Hz, H-9), 5.62 (1H, dd, 17.5, 1.5 Hz, H-1b), 5.92 (1H, ddd, 17.5, 10.0, 6.0 Hz, H-2). 13C-NMR (125 MHz, CDCl3): δc 14.0 (C-17), 22.6 (C-15), 27.6 (C-11), 29.0 (C-12), 29.1 (C-14), 29.2(C-13), 31.7(C-16), 58.4 (C-8), 63.3 (C-3), 68.6 (C-6), 70.2 (C-5), 78.3 (C-4), 79.8 (C-7), 117.2 (C-1), 127.6 (C10), 134.4 (C-9), 135.8 (C-2).
+ 213.5° (c = 0.36, CHCl3); UV (CHCl3) λmax (log ξ): 217 (0.81), 243 (1.39), 260 (1.75) and 286 (2.11) nm; IR (KBr) νmax: 3345, 3020, 2932, 2850, 2251, 1466, 1021, 938 and 875 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 2.10 (2H, q, 7.0, H-11), 4.94 (1H, brd, 5.0 Hz, H-3), 5.20 (1H, d, 8.5 Hz, H-8), 5.25 (1H, d, 10.0 Hz, H-1a), 5.46 (1H, ddt, 11.5, 7.5, 1.0 Hz, H-10), 5.50 (1H, ddt, 11.5, 5.0, 1.5 Hz, H-9), 5.62 (1H, dd, 17.5, 1.5 Hz, H-1b), 5.92 (1H, ddd, 17.5, 10.0, 6.0 Hz, H-2). 13C-NMR (125 MHz, CDCl3): δc 14.0 (C-17), 22.6 (C-15), 27.6 (C-11), 29.0 (C-12), 29.1 (C-14), 29.2(C-13), 31.7(C-16), 58.4 (C-8), 63.3 (C-3), 68.6 (C-6), 70.2 (C-5), 78.3 (C-4), 79.8 (C-7), 117.2 (C-1), 127.6 (C10), 134.4 (C-9), 135.8 (C-2). + 208.6° (c = 0.28, CHCl3); UV (CHCl3) λmax (log ξ): 210 (1.25), 245 (1.39), 253 (2.35) and 267 (4.12) nm; IR (KBr) νmax: 3350, 3015, 2934, 2860, 2250, 1468, 1025, 940 and 866 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0 Hz, H-17), 1.00 (3H, t, 7.5 Hz, H-1), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.73 (2H, m, H-2), 2.09 (2H, q, 7.0 Hz, H-11), 4.38 (1H, t, 6.5 Hz, H-3), 5.19 (1H, d, 8.5 Hz, H-8), 5.51 (1H, t, 8.5 Hz, H-10), 5.59 (1H, ddt, 11.0, 7.5, 1.0 Hz, H-9). 13C-NMR (125 MHz, CDCl3): δc 9.3 (C-1), 14.0 (C-17), 22.5 (C-15), 27.7 (C-11), 29.1 (C-12), 29.1 (C-14), 29.3 (C-13), 30.5 (C-2), 31.7 (C-16), 58.40 (C-8), 63.9 (C-3), 68.7 (C-6), 68.8 (C-5), 79.1 (C-4), 80.7 (C-7), 127.7 (C-10), 134.3 (C-9).
+ 208.6° (c = 0.28, CHCl3); UV (CHCl3) λmax (log ξ): 210 (1.25), 245 (1.39), 253 (2.35) and 267 (4.12) nm; IR (KBr) νmax: 3350, 3015, 2934, 2860, 2250, 1468, 1025, 940 and 866 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0 Hz, H-17), 1.00 (3H, t, 7.5 Hz, H-1), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.73 (2H, m, H-2), 2.09 (2H, q, 7.0 Hz, H-11), 4.38 (1H, t, 6.5 Hz, H-3), 5.19 (1H, d, 8.5 Hz, H-8), 5.51 (1H, t, 8.5 Hz, H-10), 5.59 (1H, ddt, 11.0, 7.5, 1.0 Hz, H-9). 13C-NMR (125 MHz, CDCl3): δc 9.3 (C-1), 14.0 (C-17), 22.5 (C-15), 27.7 (C-11), 29.1 (C-12), 29.1 (C-14), 29.3 (C-13), 30.5 (C-2), 31.7 (C-16), 58.40 (C-8), 63.9 (C-3), 68.7 (C-6), 68.8 (C-5), 79.1 (C-4), 80.7 (C-7), 127.7 (C-10), 134.3 (C-9). + 180.8° (c = 0.53, CHCl3); UV (CHCl3) λmax (log ξ): 206 (1.76), 235 (1.79), 248 (2.89) and 268 (4.42) nm; IR (KBr) νmax: 3410, 2920, 2856, 2246, 1718, 1628, 1560, 1465 and 1025 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.30 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.62 (2H, m, H-2), 2.05 (3H, s, COCH3), 2.10 (2H, q, 7.5 Hz, H-8), 4.06 (2H t, 7.5 Hz, H-1), 4.94 (1H, brd, 5.0 Hz, H-16), 5.19 (1H, d, 8.0 Hz, H-11), 5.24 (1H, d, 10.5 Hz, H-18a), 5.46 (1H, dd, 17.0, 1.5 Hz, H-18b), 5.51 (1H, dd, 10.5, 8.0 Hz, H-9), 5.59 (1H, ddt, 10.5, 7.5, 1.0Hz, H-10), 5.93 (1H, ddd, 17.0, 10.5, 1.5 Hz, H-17). 13C-NMR (125 MHz, CDCl3): δc 20.9 (COCH3), 25.7 (C-2), 27.5 (C-3), 28.5 (C-5), 28.9 (C-4), 29.0 (C-6), 29.1 (C-8), 29.1 (C-7), 58.4 (C-11), 63.2 (C-16), 64.7 (C-1), 68.6 (C-13), 70.0 (C-14), 78.4 (C-15), 80.7 (C-12), 117.0 (C-18), 127.9 (C-10), 134.1 (C-9), 135.9 (C-17), 171.6 (C=O).
+ 180.8° (c = 0.53, CHCl3); UV (CHCl3) λmax (log ξ): 206 (1.76), 235 (1.79), 248 (2.89) and 268 (4.42) nm; IR (KBr) νmax: 3410, 2920, 2856, 2246, 1718, 1628, 1560, 1465 and 1025 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.30 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.62 (2H, m, H-2), 2.05 (3H, s, COCH3), 2.10 (2H, q, 7.5 Hz, H-8), 4.06 (2H t, 7.5 Hz, H-1), 4.94 (1H, brd, 5.0 Hz, H-16), 5.19 (1H, d, 8.0 Hz, H-11), 5.24 (1H, d, 10.5 Hz, H-18a), 5.46 (1H, dd, 17.0, 1.5 Hz, H-18b), 5.51 (1H, dd, 10.5, 8.0 Hz, H-9), 5.59 (1H, ddt, 10.5, 7.5, 1.0Hz, H-10), 5.93 (1H, ddd, 17.0, 10.5, 1.5 Hz, H-17). 13C-NMR (125 MHz, CDCl3): δc 20.9 (COCH3), 25.7 (C-2), 27.5 (C-3), 28.5 (C-5), 28.9 (C-4), 29.0 (C-6), 29.1 (C-8), 29.1 (C-7), 58.4 (C-11), 63.2 (C-16), 64.7 (C-1), 68.6 (C-13), 70.0 (C-14), 78.4 (C-15), 80.7 (C-12), 117.0 (C-18), 127.9 (C-10), 134.1 (C-9), 135.9 (C-17), 171.6 (C=O). + 168.8° (c = 0.47, CHCl3); UV (CHCl3) λmax (log ξ): 205 (1.88), 233 (1.89), 246 (3.39) and 266 (4.05) nm; IR (KBr) νmax: 3440, 2930, 2855, 2250, 1720, 1622, 1550, 1286 and 1020 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3 H, t, 7.5 Hz, H-18), 1.30 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.62 (2H, m, H-2), 1.73 (2H, m, H-17), 2.05 (3H, s, COCH3), 2.11 (2H, q, 7.5 Hz, H-8), 4.05 (2H, t, 7.0 Hz, H-1), 4.37 (1H, t, 6.5 Hz, H-16), 5.18 (1H, d, 8.5 Hz, H-11), 5.50 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.58 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-10). 13C-NMR (125 MHz, CDCl3): δc 9.3 (C-18), 21.0 (COCH3), 25.7 (C-2), 27.5 (C-8), 28.9 (C-6), 28.9 (C-5), 29.0 (C-4), 29.1 (C-3), 29.1 (C-7), 30.5 (C-17), 58.4 (C-11), 64.7 (C-16), 68.6 (C-1), 68.7 (C-13), 70.0 (C-14), 79.1 (C-15), 80.7 (C-12), 127.9 (C-10), 134.0 (C-9), 171.6 (C=O).
+ 168.8° (c = 0.47, CHCl3); UV (CHCl3) λmax (log ξ): 205 (1.88), 233 (1.89), 246 (3.39) and 266 (4.05) nm; IR (KBr) νmax: 3440, 2930, 2855, 2250, 1720, 1622, 1550, 1286 and 1020 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3 H, t, 7.5 Hz, H-18), 1.30 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.62 (2H, m, H-2), 1.73 (2H, m, H-17), 2.05 (3H, s, COCH3), 2.11 (2H, q, 7.5 Hz, H-8), 4.05 (2H, t, 7.0 Hz, H-1), 4.37 (1H, t, 6.5 Hz, H-16), 5.18 (1H, d, 8.5 Hz, H-11), 5.50 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.58 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-10). 13C-NMR (125 MHz, CDCl3): δc 9.3 (C-18), 21.0 (COCH3), 25.7 (C-2), 27.5 (C-8), 28.9 (C-6), 28.9 (C-5), 29.0 (C-4), 29.1 (C-3), 29.1 (C-7), 30.5 (C-17), 58.4 (C-11), 64.7 (C-16), 68.6 (C-1), 68.7 (C-13), 70.0 (C-14), 79.1 (C-15), 80.7 (C-12), 127.9 (C-10), 134.0 (C-9), 171.6 (C=O). +194.4° (c = 0.16, CHCl3); UV (CHCl3) λmax (log ξ): 226 (1.10), 255 (4.09) and 261 (3.95) nm; IR (KBr) νmax: 3357, 3022, 2929, 2855, 2251, 2150, 1675, 1405 and1303 cm−1. 1H-NMR (500 MHz, CDCl3): δH 1.31 (8H, m, H-3, H-4, H-5, H-6), 1.39 (2H, m, H-7), 1.56 (2H, m, H-2), 2.11 (2H, dq, 1.5, 7.5 Hz, H-8), 3.64 (2H, t, 7.0 Hz, H-1), 4.93 (1H, brd, 5.5 Hz, H-16), 5.19 (1H, d, 8.0 Hz, H-11), 5.22 (1H, dt, 10.0, 1.0 Hz, H-18a), 5.46 (1H, dt, 17.4, 1.0 Hz, H-18b), 5.51 (1H, ddt, 10.6, 8.2, 1.5 Hz, H-9), 5.58 (1H, ddt, 10.6, 7.3, 1.5 Hz, H-10), 5.93 (1H, ddd, 17.4, 10.0, 5.5 Hz, H-17). 13C-NMR (125 MHz, CDCl3): δc 25.6 (C-3), 27.5 (C-8), 28.8 (C-7), 29.0 (C-6), 29.1 (C-4), 29.2 (C-5), 32.6 (C-2), 58.5 (C-11), 63.0 (C-1), 63.3 (C-16), 68.7 (C-13), 70.1 (C-14), 78.5 (C-15), 79.8 (C-12), 117.1 (C-18), 127.9 (C-9), 134.2 (C-10), 136.0 (C-17).
+194.4° (c = 0.16, CHCl3); UV (CHCl3) λmax (log ξ): 226 (1.10), 255 (4.09) and 261 (3.95) nm; IR (KBr) νmax: 3357, 3022, 2929, 2855, 2251, 2150, 1675, 1405 and1303 cm−1. 1H-NMR (500 MHz, CDCl3): δH 1.31 (8H, m, H-3, H-4, H-5, H-6), 1.39 (2H, m, H-7), 1.56 (2H, m, H-2), 2.11 (2H, dq, 1.5, 7.5 Hz, H-8), 3.64 (2H, t, 7.0 Hz, H-1), 4.93 (1H, brd, 5.5 Hz, H-16), 5.19 (1H, d, 8.0 Hz, H-11), 5.22 (1H, dt, 10.0, 1.0 Hz, H-18a), 5.46 (1H, dt, 17.4, 1.0 Hz, H-18b), 5.51 (1H, ddt, 10.6, 8.2, 1.5 Hz, H-9), 5.58 (1H, ddt, 10.6, 7.3, 1.5 Hz, H-10), 5.93 (1H, ddd, 17.4, 10.0, 5.5 Hz, H-17). 13C-NMR (125 MHz, CDCl3): δc 25.6 (C-3), 27.5 (C-8), 28.8 (C-7), 29.0 (C-6), 29.1 (C-4), 29.2 (C-5), 32.6 (C-2), 58.5 (C-11), 63.0 (C-1), 63.3 (C-16), 68.7 (C-13), 70.1 (C-14), 78.5 (C-15), 79.8 (C-12), 117.1 (C-18), 127.9 (C-9), 134.2 (C-10), 136.0 (C-17). + 233.0° (c = 0.3, CHCl3); UV(CHCl3) λmax (log ξ): 207 (1.07), 232 (1.17), 263 (3.98), and 288 (3.84) nm; IR (KBr) νmax: 3355, 3021, 2930, 2856, 2232, 2143, 1656, 1463, 1305, 1095 and 1017 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3H, s, H-18), 1.74 (2H, m, H-17),1.31 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.57 (2H, m, H-2), 2.11 (2H, dq, 1.5, 7.1 Hz, H-8), 3.64 (2H, t, 6.5 Hz, H-1), 4.37 (1H, t, 6.6 Hz, H-16), 5.19 (1H, d, 8.0 Hz, H-11), 5.52 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.58 (1H, ddt, 10.6, 7.3, 1.5 Hz, H-10). 13C-NMR (125 MHz, CDCl3): δc 9.3 (C-18), 25.6 (C-2), 27.5 (C-8), 28.8 (C-7), 29.0 (C-6), 29.1 (C-4), 29.2 (C-5), 30.6 (C-17), 32.6 (C-2), 58.5 (C-11), 63.0 (C-1), 63.8 (C-16), 68.8 (C-13), 68.8 (C-14), 80.9 (C-15), 79.1 (C-12), 128.0 (C-19), 134.1 (C-10).
+ 233.0° (c = 0.3, CHCl3); UV(CHCl3) λmax (log ξ): 207 (1.07), 232 (1.17), 263 (3.98), and 288 (3.84) nm; IR (KBr) νmax: 3355, 3021, 2930, 2856, 2232, 2143, 1656, 1463, 1305, 1095 and 1017 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3H, s, H-18), 1.74 (2H, m, H-17),1.31 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.57 (2H, m, H-2), 2.11 (2H, dq, 1.5, 7.1 Hz, H-8), 3.64 (2H, t, 6.5 Hz, H-1), 4.37 (1H, t, 6.6 Hz, H-16), 5.19 (1H, d, 8.0 Hz, H-11), 5.52 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.58 (1H, ddt, 10.6, 7.3, 1.5 Hz, H-10). 13C-NMR (125 MHz, CDCl3): δc 9.3 (C-18), 25.6 (C-2), 27.5 (C-8), 28.8 (C-7), 29.0 (C-6), 29.1 (C-4), 29.2 (C-5), 30.6 (C-17), 32.6 (C-2), 58.5 (C-11), 63.0 (C-1), 63.8 (C-16), 68.8 (C-13), 68.8 (C-14), 80.9 (C-15), 79.1 (C-12), 128.0 (C-19), 134.1 (C-10). + 202.8° (c = 0.86, CHCl3); UV(CHCl3) λmax (log ξ): 206 (1.27), 232 (1.37), 253 (3.73), and 267 (4.12) nm; IR (KBr) νmax: 3012, 2920, 2816, 2248, 1726, 1718, 1612, 1436, 1252, 1068 and 930 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.00 (3H, t, 7.0, H-1),1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.79 (2H, m, H-2), 2.07 (6H, s, COCH3) 2.13 (2H, dq, 7.0, 1.0 Hz, H-11), 5.33 (1H, t, 6.5 Hz, H-3), 5.46 (1H, ddt, 11.0, 8.5, 1.5 Hz, H-9), 5.66 (1H, dt, 11.0, 7.5, 1.0 Hz, H-10), 6.11 (1H, dt, 8.5, 1.0 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-1), 14.0 (C-17), 20.8 (COCH3), 20.9 (COCH3), 22.6 (C-15), 27.8 (C-11), 27.8 (C-12), 29.1 (C-14), 29.1 (C-13), 29.2 (C-2), 31.7 (C-16), 60.1 (C-8), 65.2 (C-3), 69.2 (C-6), 69.3 (C-5), 76.0 (C-4), 77.3 (C-7), 123.8 (C-10), 136.4 (C-9), 169.4 (C=O), 169.7 (C=O).
+ 202.8° (c = 0.86, CHCl3); UV(CHCl3) λmax (log ξ): 206 (1.27), 232 (1.37), 253 (3.73), and 267 (4.12) nm; IR (KBr) νmax: 3012, 2920, 2816, 2248, 1726, 1718, 1612, 1436, 1252, 1068 and 930 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.00 (3H, t, 7.0, H-1),1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.79 (2H, m, H-2), 2.07 (6H, s, COCH3) 2.13 (2H, dq, 7.0, 1.0 Hz, H-11), 5.33 (1H, t, 6.5 Hz, H-3), 5.46 (1H, ddt, 11.0, 8.5, 1.5 Hz, H-9), 5.66 (1H, dt, 11.0, 7.5, 1.0 Hz, H-10), 6.11 (1H, dt, 8.5, 1.0 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-1), 14.0 (C-17), 20.8 (COCH3), 20.9 (COCH3), 22.6 (C-15), 27.8 (C-11), 27.8 (C-12), 29.1 (C-14), 29.1 (C-13), 29.2 (C-2), 31.7 (C-16), 60.1 (C-8), 65.2 (C-3), 69.2 (C-6), 69.3 (C-5), 76.0 (C-4), 77.3 (C-7), 123.8 (C-10), 136.4 (C-9), 169.4 (C=O), 169.7 (C=O). + 243.5° (c = 0.92, CHCl3); UV (CHCl3) λmax (log ξ): 208 (1.07), 228 (1.52), 248 (3.25) and 270 (3.66) nm; IR (KBr) νmax: 2982, 2867, 2248, 1720, 1716, 1384, 1142, 950 and 862 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.36 (2H, m, H-12), 2.07 (3H, s, COCH3), 2.09 (3H, s, COCH3), 2.14 (2H, q, 7.0, H-11), 5.33 (1H, d, 10.0 Hz, H-1a), 5.46 (1H, ddt, 10.5, 8.5, 1.5Hz, H-9), 5.53 (1H, d, 17.5 Hz, H-1b), 5.66 (1H, ddt, 10.5, 7.0, 1.0 Hz, H-10), 5.84 (1H, ddd, 17.5, 10.0, 6.0 Hz, H-2), 5.90 (1H, dd, 6.0, 1.0 Hz, H-3), 6.13 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 14.1 (C-17), 20.8 (COCH3), 20.9 (COCH3), 22.6 (C-15), 27.9 (C-11), 29.0 (C-12), 29.1 (C-14), 29.1 (C-13), 31.8 (C-16), 60.0 (C-8), 64.4 (C-3), 69.1 (C-6), 70.7 (C-5), 75.1 (C-4), 77.6 (C-7), 119.7 (C-1), 123.7 (C-10), 131.9 (C-9), 136.5 (C-2), 169.4 (C=O), 169.4 (C=O).
+ 243.5° (c = 0.92, CHCl3); UV (CHCl3) λmax (log ξ): 208 (1.07), 228 (1.52), 248 (3.25) and 270 (3.66) nm; IR (KBr) νmax: 2982, 2867, 2248, 1720, 1716, 1384, 1142, 950 and 862 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.36 (2H, m, H-12), 2.07 (3H, s, COCH3), 2.09 (3H, s, COCH3), 2.14 (2H, q, 7.0, H-11), 5.33 (1H, d, 10.0 Hz, H-1a), 5.46 (1H, ddt, 10.5, 8.5, 1.5Hz, H-9), 5.53 (1H, d, 17.5 Hz, H-1b), 5.66 (1H, ddt, 10.5, 7.0, 1.0 Hz, H-10), 5.84 (1H, ddd, 17.5, 10.0, 6.0 Hz, H-2), 5.90 (1H, dd, 6.0, 1.0 Hz, H-3), 6.13 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 14.1 (C-17), 20.8 (COCH3), 20.9 (COCH3), 22.6 (C-15), 27.9 (C-11), 29.0 (C-12), 29.1 (C-14), 29.1 (C-13), 31.8 (C-16), 60.0 (C-8), 64.4 (C-3), 69.1 (C-6), 70.7 (C-5), 75.1 (C-4), 77.6 (C-7), 119.7 (C-1), 123.7 (C-10), 131.9 (C-9), 136.5 (C-2), 169.4 (C=O), 169.4 (C=O). + 164.6° (c = 0.72, CHCl3); UV(CHCl3) λmax (log ξ): 203 (0.86), 224 (1.57), 242 (4.21), and 266 (4.58) nm; IR (KBr) νmax: 3385, 3025, 2946, 2825, 2230, 1715, 1580, 1420, 1232, 1060 and 896 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.00 (3H, t, 7.0, H-1),1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.79 (2H, m, H-2), 2.07 (6H, s, COCH3) 2.13 (2H, dq, 7.0, 1.0 Hz, H-11), 4.37 (1H, t, 6.5 Hz, H-3), 5.47 (1H, ddt, 11.0, 8.5, 1.5 Hz, H-9), 5.65 (1H, ddt, 11.0, 7.5, 1.0 Hz, H-10), 6.13 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-1), 14.0 (C-17), 20.9 (COCH3), 22.6 (C-15), 27.8 (C-11), 27.8 (C-12), 29.1 (C-14), 29.2 (C-13), 30.6 (C-2), 31.7 (C-16), 60.1 (C-8), 64.0 (C-3), 68.9 (C-6), 69.4 (C-5), 75.9 (C-4), 80.9 (C-7), 123.9 (C-10), 136.3 (C-9), 169.5 (C=O).
+ 164.6° (c = 0.72, CHCl3); UV(CHCl3) λmax (log ξ): 203 (0.86), 224 (1.57), 242 (4.21), and 266 (4.58) nm; IR (KBr) νmax: 3385, 3025, 2946, 2825, 2230, 1715, 1580, 1420, 1232, 1060 and 896 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.00 (3H, t, 7.0, H-1),1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.79 (2H, m, H-2), 2.07 (6H, s, COCH3) 2.13 (2H, dq, 7.0, 1.0 Hz, H-11), 4.37 (1H, t, 6.5 Hz, H-3), 5.47 (1H, ddt, 11.0, 8.5, 1.5 Hz, H-9), 5.65 (1H, ddt, 11.0, 7.5, 1.0 Hz, H-10), 6.13 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-1), 14.0 (C-17), 20.9 (COCH3), 22.6 (C-15), 27.8 (C-11), 27.8 (C-12), 29.1 (C-14), 29.2 (C-13), 30.6 (C-2), 31.7 (C-16), 60.1 (C-8), 64.0 (C-3), 68.9 (C-6), 69.4 (C-5), 75.9 (C-4), 80.9 (C-7), 123.9 (C-10), 136.3 (C-9), 169.5 (C=O). + 213.6° (c = 1.12, CHCl3); UV(CHCl3) λmax (log ξ): 205 (0.93), 222 (2.17), 245 (4.01), and 270 (4.27) nm; IR (KBr) νmax: 3360, 3010, 2936, 2860, 2252, 1722, 1596, 1440, 1210, 1060 and 912 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.02 (3H, t, 7.0, H-1), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.79 (2H, m, H-2), 2.07 (3H, s, COCH3) 2.13 (2H, dq, 7.0, 1.0 Hz, H-11), 5.19 (1H, d, 8.0 Hz, H-8), 5.34 (1H, t, 6.5 Hz, H-3), 5.48 (1H, ddt, 11.0, 8.5, 1.5 Hz, H-9), 5.63 (1H, dt, 11.0, 7.5, 1.0 Hz, H-10). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-1), 14.0 (C-17), 20.8 (COCH3), 22.6 (C-15), 27.7 (C-11), 27.9 (C-12), 29.1 (C-14), 29.2 (C-13), 29.3 (C-2), 31.8 (C-16), 58.7 (C-8), 65.3 (C-3), 69.2 (C-6), 69.3 (C-5), 76.0 (C-4), 79.4 (C-7), 127.8 (C-10), 134.7 (C-9), 169.5 (C=O).
+ 213.6° (c = 1.12, CHCl3); UV(CHCl3) λmax (log ξ): 205 (0.93), 222 (2.17), 245 (4.01), and 270 (4.27) nm; IR (KBr) νmax: 3360, 3010, 2936, 2860, 2252, 1722, 1596, 1440, 1210, 1060 and 912 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.88 (3H, t, 7.0, H-17), 1.02 (3H, t, 7.0, H-1), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.79 (2H, m, H-2), 2.07 (3H, s, COCH3) 2.13 (2H, dq, 7.0, 1.0 Hz, H-11), 5.19 (1H, d, 8.0 Hz, H-8), 5.34 (1H, t, 6.5 Hz, H-3), 5.48 (1H, ddt, 11.0, 8.5, 1.5 Hz, H-9), 5.63 (1H, dt, 11.0, 7.5, 1.0 Hz, H-10). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-1), 14.0 (C-17), 20.8 (COCH3), 22.6 (C-15), 27.7 (C-11), 27.9 (C-12), 29.1 (C-14), 29.2 (C-13), 29.3 (C-2), 31.8 (C-16), 58.7 (C-8), 65.3 (C-3), 69.2 (C-6), 69.3 (C-5), 76.0 (C-4), 79.4 (C-7), 127.8 (C-10), 134.7 (C-9), 169.5 (C=O). + 230.2° (c = 0.91, CHCl3); UV(CHCl3) λmax (log ξ): 210 (1.56), 228 (3.17), 242 (4.31), and 265 (4.45) nm; IR (KBr) νmax: 3422, 2981, 2258, 1716, 1584, 1432, 1226, 1030 and 990 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.89 (3H, t, 7.0, H-17), 1.27 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 2.07 (3H, s, COCH3), 2.14 (2H, q, 7.0, H-11), 4.93 (1H, d, 6.0 Hz, H-3), 5.27 (1H, d, 10.0 Hz, H-1a), 5.46 (1H, ddt, 10.5, 8.5, 1.5Hz, H-9), 5.50 (1H, d, 17.5 Hz, H-1b), 5.66 (1H, ddt, 10.5, 7.0, 1.0 Hz, H-10), 5.84 (1H, ddd, 17.5, 10.0, 6.0 Hz, H-2), 6.14 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 14.1 (C-17), 20.9 (COCH3), 22.6 (C-15), 27.9 (C-11), 29.0 (C-12), 29.1 (C-14), 29.2 (C-13), 31.8 (C-16), 60.0 (C-8), 64.4 (C-3), 69.2 (C-6), 70.9 (C-5), 75.5 (C-4), 77.7 (C-7), 117.4 (C-1), 123.8 (C-10), 131.9 (C-9), 136.5 (C-2), 169.4 (C=O).
+ 230.2° (c = 0.91, CHCl3); UV(CHCl3) λmax (log ξ): 210 (1.56), 228 (3.17), 242 (4.31), and 265 (4.45) nm; IR (KBr) νmax: 3422, 2981, 2258, 1716, 1584, 1432, 1226, 1030 and 990 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.89 (3H, t, 7.0, H-17), 1.27 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 2.07 (3H, s, COCH3), 2.14 (2H, q, 7.0, H-11), 4.93 (1H, d, 6.0 Hz, H-3), 5.27 (1H, d, 10.0 Hz, H-1a), 5.46 (1H, ddt, 10.5, 8.5, 1.5Hz, H-9), 5.50 (1H, d, 17.5 Hz, H-1b), 5.66 (1H, ddt, 10.5, 7.0, 1.0 Hz, H-10), 5.84 (1H, ddd, 17.5, 10.0, 6.0 Hz, H-2), 6.14 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 14.1 (C-17), 20.9 (COCH3), 22.6 (C-15), 27.9 (C-11), 29.0 (C-12), 29.1 (C-14), 29.2 (C-13), 31.8 (C-16), 60.0 (C-8), 64.4 (C-3), 69.2 (C-6), 70.9 (C-5), 75.5 (C-4), 77.7 (C-7), 117.4 (C-1), 123.8 (C-10), 131.9 (C-9), 136.5 (C-2), 169.4 (C=O). + 210.8° (c = 0.51, CHCl3); UV(CHCl3) λmax (log ξ): 208 (1.36), 226 (2.57), 248 (4.31), and 268 (4.48) nm; IR (KBr) νmax: 3380, 3012, 2252, 1718, 1560, 1482, 1216, 1024 and 990 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.89 (3H, t, 7.0, H-17), 1.27 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 2.09 (3H, s, COCH3), 2.11 (2H, q, 7.0, H-11), 5.19 (1H, d, 6.0 Hz, H-3), 5.33 (1H, d, 10.0 Hz, H-1a), 5.48 (1H, ddt, 10.5, 8.5, 1.5Hz, H-9), 5.51 (1H, d, 17.5 Hz, H-1b), 5.60 (1H, ddt, 10.5, 7.0, 1.0 Hz, H-10), 5.86 (1H, ddd, 17.5, 10.0, 6.0 Hz, H-2), 5.91 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 14.0 (C-17), 20.8 (COCH3), 22.6 (C-15), 27.9 (C-11), 29.0 (C-12), 29.1 (C-14), 29.2 (C-13), 31.8 (C-16), 58.6 (C-8), 64.4 (C-3), 68.6 (C-6), 70.8 (C-5), 74.8 (C-4), 80.1 (C-7), 119.7 (C-1), 127.6 (C-10), 132.0 (C-9), 134.7 (C-2), 169.4 (C=O).
+ 210.8° (c = 0.51, CHCl3); UV(CHCl3) λmax (log ξ): 208 (1.36), 226 (2.57), 248 (4.31), and 268 (4.48) nm; IR (KBr) νmax: 3380, 3012, 2252, 1718, 1560, 1482, 1216, 1024 and 990 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.89 (3H, t, 7.0, H-17), 1.27 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 2.09 (3H, s, COCH3), 2.11 (2H, q, 7.0, H-11), 5.19 (1H, d, 6.0 Hz, H-3), 5.33 (1H, d, 10.0 Hz, H-1a), 5.48 (1H, ddt, 10.5, 8.5, 1.5Hz, H-9), 5.51 (1H, d, 17.5 Hz, H-1b), 5.60 (1H, ddt, 10.5, 7.0, 1.0 Hz, H-10), 5.86 (1H, ddd, 17.5, 10.0, 6.0 Hz, H-2), 5.91 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 14.0 (C-17), 20.8 (COCH3), 22.6 (C-15), 27.9 (C-11), 29.0 (C-12), 29.1 (C-14), 29.2 (C-13), 31.8 (C-16), 58.6 (C-8), 64.4 (C-3), 68.6 (C-6), 70.8 (C-5), 74.8 (C-4), 80.1 (C-7), 119.7 (C-1), 127.6 (C-10), 132.0 (C-9), 134.7 (C-2), 169.4 (C=O). + 185.4° (c = 0.41, CHCl3); UV(CHCl3) λmax (log ξ): 205 (0.76), 223 (1.35), 240 (3.21), and 265 (4.05) nm; IR (KBr) νmax: 2985, 2258, 1723, 1716, 1585, 1477, 1238, 1028 and 954 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3 H, t, 7.5 Hz, H-18), 1.29 (8H, m, H-3, H-4, H-5, H-6), 1.37 (2H, m, H-7), 1.61 (2H, m, H-2), 1.79 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.07 (6H, s, 2 × COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H, t, 7.0 Hz, H-1), 5.33 (1H, t, 6.5 Hz, H-16), 5.47 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.66 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-10), 6.13 (1H, d, 8.5 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-18), 20.8 (COCH3), 20.9 (COCH3), 21.0 (COCH3), 25.8 (C-2), 27.8 (C-8), 28.6 (C-6), 28.9 (C-5), 29.0 (C-4), 29.1 (C-3), 29.1 (C-7), 29.3 (C-17), 60.0 (C-11), 64.6 (C-16), 65.1 (C-1), 69.2 (C-13), 69.3 (C-14), 75.9 (C-15), 77.2 (C-12), 123.8 (C-10), 136.2 (C-9), 169.4 (C=O), 169.7 (C=O), 171.2 (C=O).
+ 185.4° (c = 0.41, CHCl3); UV(CHCl3) λmax (log ξ): 205 (0.76), 223 (1.35), 240 (3.21), and 265 (4.05) nm; IR (KBr) νmax: 2985, 2258, 1723, 1716, 1585, 1477, 1238, 1028 and 954 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3 H, t, 7.5 Hz, H-18), 1.29 (8H, m, H-3, H-4, H-5, H-6), 1.37 (2H, m, H-7), 1.61 (2H, m, H-2), 1.79 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.07 (6H, s, 2 × COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H, t, 7.0 Hz, H-1), 5.33 (1H, t, 6.5 Hz, H-16), 5.47 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.66 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-10), 6.13 (1H, d, 8.5 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-18), 20.8 (COCH3), 20.9 (COCH3), 21.0 (COCH3), 25.8 (C-2), 27.8 (C-8), 28.6 (C-6), 28.9 (C-5), 29.0 (C-4), 29.1 (C-3), 29.1 (C-7), 29.3 (C-17), 60.0 (C-11), 64.6 (C-16), 65.1 (C-1), 69.2 (C-13), 69.3 (C-14), 75.9 (C-15), 77.2 (C-12), 123.8 (C-10), 136.2 (C-9), 169.4 (C=O), 169.7 (C=O), 171.2 (C=O). + 236.7° (c = 0.61, CHCl3); UV(CHCl3) λmax (log ξ): 210 (1.46), 228 (2.15), 246 (3.71), and 274 (4.65) nm; IR (KBr) νmax: 2968, 2257, 1720, 1718, 1562, 1457, 1318, 1016 and 946 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.29 (8H, m, H-3, H-4, H-5, H-6), 1.37 (2H, m, H-7), 1.62 (2H, m, H-2), 2.04 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.10 (3H, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H t, 7.5 Hz, H-1), 5.33 (1H, d, 10.5 Hz, H-18a), 5.46 (1H, dt, 10.5, 7.5 Hz, Hz, H-9), 5.53 (1H, dd, 17.5, 1.5 Hz, H-18b), 5.66 (1H, ddt, 10.5, 7.5, 1.0Hz, H-10), 5.85 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-17), 5.90 (1H, brd, 5.0 Hz, H-16), 6.13 (1H, d, 8.0 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 20.8 (COCH3), 20.9 (COCH3), 20.9 (COCH3), 25.8 (C-2), 27.8 (C-3), 28.6 (C-5), 29.0 (C-4), 29.0 (C-6), 29.1 (C-8), 29.2 (C-7), 60.0 (C-11), 64.3 (C-16), 64.6 (C-1), 69.1 (C-13), 70.7 (C-14), 75.1 (C-15), 76.7 (C-12), 119.7 (C-18), 123.7 (C-10), 131.9 (C-9), 136.3 (C-17), 169.3(C=O), 169.4 (C=O), 171.6 (C=O).
+ 236.7° (c = 0.61, CHCl3); UV(CHCl3) λmax (log ξ): 210 (1.46), 228 (2.15), 246 (3.71), and 274 (4.65) nm; IR (KBr) νmax: 2968, 2257, 1720, 1718, 1562, 1457, 1318, 1016 and 946 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.29 (8H, m, H-3, H-4, H-5, H-6), 1.37 (2H, m, H-7), 1.62 (2H, m, H-2), 2.04 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.10 (3H, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H t, 7.5 Hz, H-1), 5.33 (1H, d, 10.5 Hz, H-18a), 5.46 (1H, dt, 10.5, 7.5 Hz, Hz, H-9), 5.53 (1H, dd, 17.5, 1.5 Hz, H-18b), 5.66 (1H, ddt, 10.5, 7.5, 1.0Hz, H-10), 5.85 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-17), 5.90 (1H, brd, 5.0 Hz, H-16), 6.13 (1H, d, 8.0 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 20.8 (COCH3), 20.9 (COCH3), 20.9 (COCH3), 25.8 (C-2), 27.8 (C-3), 28.6 (C-5), 29.0 (C-4), 29.0 (C-6), 29.1 (C-8), 29.2 (C-7), 60.0 (C-11), 64.3 (C-16), 64.6 (C-1), 69.1 (C-13), 70.7 (C-14), 75.1 (C-15), 76.7 (C-12), 119.7 (C-18), 123.7 (C-10), 131.9 (C-9), 136.3 (C-17), 169.3(C=O), 169.4 (C=O), 171.6 (C=O). + 285.2° (c = 1.01, CHCl3); UV(CHCl3) λmax (log ξ): 208 (1.40), 226 (3.15), 245 (4.81), and 268 (4.55) nm; IR (KBr) νmax: 3348, 3014, 2988, 2254, 1722, 1710, 1486, 1380, 1258, 1016 and 885 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.29 (8H, m, H-3, H-4, H-5, H-6), 1.37 (2H, m, H-7), 1.62 (2H, m, H-2), 2.04 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H t, 7.5 Hz, H-1), 5.19 (1H, d, 8.0 Hz, H-11), 5.34 (1H, d, 10.5 Hz, H-18a), 5.50 (1H, dt, 10.5, 7.5 Hz, Hz, H-9), 5.52 (1H, dd, 17.5, 1.5 Hz, H-18b), 5.62 (1H, ddt, 10.5, 7.5, 1.0Hz, H-10), 5.86 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-17), 5.90 (1H, brd, 5.0 Hz, H-16). 13C-NMR (125 MHz, CDCl3): δc 20.8 (COCH3), 20.9 (COCH3), 25.8 (C-2), 27.8 (C-3), 28.6 (C-5), 29.0 (C-4), 29.0 (C-6), 29.1 (C-8), 29.2 (C-7), 58.6 (C-11), 64.3 (C-16), 64.6 (C-1), 68.6 (C-13), 70.8 (C-14), 74.9 (C-15), 80.1 (C-12), 119.7 (C-18), 127.7 (C-10), 132.0 (C-9), 134.6 (C-17), 169.4(C=O), 171.3 (C=O).
+ 285.2° (c = 1.01, CHCl3); UV(CHCl3) λmax (log ξ): 208 (1.40), 226 (3.15), 245 (4.81), and 268 (4.55) nm; IR (KBr) νmax: 3348, 3014, 2988, 2254, 1722, 1710, 1486, 1380, 1258, 1016 and 885 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.29 (8H, m, H-3, H-4, H-5, H-6), 1.37 (2H, m, H-7), 1.62 (2H, m, H-2), 2.04 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H t, 7.5 Hz, H-1), 5.19 (1H, d, 8.0 Hz, H-11), 5.34 (1H, d, 10.5 Hz, H-18a), 5.50 (1H, dt, 10.5, 7.5 Hz, Hz, H-9), 5.52 (1H, dd, 17.5, 1.5 Hz, H-18b), 5.62 (1H, ddt, 10.5, 7.5, 1.0Hz, H-10), 5.86 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-17), 5.90 (1H, brd, 5.0 Hz, H-16). 13C-NMR (125 MHz, CDCl3): δc 20.8 (COCH3), 20.9 (COCH3), 25.8 (C-2), 27.8 (C-3), 28.6 (C-5), 29.0 (C-4), 29.0 (C-6), 29.1 (C-8), 29.2 (C-7), 58.6 (C-11), 64.3 (C-16), 64.6 (C-1), 68.6 (C-13), 70.8 (C-14), 74.9 (C-15), 80.1 (C-12), 119.7 (C-18), 127.7 (C-10), 132.0 (C-9), 134.6 (C-17), 169.4(C=O), 171.3 (C=O). + 285.2° (c = 1.01, CHCl3); UV(CHCl3) λmax (log ξ): 208 (1.40), 226 (3.15), 245 (4.81), and 268 (4.55) nm; IR (KBr) νmax: 3348, 3014, 2988, 2254, 1722, 1710, 1486, 1380, 1258, 1016 and 885 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.26 (8H, m, H-3, H-4, H-5, H-6), 1.41 (2H, m, H-7), 1.61 (2H, m, H-2), 2.04 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H t, 7.5 Hz, H-1), 4.94 (1H, brd, 5.0 Hz, H-16), 5.25 (1H, d, 10.5 Hz, H-18a), 5.50 (1H, dt, 10.5, 7.5 Hz, Hz, H-9), 5.55 (1H, dd, 17.5, 1.5 Hz, H-18b), 5.65 (1H, ddt, 10.5, 7.5, 1.0Hz, H-10), 5.96 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-17), 6.13 (1H, d, 8.0 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 20.8 (COCH3), 21.0 (COCH3), 25.8 (C-2), 27.8 (C-3), 28.6 (C-5), 29.0 (C-4), 29.0 (C-6), 29.1 (C-8), 30.4 (C-7), 58.6 (C-11), 64.3 (C-16), 64.6 (C-1), 68.6 (C-13), 70.8 (C-14), 74.9 (C-15), 80.1 (C-12), 117.3 (C-18), 123.9 (C-10), 130.8 (C-9), 136.2 (C-17), 169.5 (C=O), 171.3 (C=O).
+ 285.2° (c = 1.01, CHCl3); UV(CHCl3) λmax (log ξ): 208 (1.40), 226 (3.15), 245 (4.81), and 268 (4.55) nm; IR (KBr) νmax: 3348, 3014, 2988, 2254, 1722, 1710, 1486, 1380, 1258, 1016 and 885 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.26 (8H, m, H-3, H-4, H-5, H-6), 1.41 (2H, m, H-7), 1.61 (2H, m, H-2), 2.04 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H t, 7.5 Hz, H-1), 4.94 (1H, brd, 5.0 Hz, H-16), 5.25 (1H, d, 10.5 Hz, H-18a), 5.50 (1H, dt, 10.5, 7.5 Hz, Hz, H-9), 5.55 (1H, dd, 17.5, 1.5 Hz, H-18b), 5.65 (1H, ddt, 10.5, 7.5, 1.0Hz, H-10), 5.96 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-17), 6.13 (1H, d, 8.0 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 20.8 (COCH3), 21.0 (COCH3), 25.8 (C-2), 27.8 (C-3), 28.6 (C-5), 29.0 (C-4), 29.0 (C-6), 29.1 (C-8), 30.4 (C-7), 58.6 (C-11), 64.3 (C-16), 64.6 (C-1), 68.6 (C-13), 70.8 (C-14), 74.9 (C-15), 80.1 (C-12), 117.3 (C-18), 123.9 (C-10), 130.8 (C-9), 136.2 (C-17), 169.5 (C=O), 171.3 (C=O). + 225.4° (c = 1.01, CHCl3); UV(CHCl3) λmax (log ξ): 205 (0.76), 223 (1.35), 240 (3.21), and 265 (4.05) nm; IR (KBr) νmax: 3310, 2985, 2258, 1723, 1716, 1585, 1477, 1238, 1028 and 954 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.89 (3 H, t, 7.5 Hz, H-18), 1.28 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.62 (2H, m, H-2), 1.78 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.09 (3, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H, t, 7.0 Hz, H-1), 5.33 (1H, t, 6.5 Hz, H-16), 5.47 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.66 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-10), 5.90 (1H, d, 8.5 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-18), 20.8 (COCH3), 20.9 (COCH3), 25.8 (C-2), 27.8 (C-8), 28.6 (C-6), 28.9 (C-5), 29.0 (C-4), 29.1 (C-3), 29.1 (C-7), 29.3 (C-17), 60.0 (C-11), 64.6 (C-16), 65.1 (C-1), 69.2 (C-13), 69.5 (C-14), 75.6 (C-15), 77.6 (C-12), 126.1 (C-10), 134.3 (C-9), 169.4 (C=O), 171.2 (C=O).
+ 225.4° (c = 1.01, CHCl3); UV(CHCl3) λmax (log ξ): 205 (0.76), 223 (1.35), 240 (3.21), and 265 (4.05) nm; IR (KBr) νmax: 3310, 2985, 2258, 1723, 1716, 1585, 1477, 1238, 1028 and 954 cm−1; 1H-NMR (500 MHz, CDCl3): δH 0.89 (3 H, t, 7.5 Hz, H-18), 1.28 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.62 (2H, m, H-2), 1.78 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.09 (3, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-8), 4.05 (2H, t, 7.0 Hz, H-1), 5.33 (1H, t, 6.5 Hz, H-16), 5.47 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.66 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-10), 5.90 (1H, d, 8.5 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-18), 20.8 (COCH3), 20.9 (COCH3), 25.8 (C-2), 27.8 (C-8), 28.6 (C-6), 28.9 (C-5), 29.0 (C-4), 29.1 (C-3), 29.1 (C-7), 29.3 (C-17), 60.0 (C-11), 64.6 (C-16), 65.1 (C-1), 69.2 (C-13), 69.5 (C-14), 75.6 (C-15), 77.6 (C-12), 126.1 (C-10), 134.3 (C-9), 169.4 (C=O), 171.2 (C=O).  + 241.4° (c = 1.21, CHCl3); UV(CHCl3) λmax (log ξ): 204 (0.86), 225 (1.25), 242 (3.61), and 266 (4.15) nm; IR (KBr) νmax: 3326, 2986, 2252, 1720, 1715, 1565, 1447, 1232, 1120 and 880 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3 H, t, 7.5 Hz, H-18), 1.29 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.62 (2H, m, H-2), 1.74 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.07 (3, s, COCH3), 2.15 (2H, q, 7.5 Hz, H-8), 4.06 (2H, t, 7.0 Hz, H-1), 4.37 (1H, t, 6.5 Hz, H-16), 5.48 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.66 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-10), 6.13 (1H, d, 8.5 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-18), 20.8 (COCH3), 20.9 (COCH3), 25.8 (C-2), 27.6 (C-8), 28.6 (C-6), 28.9 (C-5), 29.0 (C-4), 29.1 (C-3), 29.3 (C-7), 30.6(C-17), 58.6 (C-11), 64.0 (C-16), 65.4 (C-1), 68.7 (C-13), 69.5 (C-14), 75.7 (C-15), 81.1 (C-12), 124.0 (C-10), 136.1 (C-9), 169.4 (C=O), 171.4 (C=O).
+ 241.4° (c = 1.21, CHCl3); UV(CHCl3) λmax (log ξ): 204 (0.86), 225 (1.25), 242 (3.61), and 266 (4.15) nm; IR (KBr) νmax: 3326, 2986, 2252, 1720, 1715, 1565, 1447, 1232, 1120 and 880 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3 H, t, 7.5 Hz, H-18), 1.29 (8H, m, H-3, H-4, H-5, H-6), 1.38 (2H, m, H-7), 1.62 (2H, m, H-2), 1.74 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.07 (3, s, COCH3), 2.15 (2H, q, 7.5 Hz, H-8), 4.06 (2H, t, 7.0 Hz, H-1), 4.37 (1H, t, 6.5 Hz, H-16), 5.48 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-9), 5.66 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-10), 6.13 (1H, d, 8.5 Hz, H-11). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-18), 20.8 (COCH3), 20.9 (COCH3), 25.8 (C-2), 27.6 (C-8), 28.6 (C-6), 28.9 (C-5), 29.0 (C-4), 29.1 (C-3), 29.3 (C-7), 30.6(C-17), 58.6 (C-11), 64.0 (C-16), 65.4 (C-1), 68.7 (C-13), 69.5 (C-14), 75.7 (C-15), 81.1 (C-12), 124.0 (C-10), 136.1 (C-9), 169.4 (C=O), 171.4 (C=O). + 168.6° (c = 0.50, CHCl3); UV(CHCl3) λmax (log ξ): 206 (0.56), 226 (1.55), 242 (2.86), and 265 (3.84) nm; IR (KBr) νmax: 3410, 3020, 2252, 1718, 1714, 1562, 1460, 1235, 1022 and 950 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3 H, t, 7.5 Hz, H-1), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.37 (2H, m, H-12), 1.61 (2H, m, H-17), 1.79 (2H, m, H-2), 2.04 (3H, s, COCH3), 2.09 (3H, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-11), 4.08 (2H, t, 7.0 Hz, H-18), 5.33 (1H, t, 6.5 Hz, H-3), 5.47 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-10), 5.66 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-9), 6.13 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-1), 20.8 (COCH3), 21.0 (COCH3), 25.8 (C-17), 27.8 (C-11), 28.6 (C-13), 28.9 (C-14), 29.0 (C-15), 29.1 (C-16), 29.1 (C-12), 29.3 (C-2), 60.0 (C-8), 64.6 (C-3), 65.1 (C-18), 69.1 (C-6), 69.2 (C-5), 75.7 (C-4), 77.5 (C-7), 123.6 (C-9), 136.1 (C-10), 169.4 (C=O), 169.7 (C=O).
+ 168.6° (c = 0.50, CHCl3); UV(CHCl3) λmax (log ξ): 206 (0.56), 226 (1.55), 242 (2.86), and 265 (3.84) nm; IR (KBr) νmax: 3410, 3020, 2252, 1718, 1714, 1562, 1460, 1235, 1022 and 950 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.00 (3 H, t, 7.5 Hz, H-1), 1.28 (8H, m, H-13, H-14, H-15, H-16), 1.37 (2H, m, H-12), 1.61 (2H, m, H-17), 1.79 (2H, m, H-2), 2.04 (3H, s, COCH3), 2.09 (3H, s, COCH3), 2.13 (2H, q, 7.5 Hz, H-11), 4.08 (2H, t, 7.0 Hz, H-18), 5.33 (1H, t, 6.5 Hz, H-3), 5.47 (1H, ddt, 10.6, 7.5, 1.5 Hz, H-10), 5.66 (1H, ddt, 10.6, 8.5, 1.5 Hz, H-9), 6.13 (1H, d, 8.5 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 9.2 (C-1), 20.8 (COCH3), 21.0 (COCH3), 25.8 (C-17), 27.8 (C-11), 28.6 (C-13), 28.9 (C-14), 29.0 (C-15), 29.1 (C-16), 29.1 (C-12), 29.3 (C-2), 60.0 (C-8), 64.6 (C-3), 65.1 (C-18), 69.1 (C-6), 69.2 (C-5), 75.7 (C-4), 77.5 (C-7), 123.6 (C-9), 136.1 (C-10), 169.4 (C=O), 169.7 (C=O). + 236.7° (c = 0.61, CHCl3); UV(CHCl3) λmax (log ξ): 210 (1.46), 228 (2.15), 246 (3.71), and 274 (4.65) nm; IR (KBr) νmax: 3380, 3010, 2252, 1718, 1713, 1568, 1438, 1310, 1016 and 890 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.31 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.61 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.14 (2H, q, 7.5 Hz, H-11), 4.09 (2H t, 7.5 Hz, H-17), 5.33 (1H, d, 10.5 Hz, H-1a), 5.46 (1H, dt, 10.5, 7.5 Hz, Hz, H-10), 5.53 (1H, dd, 17.5, 1.5 Hz, H-1b), 5.66 (1H, ddt, 10.5, 7.5, 1.0Hz, H-9), 5.85 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-2), 5.90 (1H, brd, 5.0 Hz, H-3), 6.13 (1H, d, 8.0 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 20.8 (COCH3), 20.9 (COCH3), 25.8 (C-17), 27.8 (C-16), 28.6 (C-14), 29.0 (C-15), 29.0 (C-13), 29.1 (C-11), 29.2 (C-12), 60.0 (C-8), 64.3 (C-3), 64.6 (C-18), 69.1 (C-6), 70.7 (C-5), 75.1 (C-4), 76.7 (C-7), 119.7 (C-1), 123.7 (C-9), 131.9 (C-10), 136.3 (C-2), 169.3(C=O), 169.4 (C=O).
+ 236.7° (c = 0.61, CHCl3); UV(CHCl3) λmax (log ξ): 210 (1.46), 228 (2.15), 246 (3.71), and 274 (4.65) nm; IR (KBr) νmax: 3380, 3010, 2252, 1718, 1713, 1568, 1438, 1310, 1016 and 890 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.31 (8H, m, H-13, H-14, H-15, H-16), 1.38 (2H, m, H-12), 1.61 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.07 (3H, s, COCH3), 2.14 (2H, q, 7.5 Hz, H-11), 4.09 (2H t, 7.5 Hz, H-17), 5.33 (1H, d, 10.5 Hz, H-1a), 5.46 (1H, dt, 10.5, 7.5 Hz, Hz, H-10), 5.53 (1H, dd, 17.5, 1.5 Hz, H-1b), 5.66 (1H, ddt, 10.5, 7.5, 1.0Hz, H-9), 5.85 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-2), 5.90 (1H, brd, 5.0 Hz, H-3), 6.13 (1H, d, 8.0 Hz, H-8). 13C-NMR (125 MHz, CDCl3): δc 20.8 (COCH3), 20.9 (COCH3), 25.8 (C-17), 27.8 (C-16), 28.6 (C-14), 29.0 (C-15), 29.0 (C-13), 29.1 (C-11), 29.2 (C-12), 60.0 (C-8), 64.3 (C-3), 64.6 (C-18), 69.1 (C-6), 70.7 (C-5), 75.1 (C-4), 76.7 (C-7), 119.7 (C-1), 123.7 (C-9), 131.9 (C-10), 136.3 (C-2), 169.3(C=O), 169.4 (C=O). + 216.8° (c = 0.26, CHCl3); UV(CHCl3) λmax (log ξ): 206 (0.86), 228 (2.35), 247 (3.75), and 270 (4.25) nm; IR (KBr) νmax: 3460, 3010, 2250, 1716, 1562, 1442, 1320, 1038 and 860 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.31 (8H, m, H-13, H-14, H-15, H-16), 1.37 (2H, m, H-12), 1.61 (2H, m, H-17), 2.07 (3H, s, COCH3), 2.14 (2H, q, 7.5 Hz, H-11), 4.09 (2H t, 7.5 Hz, H-17), 4.33 (1H, brd, 5.0 Hz, H-3), 5.33 (1H, d, 10.5 Hz, H-1a), 5.48 (1H, dt, 10.5, 7.5 Hz, Hz, H-10), 5.52 (1H, dd, 17.5, 1.5 Hz, H-1b), 5.66 (1H, ddt, 10.5, 7.5, 1.0Hz, H-9), 5.92 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-2), 6.13 (1H, d, 8.0 Hz, H-8). 13C-NMR (125 MHz, CDCl3): 20.9 (COCH3), 25.8 (C-17), 27.8 (C-16), 28.6 (C-14), 29.0 (C-15), 29.0 (C-13), 29.1 (C-11), 29.2 (C-12), 61.3 (C-8), 63.3 (C-3), 64.6 (C-18), 69.1 (C-6), 70.6 (C-5), 75.6 (C-4), 79.8 (C-7), 119.7 (C-1), 127.8 (C-10), 132.1 (C-9), 136.4 (C-2), 169.4 (C=O).
+ 216.8° (c = 0.26, CHCl3); UV(CHCl3) λmax (log ξ): 206 (0.86), 228 (2.35), 247 (3.75), and 270 (4.25) nm; IR (KBr) νmax: 3460, 3010, 2250, 1716, 1562, 1442, 1320, 1038 and 860 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.31 (8H, m, H-13, H-14, H-15, H-16), 1.37 (2H, m, H-12), 1.61 (2H, m, H-17), 2.07 (3H, s, COCH3), 2.14 (2H, q, 7.5 Hz, H-11), 4.09 (2H t, 7.5 Hz, H-17), 4.33 (1H, brd, 5.0 Hz, H-3), 5.33 (1H, d, 10.5 Hz, H-1a), 5.48 (1H, dt, 10.5, 7.5 Hz, Hz, H-10), 5.52 (1H, dd, 17.5, 1.5 Hz, H-1b), 5.66 (1H, ddt, 10.5, 7.5, 1.0Hz, H-9), 5.92 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-2), 6.13 (1H, d, 8.0 Hz, H-8). 13C-NMR (125 MHz, CDCl3): 20.9 (COCH3), 25.8 (C-17), 27.8 (C-16), 28.6 (C-14), 29.0 (C-15), 29.0 (C-13), 29.1 (C-11), 29.2 (C-12), 61.3 (C-8), 63.3 (C-3), 64.6 (C-18), 69.1 (C-6), 70.6 (C-5), 75.6 (C-4), 79.8 (C-7), 119.7 (C-1), 127.8 (C-10), 132.1 (C-9), 136.4 (C-2), 169.4 (C=O). + 176.4° (c = 0.16, CHCl3); UV(CHCl3) λmax (log ξ): 203 (0.75), 225 (2.10), 248 (2.65), and 266 (4.86) nm; IR (KBr) νmax :3410, 3022, 2258, 1720, 1582, 1432, 1360, 1024 and 860 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.31 (8H, m, H-13, H-14, H-15, H-16), 1.37 (2H, m, H-12), 1.61 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.14 (2H, q, 7.5 Hz, H-11), 4.09 (2H t, 7.5 Hz, H-17), 5.13 (1H, d, 8.0 Hz, H-8) 5.33 (1H, d, 10.5 Hz, H-1a), 5.48 (1H, dt, 10.5, 7.5 Hz, Hz, H-10), 5.52 (1H, dd, 17.5, 1.5 Hz, H-1b), 5.68 (1H, ddt, 10.5, 7.5, 1.0Hz, H-9), 5.90 (1H, brd, 5.0 Hz, H-3), 5.92 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-2). 13C-NMR (125 MHz, CDCl3): 20.9 (COCH3), 25.8 (C-17), 27.8 (C-16), 28.6 (C-14), 29.0 (C-15), 29.0 (C-13), 29.1 (C-11), 29.2 (C-12), 61.3 (C-8), 63.3 (C-3), 64.6 (C-18), 69.1 (C-6), 70.6 (C-5), 75.5 (C-4), 77.8 (C-7), 117.2 (C-1), 129.2 (C-10), 134.4 (C-9), 136.4 (C-2), 169.3 (C=O).
+ 176.4° (c = 0.16, CHCl3); UV(CHCl3) λmax (log ξ): 203 (0.75), 225 (2.10), 248 (2.65), and 266 (4.86) nm; IR (KBr) νmax :3410, 3022, 2258, 1720, 1582, 1432, 1360, 1024 and 860 cm−1; 1H-NMR (500 MHz, CDCl3): δH 1.31 (8H, m, H-13, H-14, H-15, H-16), 1.37 (2H, m, H-12), 1.61 (2H, m, H-17), 2.04 (3H, s, COCH3), 2.14 (2H, q, 7.5 Hz, H-11), 4.09 (2H t, 7.5 Hz, H-17), 5.13 (1H, d, 8.0 Hz, H-8) 5.33 (1H, d, 10.5 Hz, H-1a), 5.48 (1H, dt, 10.5, 7.5 Hz, Hz, H-10), 5.52 (1H, dd, 17.5, 1.5 Hz, H-1b), 5.68 (1H, ddt, 10.5, 7.5, 1.0Hz, H-9), 5.90 (1H, brd, 5.0 Hz, H-3), 5.92 (1H, ddd, 17.0, 10.0, 1.5 Hz, H-2). 13C-NMR (125 MHz, CDCl3): 20.9 (COCH3), 25.8 (C-17), 27.8 (C-16), 28.6 (C-14), 29.0 (C-15), 29.0 (C-13), 29.1 (C-11), 29.2 (C-12), 61.3 (C-8), 63.3 (C-3), 64.6 (C-18), 69.1 (C-6), 70.6 (C-5), 75.5 (C-4), 77.8 (C-7), 117.2 (C-1), 129.2 (C-10), 134.4 (C-9), 136.4 (C-2), 169.3 (C=O).3.7. Anticancer Assay
3.7.1. Cancer Cell Lines and Culture
3.7.2. Measurement of Cell Viability
3.7.3. Hoechst Staining
3.7.4. Measurement of Mitochondrial Membrane Potential
3.7.5. Cell Cycle Analysis by Flow Cytometry
3.7.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christensen, L.P. Aliphatic C(17)-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat. Food Nutr. Agric. 2011, 3, 64–77. [Google Scholar] [CrossRef]
- Pan, Y.; Lowary, T.L.; Tykwinski, R.R. Naturally occurring and synthetic polyyne glycosides. Can. J. Chem. 2009, 87, 1565–1582. [Google Scholar] [CrossRef]
- Siddiq, A.; Dembitsky, V. Acetylenic anticancer agents. Anti-Cancer Agent. Med. Chem. 2008, 8, 132–170. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef]
- Shi Shun, A.L.K.; Tykwinski, R.R. Synthesis of naturally occurring polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Metzger, B.T.; Barnes, D.M. Polyacetylene diversity and bioactivity in orange market and locally grown colored carrots (Daucus carota L.). J. Agric. Food Chem. 2009, 57, 11134–11139. [Google Scholar] [CrossRef]
- Metzger, B.T.; Barnes, D.M.; Reed, J.D. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008, 56, 3554–3560. [Google Scholar] [CrossRef]
- Li, C.; Lee, D.; Graf, T.N.; Phifer, S.S.; Nakanishi, Y.; Riswan, S.; Setyowati, F.M.; Saribi, A.M.; Soejarto, D.D.; Farnsworth, N.R.; et al. Bioactive constituents of the stem bark of mitrephora glabra. J. Nat. Prod. 2009, 72, 1949–1953. [Google Scholar] [CrossRef]
- Lantz, T.C.; Antos, J.A. Clonal expansion in the deciduous understory shrub, Devil's club (Oplopanax horridus; Araliaceae). Can. J. Bot. 2002, 80, 1052–1062. [Google Scholar] [CrossRef]
- Nukhimovskii, E.L.; Zhurba, O.V. Ecological morphology of certain medicinal plants growing under natural conditions 8. Oplopanax elatus. Rastitel'nye Resursy 1979, 15, 507–516. [Google Scholar]
- Smith, G.W. Arctic pharmacognosia .2. devils club, Oplopanax horridus. J. Ethnopharm. 1983, 7, 313–320. [Google Scholar]
- Gottesfeld, L.M.J. The importance of bark products in the aboriginal economies of northwestern british-columbia, Canada. Econ. Bot. 1992, 46, 148–157. [Google Scholar] [CrossRef]
- Gottesfeld, L.M.J. Wet'Suwet'en ethnobotany: Traditional plant uses. J. Ethnobiol. 1994, 14, 185–210. [Google Scholar]
- McCutcheon, A.R.; Roberts, T.E.; Gibbons, E.; Ellis, S.M.; Babiuk, L.A.; Hancock, R.E.W.; Towers, G.H.N. Antiviral screening of British Columbian medicinal plants. J. Ethnopharm. 1995, 49, 101–110. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.L.; Wang, C.Z.; Williams, S.; Yuan, C.S. Improving anticancer activities of Oplopanax horridus root bark extract by removing water-soluble components. Phytother. Res. 2010, 24, 1166–1174. [Google Scholar]
- Sun, S.; Du, G.J.; Qi, L.W.; Williams, S.; Wang, C.Z.; Yuan, C.S. Hydrophobic constituents and their potential anticancer activities from Devil's Club (Oplopanax horridus Miq.). J. Ethnopharm. 2010, 132, 280–285. [Google Scholar] [CrossRef]
- Li, X.L.; Sun, S.; Du, G.J.; Qi, L.W.; Williams, S.; Wang, C.Z.; Yuan, C.S. Effects of Oplopanax horridus on human colorectal cancer cells. Anticancer Res. 2010, 30, 295–302. [Google Scholar]
- Huang, W.H.; Zhang, Q.W.; Wang, C.Z.; Yuan, C.S.; Li, S.P. Isolation and identification of two new polyynes from a north american ethnic medicinal plant-Oplopanax horridus (Smith) miq. Molecules 2010, 15, 1089–1096. [Google Scholar] [CrossRef]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C(17)-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar]
- Wang, C.Z.; Zhang, Z.; Huang, W.H.; Du, G.J.; Wen, X.D.; Calway, T.; Yu, C.; Nass, R.; Zhao, J.; Du, W.; et al. Identification of potential anticancer compounds from Oplopanax horridus. Phytomedicine 2013, 20, 999–1006. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S.S.; Ou, D.; Warnock, G.L.; Hasman, D. Antiproliferation activity of Devil's club (Oplopanax horridus) and anticancer agents on human pancreatic cancer multicellular spheroids. Phytomedicine 2014, 21, 506–514. [Google Scholar] [CrossRef]
- Huang, W.H.; Zhang, Q.W.; Yuan, C.S.; Wang, C.Z.; Li, S.P.; Zhou, H.H. Chemical constituents of the plants from the genus Oplopanax. Chem. Biodiver. 2014, 11, 181–196. [Google Scholar] [CrossRef]
- McGill, C.M.; Alba-Rodriguez, E.J.; Li, S.; Benson, C.J.; Ondrasik, R.M.; Fisher, L.N.; Claxton, D.F.; Barth, B.M. Extracts of Devil's club (Oplopanax horridus) exert therapeutic efficacy in experimental models of acute myeloid leukemia. Phytother. Res. 2014, 28. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Meng, L.-Z.; Huang, W.-H.; Wang, C.-Z.; Yuan, C.-S.; Li, S.-P. Anticancer Activities of Polyynes from the Root Bark of Oplopanax horridus and Their Acetylated Derivatives. Molecules 2014, 19, 6142-6162. https://doi.org/10.3390/molecules19056142
Meng L-Z, Huang W-H, Wang C-Z, Yuan C-S, Li S-P. Anticancer Activities of Polyynes from the Root Bark of Oplopanax horridus and Their Acetylated Derivatives. Molecules. 2014; 19(5):6142-6162. https://doi.org/10.3390/molecules19056142
Chicago/Turabian StyleMeng, Lan-Zhen, Wei-Hua Huang, Chong-Zhi Wang, Chun-Su Yuan, and Shao-Ping Li. 2014. "Anticancer Activities of Polyynes from the Root Bark of Oplopanax horridus and Their Acetylated Derivatives" Molecules 19, no. 5: 6142-6162. https://doi.org/10.3390/molecules19056142
APA StyleMeng, L.-Z., Huang, W.-H., Wang, C.-Z., Yuan, C.-S., & Li, S.-P. (2014). Anticancer Activities of Polyynes from the Root Bark of Oplopanax horridus and Their Acetylated Derivatives. Molecules, 19(5), 6142-6162. https://doi.org/10.3390/molecules19056142