Distribution of Nucleosides in Populations of Cordyceps cicadae
Abstract
:1. Introduction


| Province | Location |
|---|---|
| Yunnan | Mojiang, Fengyang [13] Lanping, Weixi, Xianggelila, Zhaotong and Kunming |
| Sichuan | Mount Emei, Qingcheng mountain and Qingyun mountain [13] and Xiangcheng |
| Guizhou | Fanjing mountain, Libo karst geopark, Guiyang forest park and Huaxi [13,14] |
| Jiangsu | Yixing |
| Guangxi | Leye [12] |
| Hainan | Wuzhi mountain [12] |
| Fujian | Wushan in Fuzhou [13] |
| Shanghai | Tianma mountain [15] |
| Zhejiang | Hangzhou [16] |
| Guangdong [11] | — a |
| Hunan [11] | — |
| Hubei [11] | — |
2. Results and Discussions
2.1. Descriptive Statistical Analysis
| Pop | Position | Contents (mean (µg/g)/CV (%)) | Total amount (µg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uracil | Uridine | 2'-Deoxyuridine | Inosine | Guanosine | Adenine | Thymidine | Adenosine | 2'-deoxyadenosine | Cordycepin | |||
| CCKSG | coremium | 444.47/33.73 | 1577.49/19.00 | 350.41/95.22 | 1166.62/10.33 | 733.03/42.09 | 166.17/32.36 | 83.81/29.20 | 837.00/39.93 | 54.62/25.13 | — b | 5413.62 |
| sclerotium | 315.63/62.24 | 774.72/41.32 | 57.24/49.62 | 198.44/77.06 | 314.61/48.99 | 99.15/34.95 | 75.02/39.66 | 510.99/74.40 | 52.94/26.47 | — | 2398.75 | |
| CCLHH | coremium | 334.54/27.49 | 1587.46/12.93 | 119.21/40.45 | 291.28/54.31 | 1330.38/12.14 | 72.39/32.70 | 43.05/34.95 | 857.36/21.13 | 32.81/28.25 | — | 4668.47 |
| sclerotium | 149.32/26.57 | 1353.86/36.23 | 62.34/81.92 | 98.06/44.89 | 720.48/34.92 | 77.91/71.95 | 138.32/126.94 | 742.77/36.52 | 120.84/155.87 | — | 3463.90 | |
| CCLHQ | coremium | 371.92/38.41 | 1560.08/29.22 | 299.71/70.19 | 490.96/65.31 | 1003.10/37.98 | 67.06/44.30 | 33.06/53.97 | 723.85/29.64 | 15.61/66.45 | — | 4565.36 |
| sclerotium | 266.09/29.50 | 1221.69/17.30 | 85.03/33.91 | 152.94/33.56 | 648.18/18.81 | 62.09/43.60 | 43.49/84.05 | 575.99/16.42 | 22.74/47.99 | — | 3078.23 | |
| CCLHX | coremium | 351.85/22.23 | 1365.09/14.30 | 100.01/41.75 | 267.39/20.71 | 867.27/8.36 | 58.80/32.49 | 28.27/75.87 | 811.56/10.33 | 25.17/42.68 | — | 3875.42 |
| sclerotium | 243.02/25.84 | 1224.83/17.04 | 48.13/62.70 | 95.47/16.39 | 626.64/10.77 | 55.06/39.08 | 68.09/108.80 | 629.61/11.29 | 34.13/98.29 | — | 3024.97 | |
| CCLSH | coremium | 279.84/40.87 | 1676.29/36.07 | 88.49/61.11 | 456.96/65.69 | 1091.44/40.48 | 66.65/57.11 | 21.56/55.73 | 727.60/39.93 | 14.31/53.31 | — | 4423.14 |
| sclerotium | 237.62/20.18 | 1387.93/20.31 | 53.99/48.53 | 181.70/43.04 | 614.19/12.73 | 76.20/29.13 | 45.70/44.16 | 585.88/26.66 | 25.17/41.58 | — | 3208.38 | |
| CCLTD | coremium | 305.86/23.19 | 1278.23/21.53 | 94.33/33.31 | 409.24/58.69 | 751.05/17.91 | 43.21/41.17 | 27.58/64.09 | 749.17/21.50 | 35.72/46.07 | — | 3694.39 |
| sclerotium | 204.83/21.19 | 1117.27/22.69 | 40.83/39.27 | 159.18/52.50 | 661.92/11.23 | 43.13/54.55 | 52.58/73.60 | 678.05/18.48 | 44.48/48.67 | — | 3002.28 | |
| CCLTL | coremium | 289.52/37.70 | 1460.48/21.09 | 169.42/62.02 | 418.79/82.36 | 1207.12/39.58 | 51.70/39.76 | 17.73/91.67 | 923.07/21.93 | 13.01/53.70 | — | 4550.85 |
| sclerotium | 191.12/28.90 | 1159.95/28.57 | 63.93/53.47 | 96.83/33.11 | 608.91/16.31 | 61.13/100.84 | 59.21/73.47 | 564.37/15.25 | 32.67/57.02 | — | 2838.13 | |
| CCLZD | coremium | 404.82/23.43 | 1928.73/19.57 | 263.10/84.10 | 587.61/66.12 | 1347.46/36.69 | 75.06/66.30 | 45.65/60.17 | 994.31/27.95 | 31.48/47.22 | — | 5678.21 |
| sclerotium | 261.29/23.35 | 1587.68/26.09 | 80.34/50.33 | 179.66/42.85 | 802.61/14.57 | 60.07/43.57 | 91.87/74.81 | 715.34/13.00 | 55.68/43.89 | — | 3834.54 | |
| CCWYJ | coremium | 334.14/38.45 | 1890.68/35.48 | 177.81/85.93 | 391.80/61.14 | 1483.06/36.78 | 67.02/43.24 | 49.37/40.09 | 1153.78/37.73 | 42.67/39.45 | — | 5590.34 |
| sclerotium | 207.19/25.52 | 1516.08/28.42 | 40.94/81.69 | 105.91/43.88 | 859.40/25.08 | 52.03/40.70 | 101.56/62.21 | 961.26/23.62 | 97.28/67.09 | — | 3941.64 | |
| CCYTS | coremium | 329.00/39.83 | 363.30/81.78 | 49.89/78.96 | 79.65/72.59 | 351.44/58.24 | 33.63/44.80 | 39.51/51.17 | 201.54/56.48 | 15.94/112.36 | — | 1463.89 |
| sclerotium | 187.25/32.74 | 520.61/19.82 | 23.97/44.95 | 74.61/42.69 | 260.00/30.74 | 33.93/32.31 | 29.55/77.86 | 230.20/35.14 | 9.68/89.86 | — | 1369.80 | |
| OSDQI | stroma | 353.39/45.17 | 2276.38/23.94 | 6.67/138.48 | 155.27/22.06 | 1599.02/9.48 | 178.67/65.91 | 63.85/15.68 | 1685.50/8.50 | 47.01/18.41 | — | 6365.76 |
| sclerotium | 213.93/60.96 | 1432.24/22.90 | 16.76/136.97 | 430.95/9.82 | 864.00/44.40 | 91.23/25.40 | 124.32/19.25 | 675.15/70.58 | 49.26/25.25 | — | 3897.84 | |
| OSLTA | stroma | 55.31/24.31 | 1572.67/13.23 | — | 141.80/50.78 | 1176.66/10.46 | 79.75/24.11 | 24.03/44.75 | 1388.17/11.95 | 46.75/22.65 | — | 4485.14 |
| sclerotium | 48.18/43.55 | 1468.93/19.52 | — | 615.71/20.66 | 978.12/4.53 | 62.08/29.11 | 69.50/24.76 | 451.90/47.68 | 52.18/33.81 | — | 3746.60 | |
| OSMNI | stroma | 170.45/47.78 | 1387.75/7.94 | — | 96.01/40.87 | 1481.03/8.16 | 108.00/17.00 | 52.05/29.99 | 1619.77/12.70 | 44.54/32.96 | — | 4959.60 |
| sclerotium | 116.40/15.01 | 1255.39±/5.47 | — | 399.60/12.43 | 1070.80/3.27 | 99.19/24.49 | 147.95/12.60 | 752.39/8.92 | 58.51/17.55 | — | 3900.23 | |
| OSNBE | stroma | 149.76/11.15 | 2765.61/2.92 | 7.70/19.07 | 580.85/17.06 | 2130.34/3.59 | 113.59/21.67 | 58.34/26.80 | 2544.76/4.02 | 46.77/24.73 | — | 8397.72 |
| sclerotium | 127.95/15.59 | 1599.98/6.53 | 9.92/27.35 | 2073.63/18.43 | 1131.88/4.76 | 154.86/11.56 | 177.09/9.04 | 1081.60/8.63 | 106.28/17.61 | — | 6463.19 | |
| CMSMB | stroma | 319.18/19.51 | 1900.92/11.02 | 5.01/24.88 | 85.08/20.14 | 1215.38/16.31 | 313.75/20.12 | 69.41/14.69 | 1613.28/13.51 | 58.18/19.15 | 659.29/19.11 | 6239.49 |
| sclerotium | 332.93/20.04 | 1743.60/13.87 | 12.77/18.48 | 189.93/13.14 | 1075.99/16.58 | 264.18/20.17 | 68.87/16.74 | 1655.93/12.37 | 76.98/15.84 | 4173.57/13.81 | 9594.75 | |
| Species | Position | Mean content (µg/g)/CV (%) | Total amount (µg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uracil | Uridine | 2'-Deoxyuridine | Inosine | Guanosine | Adenine | Thymidine | Adenosine | 2'-deoxyadenosine | Cordycepin | |||
| C. cicadae (n c = 10) | coremium | 344.60/34.66 | 1468.78/38.58 | 171.24/104.35 | 456.03/79.55 | 1016.53/46.92 | 70.17/65.99 | 38.96/66.95 | 797.92/42.21 | 28.13/64.84 | — b | 4392.37 |
| sclerotium | 226.34/40.18 | 1186.46/37.05 | 55.68/63.15 | 134.28/60.32 | 611.69/36.79 | 62.07/59.85 | 70.54/106.70 | 619.45/40.64 | 49.56/142.54 | — | 3016.06 | |
| O. sinensis (n = 4) | stroma | 182.23/75.88 | 2000.60/31.48 | 3.59/157.80 | 243.48/86.32 | 1596.76/23.20 | 120.00/56.37 | 49.57/40.12 | 1809.55/26.14 | 46.27/23.07 | — | 6052.06 |
| sclerotium | 126.61/68.28 | 1439.14/16.90 | 6.67/192.88 | 879.97/83.68 | 1011.20/20.51 | 101.84/38.79 | 129.71/34.04 | 740.26/45.62 | 66.56/41.41 | — | 4501.96 | |
| C. militaris (n = 1) | stroma | 319.18/19.51 | 1900.92/11.02 | 5.01/24.88 | 85.08/20.14 | 1215.38/16.31 | 313.75/20.12 | 69.41/14.69 | 1613.28/13.51 | 58.18/19.15 | 659.29/19.11 | 6239.49 |
| sclerotium | 332.93/20.04 | 1743.60/13.87 | 12.77/18.48 | 189.93/13.14 | 1075.99/16.58 | 264.18/20.17 | 68.87/16.74 | 1655.93/12.37 | 76.98/15.84 | 4173.57/13.81 | 9594.75 | |
2.2. Nested Analysis
| Position | Analyte | Percent of total variance (%) | Percent of population variance (%) | Percent of individual variance (%) | F Value | Pr > F |
|---|---|---|---|---|---|---|
| coremium | uracil | 100.00 | 9.13 | 90.87 | 2.00 | 0.0477 |
| uridine | 100.00 | 52.45 | 47.55 | 12.03 | <0.0001 | |
| 2'-deoxyuridine | 100.00 | 23.83 | 76.17 | 4.13 | 0.0002 | |
| inosine | 100.00 | 54.48 | 45.52 | 12.97 | <0.0001 | |
| guanosine | 100.00 | 45.25 | 54.75 | 9.27 | <0.0001 | |
| adenine | 100.00 | 53.57 | 46.43 | 12.54 | <0.0001 | |
| thymidine | 100.00 | 44.82 | 55.18 | 9.12 | <0.0001 | |
| adenosine | 100.00 | 46.69 | 53.31 | 9.76 | <0.0001 | |
| 2'-deoxyadenosine | 100.00 | 50.39 | 49.61 | 11.16 | <0.0001 | |
| Mean | 100.00 | 42.29 | 57.71 | — | — | |
| sclerotium | uracil | 100.00 | 19.25 | 80.75 | 3.38 | 0.0013 |
| uridine | 100.00 | 47.52 | 52.48 | 10.06 | <0.0001 | |
| 2'-deoxyuridine | 100.00 | 19.19 | 80.81 | 3.37 | 0.0013 | |
| inosine | 100.00 | 22.04 | 77.96 | 3.83 | 0.0004 | |
| guanosine | 100.00 | 63.70 | 36.30 | 18.55 | <0.0001 | |
| adenine | 100.00 | 16.48 | 83.52 | 2.97 | 0.0038 | |
| thymidine | 100.00 | 9.45 | 90.55 | 2.04 | 0.0432 | |
| adenosine | 100.00 | 47.44 | 52.56 | 10.03 | <0.0001 | |
| 2'-deoxyadenosine | 100.00 | 15.38 | 84.62 | 2.82 | 0.0058 | |
| Mean | 100.00 | 28.94 | 71.06 | — | — |
2.3. Q Cluster Analysis
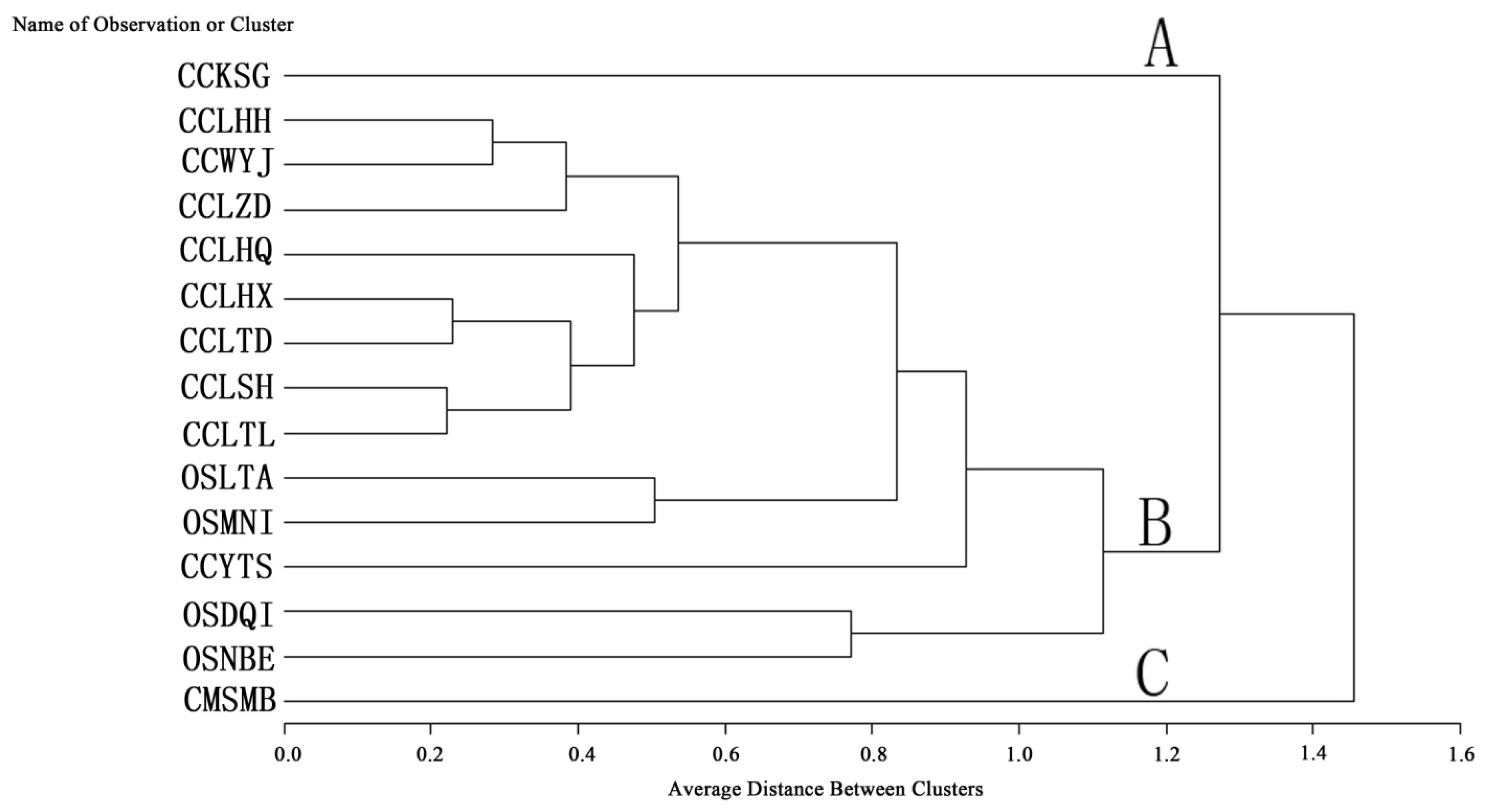

3. Experimental
3.1. Sample Preparation
| Species | NO. of populations | Samlpe size | Locus of extraction | Locality |
|---|---|---|---|---|
| C. cicadae | CCKSG | 10 | Coremium | Gelezicun, Shuanglong township, Kunming City, Yunnan |
| 10 | Sclerotium | |||
| CCLHH | 10 | Coremium | Hedongqingcun, Hexi township, Lanping county, Yunan | |
| 10 | Sclerotium | |||
| CCLHQ | 10 | Coremium | Qidenglongcun, Hexi township, Lanping county, Yunan | |
| 10 | Sclerotium | |||
| CCLHX | 10 | Coremium | Xiaqingtoucun, Hexi township, Lanping county, Yunan | |
| 10 | Sclerotium | |||
| CCLSH | 10 | Coremium | Huilongcun, Shideng township, Lanping county, Yunan | |
| 10 | Sclerotium | |||
| CCLTD | 10 | Coremium | Deqingcun, Tongdian township, Lanping county, Yunan | |
| 10 | Sclerotium | |||
| CCLTL | 10 | Coremium | Lianqiaoshacun, Tongdian township, Lanping county, Yunan | |
| 10 | Sclerotium | |||
| CCLZD | 10 | Coremium | Datujicun, Zhongpai township, Langping county, Yunnan | |
| 10 | Sclerotium | |||
| CCWYJ | 10 | Coremium | Juxiangcun, Yongchun township, Weixi county, Yunan | |
| 10 | Sclerotium | |||
| CCYTS | 10 | Coremium | Sanzhou Mountain, Taihua town, Yixing city, Jiangsu | |
| 10 | Sclerotium | |||
| O.sinensis | OSDQI | 5 | Stroma | Deqin, county, Yunnan |
| 5 | Sclerotium | |||
| OSMNI | 5 | Stroma | Manicun, Lengda township, Jiacha county, Tibet | |
| 5 | Sclerotium | |||
| OSLTA | 5 | Stroma | Litang county, Sichuan | |
| 5 | Sclerotium | |||
| OSNBE | 5 | Stroma | Nepal | |
| 5 | Sclerotium | |||
| C.militaris | CMSMB | 5 | Stroma | Baiyi township, Songming county, Yunnan |
| 5 | Sclerotium |
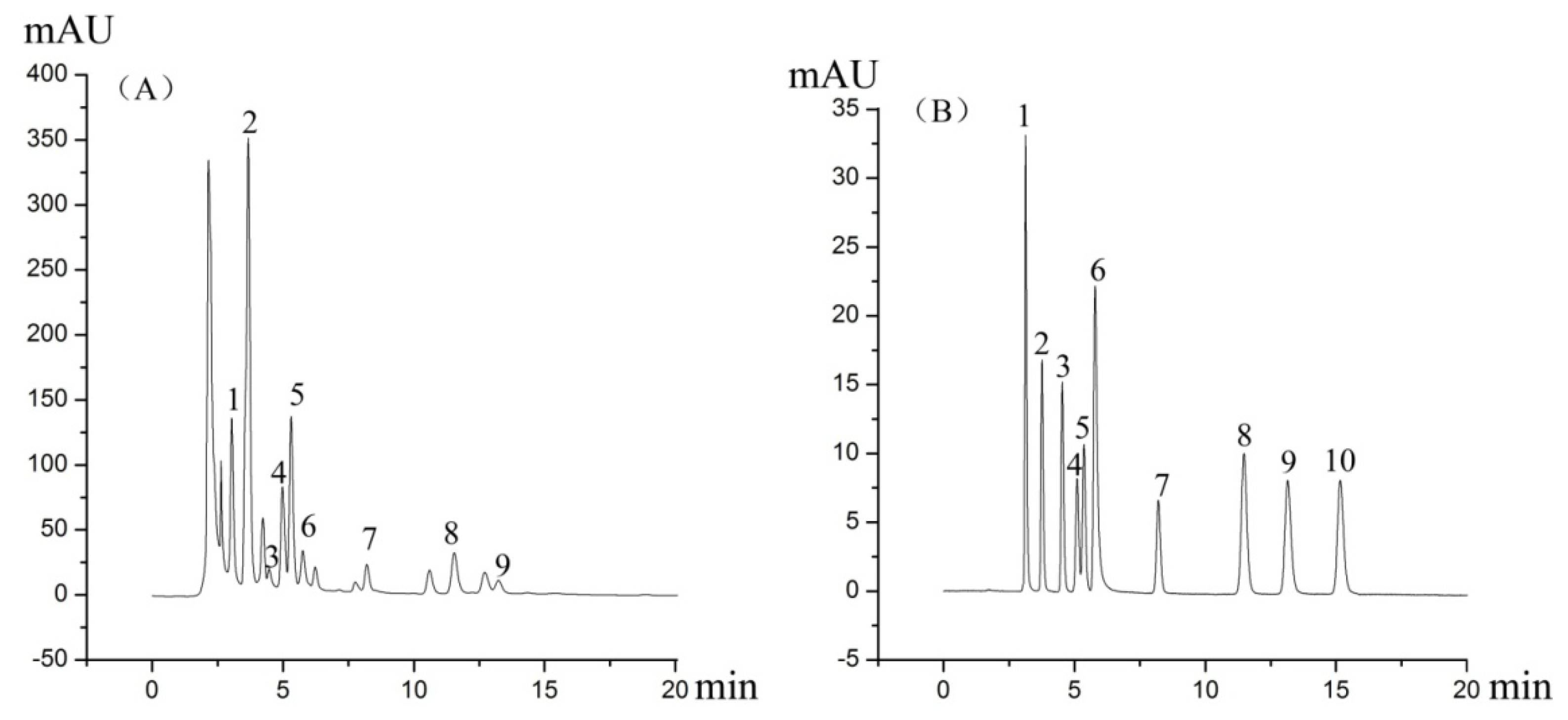
3.2. Chemicals and Reagents
3.3. Liquid Chromatography Conditions
3.4. Method Validation
3.4.1. Calibration Curves
| Analyte | λ max (nm) | Linear Regression Equation | r2 | Test Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| uracil | 260.1 | y = 0.0145x − 0.0076 | 0.9999 | 8.00–240.00 | 0.006 | 0.018 |
| uridine | 263.1 | y = 0.0233x + 0.008 | 0.9999 | 8.00–240.00 | 0.008 | 0.024 |
| 2'–deoxyuridine | 263.2 | y = 0.0234x − 0.0016 | 0.9999 | 7.00–140.00 | 0.008 | 0.024 |
| inosine | 249.8 | y = 0.0368x − 0.0479 | 0.9995 | 7.60–380.00 | 0.015 | 0.045 |
| guanosine | 254.2 | y = 0.023x + 0.029 | 0.9997 | 8.00–400.00 | 0.008 | 0.024 |
| adenine | 261.5 | y = 0.0108x | 0.9999 | 8.00–400.00 | 0.004 | 0.012 |
| thymidine | 268.2 | y = 0.0315x + 0.0009 | 0.9999 | 8.60–172.00 | 0.012 | 0.036 |
| adenosine | 261.0 | y = 0.0178x − 0.0009 | 0.9999 | 8.40–420.00 | 0.006 | 0.018 |
| 2'–deoxyadenosine | 261.1 | y = 0.0171x + 0.0025 | 0.9999 | 8.40–420.00 | 0.006 | 0.018 |
| cordycepin | 261.2 | y = 0.0189x − 0.0005 | 0.9999 | 8.40–420.00 | 0.007 | 0.021 |
3.4.2. Limits of Detection and Quantification
3.4.3. Reproducibility and Accuracy
| Analyte | Nominal Concentration (µg/mL) | Assay Value (mean ± SD) (µg/mL) | Coefficient of Variation (%) | Accuracy (%) |
|---|---|---|---|---|
| intra-day d | ||||
| uracil | 40.00 | 39.84 ± 0.50 | 1.26 | 99.60 |
| uridine | 40.00 | 39.65 ± 0.88 | 2.22 | 99.13 |
| 2'-deoxyuridine | 70.00 | 70.71 ± 1.37 | 1.94 | 101.01 |
| inosine | 38.00 | 37.65 ± 0.80 | 2.12 | 99.08 |
| guanosine | 40.00 | 40.55 ± 1.01 | 2.49 | 101.38 |
| adenine | 40.00 | 39.59 ± 0.97 | 2.45 | 98.98 |
| thymidine | 86.00 | 85.32 ± 1.15 | 1.35 | 99.21 |
| adenosine | 42.00 | 41.65 ± 0.27 | 0.65 | 99.17 |
| 2'-deoxyadenosine | 40.00 | 39.77 ± 0.62 | 1.56 | 99.43 |
| cordycepin | 42.00 | 42.39 ± 0.54 | 1.27 | 100.93 |
| Inter-day d | ||||
| uracil | 40.00 | 39.71 ± 0.48 | 1.21 | 99.28 |
| uridine | 40.00 | 39.55 ± 0.73 | 1.85 | 98.88 |
| 2'-deoxyuridine | 70.00 | 70.82 ± 1.25 | 1.77 | 101.17 |
| inosine | 38.00 | 37.62 ± 0.74 | 1.97 | 99.00 |
| guanosine | 40.00 | 40.61 ± 0.93 | 2.29 | 101.53 |
| adenine | 40.00 | 39.63 ± 0.68 | 1.72 | 99.08 |
| thymidine | 86.00 | 85.17 ± 1.27 | 1.49 | 99.03 |
| adenosine | 42.00 | 41.57 ± 0.45 | 1.08 | 98.98 |
| 2'-deoxyadenosine | 40.00 | 39.62 ± 0.84 | 2.12 | 99.05 |
| cordycepin | 42.00 | 42.49 ± 0.69 | 1.62 | 101.17 |
3.4.4. Extraction Recoveries
| Analyte | Original (µg) | Spiked Amount (µg) | Found e (mean ± SD) (µg) | Recovery f (%) | Coefficient of Variation (%) |
|---|---|---|---|---|---|
| uracil | 145.35 | 140.00 | 282.08 ± 4.89 | 98.85 | 1.73 |
| uridine | 714.12 | 700.00 | 1398.28 ± 11.60 | 98.88 | 0.83 |
| 2'-deoxyuridine | 63.85 | 60.00 | 126.87 ± 2.36 | 102.44 | 1.86 |
| inosine | 140.62 | 140.00 | 276.66 ± 2.19 | 98.59 | 0.79 |
| guanosine | 415.96 | 400.00 | 806.39 ± 3.99 | 98.83 | 0.50 |
| thymidine | 39.61 | 40.00 | 80.55 ± 1.28 | 101.18 | 1.59 |
| adenine | 31.96 | 30.00 | 61.05 ± 0.70 | 98.53 | 1.14 |
| adenosine | 375.96 | 370.00 | 736.62 ± 4.81 | 98.75 | 0.65 |
| 2'-deoxyadenosine | 25.31 | 30.00 | 54.20 ± 0.76 | 97.99 | 1.40 |
| cordycepin | — g | 150.00 | 152.12 ± 0.78 | 101.41 | 0.51 |
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Manoch, L.; Samson, R.A. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 2005, 109, 581–589. [Google Scholar] [CrossRef]
- Li, B.L. Herbal textuals research on “Chan Hua”. Chin. J. Med. Appl. Pharm. 1993, 10, 21–22. [Google Scholar]
- Chen, Z.A.; Liu, G.Y.; Hu, S.Y. Study on cultivation of Paecilomyces cicadae and its pharmacological function. Acta Mycol. Sin. 1993, 12, 138–144. [Google Scholar]
- Li, S.P.; Yang, F.Q.; Tsim, K.W.K. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. 2006, 41, 1571–1584. [Google Scholar]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef]
- Ukai, S.; Kiho, T.; Hara, C.; Morita, M.; Goto, A.; Imaizumi, N.; Hasegawa, Y. Polysaccharides in Fungi. XIII. Antitumor activity of various polysaccharides isolated from Dictyophora indusiata, Ganoderma japonicum, Cordyceps cicadae, Auricularia auricula-judae and Auricularia species. Chem. Pharm. Bull. 1983, 31, 741–744. [Google Scholar] [CrossRef]
- Weng, S.C.; Chou, C.J.; Lin, L.C.; Tsai, W.J.; Kuo, Y.C. Immunomodulatory functions of extracts from the Chinese medicinal fungus Cordyceps cicadae. J. Ethnopharmacol. 2002, 83, 79–85. [Google Scholar] [CrossRef]
- Liu, G.Y.; Hu, S.Y. Comparison of sedative and analgesic effects between Cordyceps cicadae and its cultured product. Chin. J. Med. Appl. Pharm. 1991, 8, 4–8. [Google Scholar]
- Zhu, R.; Chen, Y.P.; Deng, Y.Y.; Zheng, R.; Zhong, Y.F.; Wang, L.; Du, L.P. Cordyceps cicadae extracts ameliorate renal malfunction in a remnant kidney model. J. Zhejiang Univ. Sci. B 2011, 12, 1024–1033. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.J.; Tang, F.D. Primary exploring on pharmic effect of Cordyceps cicadae. Zhejiang J. Chin. Tradit. Med. 2001, 36, 219–220. [Google Scholar]
- Bi, S.Z.; Liu, B.; Ying, J.Z.; Shao, L.P.; Huang, N.L.; Zhang, D.Z.; Xie, Z.X.; Zang, M.; Wei, R.Q. Edible Fungal Flora of China; China Forestry Publishing House: Shanghai, China, 1991; p. 13. [Google Scholar]
- Liu, A.Y.; Li, Z.; Zhou, X.; Zhao, H.J.; Hu, H.Y.; Tan, A.J. Research and Application of Cordyceps cicadae Resources in China; Guizhou science and Technology Press: Guiyang, China, 2012; pp. 34–52. [Google Scholar]
- Liu, A.Y.; Zhou, X.; Zhao, H.J.; Liang, Z.Q.; Tan, A.J.; Zheng, Q.Y. Biological diversity of Paecilomyces cicadae I. Morphological diversity of cicadae flower and Paecilomyces cicadae. Guizhou Agric. Sci. 2007, 35, 9–11. [Google Scholar]
- Zhang, Y.J.; Liu, A.Y.; Liang, Z.Q. Formation and regeneration of protoplasts from Paecilomyces cicadae. Guizhou Agric. Sci. 1998, 26, 1–4. [Google Scholar]
- Feng, L.C. Study on TianMa Hill Cordyceps cicadae. J. Shanghai Inst. Technol. 2002, 2, 125–127. [Google Scholar]
- Chen, D.Q.; Ding, Z.S.; Lin, A.M.; Pan, P.L.; Chen, Y.T. Isolation and fermentation culture of fungi from Cordyceps cicadae. J. Chin. Med. Mater. 2006, 29, 99–101. [Google Scholar]
- Wang, Q.; Liu, Z.Y. Advances in studies on medicinal fungi Cordyceps cicadae. Chin. Tradit. Herb. Drugs 2004, 34, 469–471. [Google Scholar]
- Kiho, T.; Ito, M.; Nagai, K.; Hara, C.; Ukai, S. Polysaccharides in fungi. X XII. a water soluble polysaccharide from the alkaline extract of the insect-body portion of Chan hua (fungus: Cordyceps cicadae). Chem. Pharm. Bull. 1988, 36, 3032–3037. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Ryu, H.S.; Shin, B.R.; Kang, J.S.; Kim, H.M.; Kim, Y.O.; Hong, J.T.; Kim, Y.; Han, S.B. Phenotypic and functional maturation of dendritic cells induced by polysaccharide isolated from Paecilomyces cicadae. J. Med. Food. 2011, 14, 847–856. [Google Scholar] [CrossRef]
- Kiho, T.; Nagai, K.; Miyamoto, I.; Watanabe, T.; Ukai, S. Polysaccharides in fungi. XXV. Biological activities of two galactomannans from the insect-body portion of Chan hua (fungus: Cordyceps cicadae). Yakugaku Zasshi 1990, 110, 286–288. [Google Scholar]
- Ge, F.; Xia, C.R.; LI, C.R.; Ding, T.; Shao, Y.; Fan, M.Z. Analysis of the chemical compositions of Paecilomyces cicadae fermented mycelia and Cordyceps cicadae fruit body. Mycosystema 2007, 26, 68–75. [Google Scholar]
- Osuchowski, M.F.; Johnson, V.J.; He, Q.R.; Sharma, R.P. Myriocin, a serine palmitoyltransferase inhibitor, alters regional brain neurotransmitter levels without concurrent inhibition of the brain sphingolipid biosynthesis in mice. Toxicol. Lett. 2004, 147, 87–94. [Google Scholar] [CrossRef]
- Yu, J.W.; Xu, H.J.; Mo, Z.H.; Zhu, H.L.; Mao, X.B. Determination of myriocin in natural and cultured Cordyceps cicadae using 9-fluorenylmethyl chloroformate derivatization and high-performance liquid chromatography with UV-detection. Anal. Sci. 2009, 25, 855–859. [Google Scholar]
- Kuo, Y.C.; Weng, S.C.; Chou, C.J.; Chang, T.T.; Tsai, W.J. Activation and proliferation signals in primary human T lymphocytes inhibited by ergosterol peroxide isolated from Cordyceps cicadae. Br. J. Pharmacol. 2003, 140, 895–906. [Google Scholar] [CrossRef]
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.A. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. [Google Scholar]
- Fan, H.; Li, S.P.; Xiang, J.J.; Lai, C.M.; Yang, F.Q.; Gao, J.L.; Wang, Y.T. Qualitative and quantitative determination of nucleosides, bases and their analogues in natural and cultured Cordyceps by pressurized liquid extraction and high performance liquid chromatography–electrospray ionization tandem mass spectrometry (HPLC–ESI–MS/MS). Anal. Chim. Acta 2006, 567, 218–228. [Google Scholar] [CrossRef]
- Ikeda, R.; Nishimura, M.; Sun, Y.; Wada, M.; Nakashima, K. Simple HPLC-UV determination of nucleosides and its application to the authentication of Cordyceps and its allies. Biomed. Chromatogr. 2008, 22, 630–636. [Google Scholar] [CrossRef]
- Coradetti, R.; Conte, G.L.; Moroni, F.; Passani, M.B.; Pepeu, G. Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur. J. Pharmacol. 1984, 104, 19–26. [Google Scholar] [CrossRef]
- Schmidt, C.; Bellingham, M.C.; Richter, D.W. Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. J. Physiol. 1995, 483, 769–781. [Google Scholar]
- The Pharmacopoeia Commission of PRC. Pharmacopoeia of the People’s Republic of China, 9th ed.; Chemical Industry Publishing House: Beijing, China, 2010; p. 106. [Google Scholar]
- Benowitz, L.I.; Goldberg, D.E.; Irwin, N. Inosine stimulates axon growth in vitro and in the adult CNS. Prog. Brain Res. 2002, 137, 389–399. [Google Scholar] [CrossRef]
- Zhou, X.X.; Meyer, C.U.; Schmidtke, P.; Zepp, F. Effect of cordycepin on interleukin-10 production of human peripheral blood mononuclear cells. Eur. J. Pharmacol. 2002, 453, 309–317. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Park, S.J.; Lee, S.G.; Shin, S.C.; Choi, D.H. Cordycepin: Selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostridium spp. J. Agric. Food Chem. 2000, 48, 2744–2748. [Google Scholar] [CrossRef]
- Kodama, E.N.; McCaffrey, R.P.; Yusa, K.; Mitsuya, H. Antileukemic activity and mechanism of action of cordycepin against terminal deoxynucleotidal transferase-positive (TdT+) leukemic cells. Biochem. Pharmacol. 2000, 59, 273–281. [Google Scholar] [CrossRef]
- Li, S.P.; Li, P.; Lai, C.M.; Gong, Y.X.; Kan, K.K.W.; Dong, T.T.X.; Tsim, K.W.K.; Wang, Y.T. Simultaneous determination of ergosterol, nucleosides and their bases from natural and cultured Cordyceps by pressurised liquid extraction and high-performance liquid chromatography. J. Chromatogr. A 2004, 1036, 239–243. [Google Scholar] [CrossRef]
- Guo, F.Q.; Li, A.; Huang, L.F.; Liang, Y.Z.; Chen, B.M. Identification and determination of nucleosides in Cordyceps sinensis and its substitutes by high performance liquid chromatography with mass spectrometric detection. J. Pharm. Biomed. 2006, 40, 623–630. [Google Scholar] [CrossRef]
- Yang, F.Q.; Li, S.P. Effects of sample preparation methods on the quantification of nucleosides in natural and cultured Cordyceps. J. Pharm. Biomed. 2008, 48, 231–235. [Google Scholar] [CrossRef]
- Uauy, R.; Stringel, G.; Thomas, R.; Quan, R. Effect of dietary nucleosides on growth and maturation of the developing Gut in the Rat. J. Pediatr. Gastroenterol. Nutr. 1994, 10, 497–503. [Google Scholar]
- Carver, J.D.; Walker, W.A. The role of nucleotides in human nutrition. J. Nutr. Biochem. 1995, 6, 58–72. [Google Scholar] [CrossRef]
- Wang, S.; Yang, F.Q.; Feng, K.; Li, D.Q.; Zhao, J.; Li, S.P. Simultaneous determination of nucleosides, myriocin, and carbohydrates in Cordyceps by HPLC coupled with diode array detection and evaporative light scattering detection. J. Sep. Sci. 2009, 32, 4069–4076. [Google Scholar] [CrossRef]
- Wang, Z.B.; Li, N.; Wang, M.; Wang, Y.; Du, L.; Ji, X.F.; Yu, A.M.; Zhang, H.Q.; Qiu, F.P. Simultaneous determination of nucleosides and their bases in Cordyceps sinensis and its substitutes by matrix solid-phase dispersion extraction and HPLC. J. Sep. Sci. 2013, 36, 2348–2357. [Google Scholar] [CrossRef]
- Yang, F.Q.; Li, D.Q.; Feng, K.; Hu, D.J.; Li, S.P. Determination of nucleotides, nucleosides and their transformation products in Cordyceps by ion-pairing reversed-phase liquid chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 5501–5510. [Google Scholar] [CrossRef]
- Huang, L.F.; Liang, Y.Z.; Guo, F.Q.; Zhou, Z.F.; Cheng, B.M. Simultaneous separation and determination of active components in Cordyceps sinensis and Cordyceps militaris by LC/ESI-MS. J. Pharm. Biomed. 2003, 33, 1155–1162. [Google Scholar] [CrossRef]
- Yang, F.Q.; Ge, L.Y.; Yong, J.W.H.; Tan, S.N.; Li, S.P. Determination of nucleosides and nucleobases in different species of Cordyceps by capillary electrophoresis-mass spectrometry. J. Pharm. Biomed. 2009, 50, 307–314. [Google Scholar] [CrossRef]
- Gong, Y.X.; Li, S.P.; Li, P.; Liu, J.J.; Wang, Y.T. Simultaneous determination of six main nucleosides and bases in natural and cultured Cordyceps by capillary electrophoresis. J. Chromatogr. A 2004, 1055, 215–221. [Google Scholar] [CrossRef]
- Yang, F.Q.; Li, S.P.; Li, P.; Wang, Y.T. Optimization of CEC for simultaneous determination of eleven nucleosides and nucleobases in Cordyceps using central composite design. Electrophoresis 2007, 28, 1681–1688. [Google Scholar] [CrossRef]
- Yang, F.Q.; Guan, J.; Li, S.P. Fast simultaneous determination of 14 nucleosides and nucleobases in cultured Cordyceps using ultra-performance liquid chromatography. Talanta 2007, 73, 269–273. [Google Scholar] [CrossRef]
- Ling, J.Y.; Sun, Y.J.; Zhang, H.; Lv, P.; Zhang, C.K. Measurement of cordycepin and adenosine in stroma of Cordyceps sp. by capillary zone electrophoresis (CZE). J. Biosci. Bioeng. 2002, 94, 371–374. [Google Scholar] [CrossRef]
- Li, S.P.; Li, P.; Ji, H.; Zhang, P.; Dong, T.T.X.; Tsim, K.W.K. The contents and their change of nucleosides from natural Cordyceps sinensis and cultured Cordyceps mycelia. Acta Pharm. Sin. 2001, 36, 436–439. [Google Scholar]
- Rao, Y.K.; Chou, C.H.; Tzeng, Y.M. A simple and rapid method for identification and determination of cordycepin in Cordyceps militaris by capillary electrophoresis. Anal. Chim. Acta 2006, 566, 253–258. [Google Scholar] [CrossRef]
- Yuan, J.P.; Zhao, S.Y.; Wang, J.H.; Kuang, H.C.; Liu, X. Distribution of nucleosides and nucleobases in Edible Fungi. J. Agric. Food Chem. 2008, 56, 809–815. [Google Scholar] [CrossRef]
- Gu, Y.X.; Wang, Z.S.; Li, S.X.; Yuan, Q.S. Effect of multiple factors on accumulation of nucleosides and bases in Cordyceps militaris. Food Chem. 2007, 102, 1304–1309. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, J.; Li, S.P.; Fan, H.; Hong, M.; Wang, Y.T.; Zhu, Q. Quality evaluation of Cordyceps through simultaneous determination of eleven nucleosides and bases by RP-HPLC. J. Sep. Sci. 2006, 29, 953–958. [Google Scholar] [CrossRef]
- Li, S.P.; Su, Z.R.; Dong, T.T.X.; Tsim, K.W.K. The fruiting body and its caterpillar host of Cordyceps sinensis show close resemblance in main constituents and anti-oxidation activity. Phytomedicine 2002, 9, 319–324. [Google Scholar] [CrossRef]
- Xie, J.W.; Huang, L.F.; Hu, W.; He, Y.B.; Wong, K.P. Analysis of the main nucleosides in Cordyceps sinensis by LC/ESI-MS. Molecules 2010, 15, 305–314. [Google Scholar] [CrossRef]
- Hsu, T.H.; Shiao, L.H.; Hsieh, C.; Chang, D.M. A comparison of the chemical composition and bioactive ingredients of the Chinese medicinal mushroom DongChongXiaCao, its counterfeit and mimic, and fermented mycelium of Cordyceps sinensis. Food Chem. 2002, 78, 463–469. [Google Scholar] [CrossRef]
- Zhu, J.S.; Halpern, G.M.; Jones, K. The scientific rediscovery of an ancient Chinese herbal medicine: Cordyceps sinensis: Part I. J. Altern. Complement. Med. 1998, 4, 289–303. [Google Scholar] [CrossRef]
- Zhu, J.S.; Halpern, G.M.; Jones, K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: Part II. J. Altern. Complement. Med. 1998, 4, 429–457. [Google Scholar] [CrossRef]
- Li, S.P.; Li, P.; Dong, T.T.X.; Tsim, K.W.K. Determination of nucleosides in natural Cordyceps sinensis and cultured Cordyceps mycelia by capillary electrophoresis. Electrophoresis 2001, 22, 144–150. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, K.; Meng, S.; Chu, Z.Y. Chemical constituents of the dry sorophore of Cordyceps militaris. Acta Pharm. Sin. 2000, 35, 663–668. [Google Scholar]
- Yu, H.M.; Wang, B.S.; Huang, S.C.; Duh, P.D. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis on oxidative damage. J. Agric. Food Chem. 2006, 54, 3132–3138. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zeng, W.-B.; Yu, H.; Ge, F.; Yang, J.-Y.; Chen, Z.-H.; Wang, Y.-B.; Dai, Y.-D.; Adams, A. Distribution of Nucleosides in Populations of Cordyceps cicadae. Molecules 2014, 19, 6123-6141. https://doi.org/10.3390/molecules19056123
Zeng W-B, Yu H, Ge F, Yang J-Y, Chen Z-H, Wang Y-B, Dai Y-D, Adams A. Distribution of Nucleosides in Populations of Cordyceps cicadae. Molecules. 2014; 19(5):6123-6141. https://doi.org/10.3390/molecules19056123
Chicago/Turabian StyleZeng, Wen-Bo, Hong Yu, Feng Ge, Jun-Yuan Yang, Zi-Hong Chen, Yuan-Bing Wang, Yong-Dong Dai, and Alison Adams. 2014. "Distribution of Nucleosides in Populations of Cordyceps cicadae" Molecules 19, no. 5: 6123-6141. https://doi.org/10.3390/molecules19056123
APA StyleZeng, W.-B., Yu, H., Ge, F., Yang, J.-Y., Chen, Z.-H., Wang, Y.-B., Dai, Y.-D., & Adams, A. (2014). Distribution of Nucleosides in Populations of Cordyceps cicadae. Molecules, 19(5), 6123-6141. https://doi.org/10.3390/molecules19056123




