Abstract
Diastereoselective reactions between 4-formylpyrazoles, N-substituted maleimides and glycine derivates led to new series of pyrazolyldipyrrolo [3,4-a:3',4'-f]pyrrolizines and pyrazolylpyrrolo[3,4-c]pyrroles in good yields. The reactions proceeded by a domino process through azomethine ylides formed in situ via a 1,3-dipolar cycloaddition reaction.
1. Introduction
Multicomponent reactions (MCRs) are one of the most powerful tools in organic synthesis allowing the formation of several bonds in one step to obtain products with high structural diversity and/or molecular complexity [1,2,3]. Furthermore, the development of fast, selective and environmentally friendly MCRs involving domino processes with step and atom economy are of great importance for medicinal and synthetic chemistry [4,5,6].
The dipolar cycloaddition reaction is a known and widely studied method in organic synthesis to obtain pyrrolidine derivatives from the reaction of azomethine ylides, generated in situ, and electron-deficient olefins [7,8,9]. These five-membered ring systems belong to an important class of aza-compounds with multiple applications, for example, in the development of bioactive molecules, organocatalysts, new materials and as scaffolds in total organic synthesis [10,11,12,13].
Some interesting fused pyrrolidine systems as cyclopiazonic acid, granulatimide and isogranulatimide (Figure 1) are natural alkaloids that displayed important activities as Chk1 inhibitors and antiplasmodial agents [14,15]. Pyrrolizines are alkaloids generally isolated from plants, insects, bacteria or fungi [16,17] that have exhibited important antiproliferative activities [18,19]. In the same way, the pyrrolo[3,4-c]pyrroles have been widely applied in a variety of fields such as materials sciences, pharmaceuticals and agrochemistry [20,21,22].

Figure 1.
Pyrrolidine systems present in natural alkaloids.
Pyrazole is another five-membered ring with many applications in chemistry, especially as pharmaceuticals, pesticides and lubricants [23,24,25,26,27]. In connection with our current studies on the development of new, selective, and environmentally friendly methodologies for the synthesis of fused heterocycles [28,29,30,31], herein we report a procedure for the preparation of the scarcely studied pyrazolylpyrrolizine derivates 4 and pyrazolylpyrrolidine derivatives 5 and 6 where three moieties of known importance (i.e., pyrazole, pyrrolidine, pyrrolizine) are incorporated into a single structure. The new compounds were obtained in good yields and high diastereoselectivity by a catalyst-free three-component domino reaction between formylpyrazoles 1, N-arylmaleimides 2 and glycine-derived esters 3 (Scheme 1).
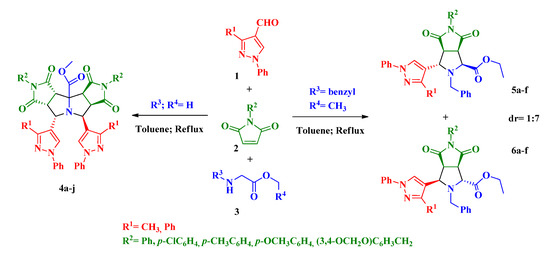
Scheme 1.
Proposed synthesis of new pyrazolylpyrrolopyrrolizine derivates 4 and pyrazolylpyrrolopyrrolidine derivates 5 and 6.
2. Results and Discussion
2.1. Synthesis of Pyrazolyldipyrrolo[3,4-a:3',4'-f]Pyrrolizines from Glycine Methyl Ester
To the best of our knowledge, not many pyrrolo[3,4-a:3',4'-f]pyrrolizine derivatives have been synthesized and few of them via 1,3-dipolar reactions [32,33,34]. Recently Zhang and coworkers reported the synthesis of pyrrolopyrrolizines from 2-furanyl and 2-thiophenylpyrrolizines by a multicomponent reaction under microwave irradiation using hetarylcarbaldehydes [33]. Similarly, our synthesis (Scheme 2) was carried out by a three-component combinatorial methodology between formylpyrazoles 1, N-substituted-maleimides 2 and glycine methyl ester 3 in refluxing toluene affording the products 4 via a double cycloaddition reaction (Table 1).
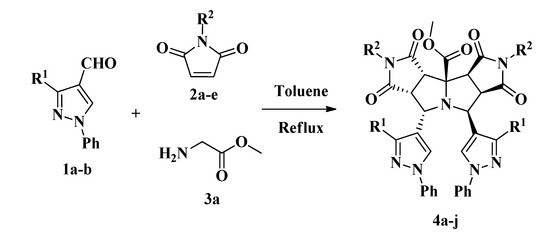
Scheme 2.
Three-component synthesis of pyrazolyldipyrrolo[3,4-a:3',4'-f]pyrrolizines 4a-j.

Table 1.
Synthesis of diverse pyrazolyldipyrrolo[3,4-a:3',4'-f]pyrrolizines 4a–j.
| Entry | R1 | R2 | Yield (%) |
|---|---|---|---|
| 4a | -CH3 | C6H5 | 96 |
| 4b | -CH3 | p-ClC6H4 | 73 |
| 4c | -CH3 | p-CH3C6H4 | 84 |
| 4d | -CH3 | p-CH3OC6H4 | 90 |
| 4e | -CH3 | (3,4-OCH2O)C6H3CH2 | 78 |
| 4f | C6H5 | C6H5 | 81 |
| 4g | C6H5 | p-ClC6H4 | 75 |
| 4h | C6H5 | p-CH3C6H4 | 93 |
| 4i | C6H5 | p-CH3OC6H4 | 94 |
| 4j | C6H5 | (3,4-OCH2O)C6H3CH2 | 75 |
The reaction consists of a domino process as shown in Scheme 3. We propose that initially the condensation of the glycine derivative 3a with the formylpyrazole 1a produced the imine 7a; subsequently, a 1,2-proton shift in imine 7a should afford the azomethine ylide 7b, which in turn should be trapped by the maleimide 2a to generate the intermediate pyrrolopyrrolidine derivative 8 (Scheme 3) [28].
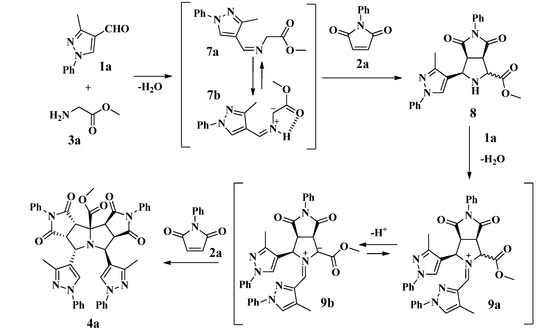
Scheme 3.
Proposed mechanism for the synthesis of pyrazolyldipyrrolo[3,4-a:3',4'-f] pyrrolizine 4a.
A second condensation should take place between the pyrrolidine intermediate 8 and the formylpyrazole 1a affording the iminium ion 9a, which should generate the 1,3-dipolar azomethine ylide 9b by deprotonation of its acidic proton adjacent to the carbomethoxy group. Finally, the tetracyclic product 4a would be formed by the diastereoselective 1,3-cycloaddition of a second molecule of maleimide 2a to the ylide 9b. During the process, the formation of the azomethine ylides 7b and 9b should be favored by the acidity of the α-protons in the moieties 7a and 9a and the in situ stability of these species due to an intramolecular H-bond and π-conjugation [8,35,36,37].
The structural elucidation of the new compounds 4a–j was made by analysis of the corresponding NMR, infrared and mass spectrometry data. The 1H-NMR spectra of compounds 4a–j show six aliphatic signals corresponding to the protons on the stereogenic centers of the dipyrrolopyrrolizine framework. Two of them appear as triplets corresponding to the H-6a and H-9a protons, and the remaining four appear as doublets assigned to the H-3a, H-3c, H-7 and H-9 protons. Crystals of compound 4g suitable for single-crystal X-ray diffraction were obtained by slow evaporation from a DMF:EtOH (1:1), thus solution confirming the structure and stereochemistry of the racemic compounds 4a–j (Figure 2) [38].
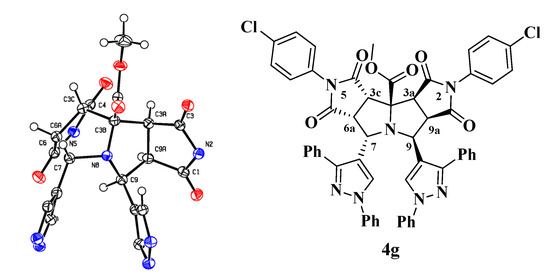
Figure 2.
ORTEP drawing of the compound 4g with 50% probability elipsoids. In the ORTEP view of the tetracyclic scaffold, the aryl pendant groups have been removed for the sake of clarity.
2.2. Synthesis of Pyrazolylpyrrolo[3,4-c]Pyrroles from N-benzylglicine Ethyl Ester
On the other hand, it is known that a similar three-component reaction using α-amino acids can be stopped at the pyrazolyl-pyrrolo[3,4-c]pyrroles 8 if the second cycloaddition is blocked by replacing the proton on the α-carbon atom by an alkyl group [28,29]. Thus, in order to preclude the formation of compounds type 4, we performed the reaction with N-benzylglycine ethyl ester 3b instead of 3a, along with the formylpyrazoles 1a–b and maleimides 2a–e (Scheme 4). As anticipated, the second amino condensation on the pyrrolidine nitrogen was blocked and the new compounds 5a–f and 6a–f were obtained in a diastereoselective manner with good yields (Table 2).
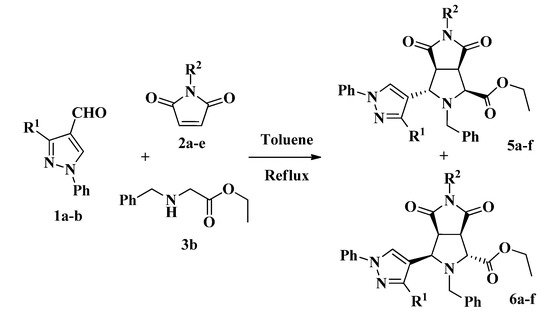
Scheme 4.
Three-component synthesis of pyrazolylpyrrolo[3,4-c]pyrroles 5 and 6.

Table 2.
Synthesis of diverse pyrazolylpyrrolo[3,4-c]pyrroles 5 and 6.
| Entry | R1 | R2 | d:r a (5:6) | Yield (%) 5 + 6 |
|---|---|---|---|---|
| 1 | -CH3 | C6H5 | 1:7 | 95 |
| 2 | -CH3 | p-ClC6H4 | 1:7 | 82 |
| 3 | -CH3 | p-CH3OC6H4 | 1:7 | 96 |
| 4 | C6H5 | C6H5 | 1:7 | 75 |
| 5 | C6H5 | p-ClC6H4 | 1:7 | 72 |
| 6 | C6H5 | p-CH3OC6H4 | 1:7 | 90 |
a Determinated by NMR.
In this approach, the first step is a condensation between the formylpyrazole 1a and the N-benzyl glycine ethyl ester 3b affording the iminium ion 10a, which is subsequently deprotonated giving azomethine ylide 10b (Scheme 5). Then, the 1,3-dipolar cycloaddition of 10b with the N-phenyl maleimide 2a afforded the diastereomers 5a and 6a. In all cases, the reaction showed good diastereoselectivity toward isomers 6, in which repulsive interactions between the ester group on the C-1 and C-6 carbonyl group are avoided due to the trans configuration between them as shown in 6a (Scheme 5) [17].
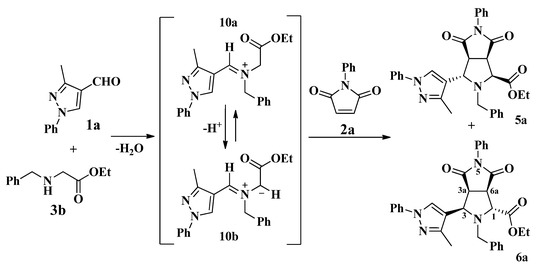
Scheme 5.
Proposed mechanism for the synthesis of pyrazolylpyrrolo[3,4-c]pyrroles 5a and 6a.
According to the 1H-NMR analysis of compounds 6 the H-1 proton on the stereogenic center appears as a singlet due to the dihedral angle (aprox. 90°) with the H-6a proton indicating a trans configuration. On the other hand, both the H-3 and H-6a protons appear as doublets with coupling constants about 9.0 Hz corresponding to a cis configuration with the H-3a proton, which in turn appears as a double doublet. Meantime, in compounds 5 the H-1 proton is observed as a doublet with a coupling constant J ≈ 9.0 Hz because of its cis configuration with respect to the H-6a proton, while the H-3 proton appears as a doublet with a smaller coupling constant (J ≈ 5.5 Hz) due to a trans configuration with respect to the H-3a proton [39].
2.3. Theoretical Calculations
To confirm our findings about the stereochemistry of the reactions as well as the obtained compounds 4–6, theoretical calculations were carried out with the DFT approach using the B.01 revision of the Gaussian 09 program package [40]. DFT calculations were performed using Becke’s three-parameter B3LYP exchange-correlation functional and the 6-311G++ basis set. The geometry of compound 6d was theoretically optimized and the most stable configuration is depicted in Figure 3. Although the found energy values for the cis and trans configurations of the stereoisomers 5d and 6d are very close, the trans configuration for 6d is slightly favored by 7.82696 × 10−17 Kcal over the cis form for 5d. This finding is in agreement with the Karplus theory [41] and with our experimental 1H-NMR measurements, since the dihedral angle 1H-C-C-6aH for the stereoisomer 6d is −94.41° and therefore in its 1H-NMR spectrum the coupling constant between the H-1 and H-6a protons has a value J3~0.0 Hz.
In order to verify the accuracy of the theoretical method, the geometry of compound 4g was theoretically optimized and the most stable configuration is depicted in Figure 4. Geometrical parameters such as bond length and molecular angles were calculated. We observed that the calculated parameters were very close to the experimental values, measured by X-ray diffraction. Some of these geometrical stocks are listed in Table 3.
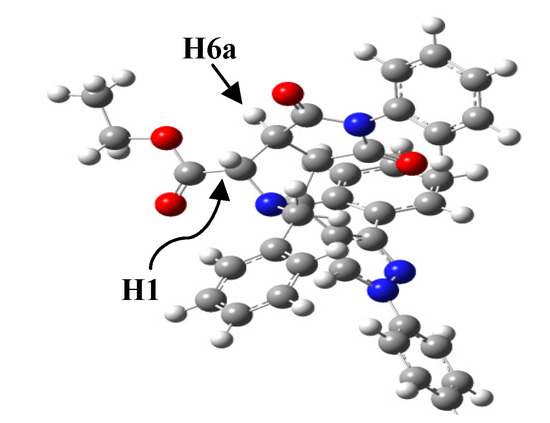
Figure 3.
Minimum-energy configuration calculated for the compound 6d at the B3LYP/6-311G++ level. Dihedral Angle (1H-C-C-6aH) = −94.41°.
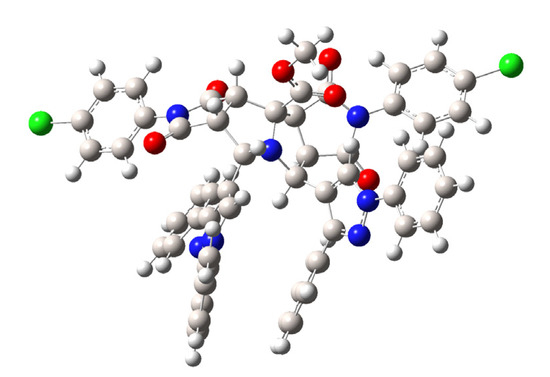
Figure 4.
Minimum-energy configuration calculated for compound 4g at the B3LYP/6-311G++ level.

Table 3.
Geometrical parameters of compound 4g; X-ray data is compared with theoretical calculated parameters.
| Bond | X-Ray Length (Å) | Calculated Length (Å) | Angle Atoms | X-Ray Angle (°) | Calculated Angle (°) |
|---|---|---|---|---|---|
| C1-O1 | 1.210(2) | 1.213 | O1-C1-N2 | 124.61(17) | 125.02 |
| C1-N2 | 1.393(2) | 1.398 | O1-C1-C8a | 128.00(16) | 127.64 |
| C1-C8a | 1.508(3) | 1.512 | N2-C1-C8a | 107.36(14) | 110.73 |
| N2-C3 | 1.399(2) | 1.409 | C1-N2-C3 | 112.48(15) | 114.34 |
| C3-O3 | 1.209(2) | 1.211 | C1-N2-C21 | 122.84(14) | 120.21 |
| C3-C3a | 1.529(3) | 1.534 | C3-N2-C21 | 124.47(15) | 124.88 |
| C3c-C4 | 1.507(3) | 1.511 | O3-C3-N2 | 124.53(17) | 123.00 |
3. Experimental
3.1. General Information
The pyrazole-4-carbaldehydes 1a–b and the glycine ester derivates 3a–b (analytical reagent grade) were purchased from Aldrich (St. Louis, Missouri, United State), Fluka (St. Louis, MO, USA) and Merck (Darmstadt, Alemania) and were used without further purification. The maleimides 2a–e were obtained according to the already reported procedure [42]. Solvents and other chemicals commercially available were used as shipped. Silica gel aluminium plates (Merck 60 F254) were used for analytical TLC. Melting points were taken on Stuart SMP10 Melting point apparatus and are uncorrected. IR spectra were recorded on a Shimadzu FTIR 8400 spectrophotometer using KBr disks or CH2Cl2 as solvent. 1H- and 13C-NMR were recorded on a Bruker Avance 400 spectrometer operating at 400 and 100 MHz respectively, using CDCl3 as solvent. Mass spectra were obtained on a Shimadzu GCMS-QP 2010 spectrometer (equipped with a direct inlet probe) operating at 70 eV and with a Bruker Esquire 6000 spectrometer equipped with an electrospray ionization source and an ion-trap detector. Microanalyses were performed on a LECO CHNS-900 elemental analyzer and the values are within ± 0.4% of theoretical values.
3.2. Synthesis and Characterization Data for Pyrazolyldipyrrolo[3,4-a:3',4'-f]Pyrrolizines 4a–j
General Synthetic Procedure
To a 25.0 mL round bottom flask equipped with a magnetic stirring bar and a reflux condenser were added pyrazole-4-carboxaldehyde 1a–b (0.2 mmol), N-substituted-maleimide 2a–e (0.2 mmol), glycine methyl ester 3a (0.2 mmol) and toluene (8 mL). The mixture was heated under reflux for 8–10 h. The reaction mixture was cooled to ambient temperature and the resulting precipitate was collected by filtration and washed with hexane-toluene (1:1) to obtain the pure compounds. In some cases, the solid was recrystallized from a mixture ethanol-DMF (1:1) to obtain the pure compound 4.
Methyl 7,9-bis(3-methyl-1-phenyl-1H-pyrazol-4-yl)-1,3,4,6-tetraoxo-2,5-diphenyldodecahydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4a). Beige solid. Yield: 96%; m.p.: 182–184 °C. IR (KBr): ν 3473, 2954, 1753, 1715 cm−1. 1H-NMR (400 MHz, CDCl3) δ ppm: 2.01 (s, 3H), 2.26 (s, 3H), 3.51 (dd, J = 8.0 Hz; J = 8.3 Hz, 1H), 3.74 (t, J = 8.0 Hz, 1H), 3.99 (s, 3H), 4.29 (d, J = 8.3 Hz, 1H), 4.46 (d, J = 10.5 Hz, 1H), 4.64 (d, J = 8.3 Hz, 1H), 4.91 (d, J = 7.8 Hz, 1H), 6.81 (d, J = 8.8 Hz, 2H), 7.18 (d, J = 8.5 Hz, 2H), 7.20–7.26 (m, 2H), 7.28–7.39 (m, 12H), 7.53 (s, 1H), 7.57 (d, J = 8.8 Hz, 2H), 7.98 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ ppm: 11.5 (CH3), 11.9 (CH3), 48.2 (CH), 48.9 (CH), 49.5 (CH), 53.2 (CH), 53.5 (OCH3), 58.1 (CH), 60.6 (CH), 79.34 (C), 112.2 (C), 118.4 (CH), 118.8 (CH), 119.4 (CH), 120.1 (C), 125.9 (CH), 126.3 (CH), 126.4 (CH), 126.8 (CH), 126.9 (CH), 127.3 (CH), 129.1 (CH), 129.2 (CH), 129.3 (CH), 129.40 (CH), 130.0 (CH), 134.3 (C), 135.4 (C), 139.6 (C), 139.7 (C), 146.6 (C), 150.7 (C), 170.2 (C), 171.7 (C), 173.2 (C), 174.7 (C), 175.7 (C). MS (EI, 70 eV) m/z (%): 771 (M+, 2), 712 (13), 598 (100), 539 (70), 268 (27), 211 (32), 171 (75). Elemental Analyses calcd. for C45H37N7O6.2H2O: C: 66.90, H: 5.12, N: 12.14. Found: C: 66.75, H: 5.47, N: 12.07.
Methyl 2,5-bis(4-chlorophenyl)-7,9-bis(3-methyl-1-phenyl-1H-pyrazol-4-yl)-1,3,4,6 tetraoxododeca-hydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4b). Pale yellow solid. Yield: 73%; m.p.: 170–172 °C. IR (KBr): ν 3479, 2953, 1716 cm−1. 1H-NMR (400 MHz, CDCl3) δ ppm: 2.03 (s, 3H), 2.29 (s, 3H), 3.53 (dd, J = 10.7, 8.2, 1H), 3.76 (t, J = 8.0 Hz, 1H), 4.02 (s, 3H), 4.31 (d, J = 8.3 Hz, 1H), 4.48 (d, J = 10.5 Hz, 1H), 4.66 (d, J = 8.3 Hz, 1H), 4.94 (d, J = 7.8 Hz, 1H), 6.83 (d, J = 8.8 Hz, 2H), 7.21 (d, J = 8.5 Hz, 2H), 7.26 (m, 2H), 7.37 (m, 10H), 7.55 (s, 1H), 7.60 (d, J = 8.8 Hz, 2H), 8.00 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ ppm: 11.5 (CH3), 11.9 (CH3), 48.2 (CH), 48.9 (CH), 49.5 (CH), 53.2 (CH); 53.5 (OCH3), 58.1 (CH), 60.6 (CH), 79.3 (C), 112.2 (C) 118.8 (CH), 119.4 (CH), 120.1 (C), 125.9 (CH), 126.3 (CH), 126.4 (CH), 126.8 (CH), 126.9 (CH), 127.3 (CH), 129.1 (CH), 129.2 (CH), 129.3 (CH), 129.4 (C), 129.7 (C), 130.0 (CH), 134.3 (C), 135.4 (C), 139.6 (C), 139.7 (C), 146.6 (C), 150.7 (C), 170.2 (C), 171.7 (C), 173.2 (C), 174.7 (C), 175.7 (C). MS (EI, 70 eV) m/z (%): 839 (M+, 2), 780 (18), 632 (42), 268 (22), 211 (39), 171 (100). Elemental Analyses calcd. for C45H35Cl2N7O6.H2O: C: 62.94, H: 4.34, N: 11.42. Found: C: 63.32, H: 4.36, N: 11.34.
Methyl 7,9-bis(3-methyl-1-phenyl-1H-pyrazol-4-yl)-1,3,4,6-tetraoxo-2,5-di-p-tolyldodecahydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4c). Beige solid. Yield: 84%; m.p.: 179–181 °C. IR (KBr): ν 3647, 2952, 1784, 1756, 1696 cm−1. 1H-NMR (400 MHz, CDCl3) δ ppm: 2.02 (s, 3H), 2.25 (s, 3H), 2.27 (s, 3H), 2.46 (s, 3H), 3.56 (dd, J = 10.7, 8.2 Hz, 1H), 3.71–3.77 (m, 1H), 4.00 (s, 3H), 4.28 (d, J = 8.3 Hz, 1H), 4.51 (d, J = 10.8 Hz, 1H), 4.66 (d, J = 8.3 Hz, 1H), 4.93 (d, J = 8.0 Hz, 1H), 6.75 (d, J = 8.3 Hz, 2H), 7.03 (d, J = 7.5 Hz, 2H), 7.21–7.25 (m, 4H), 7.33–7.39 (m, 5H), 7.39–7.44 (m, 5H), 7.58 (s, 1H), 8.04 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ ppm: 11.5 (CH3), 11.9 (CH3), 21.0 (CH3), 21.3 (CH3), 48.2 (CH), 48.8 (CH), 49.6 (CH), 53.3 (CH), 53.4 (OCH3), 58.1 (CH), 60.5 (CH), 79.3 (C), 112.5 (C), 118.4 (CH), 118.9 (CH), 119.4 (CH), 120.3 (C), 125.4 (CH), 125.5 (CH), 125.7 (CH), 126.1 (CH), 126.3 (CH), 127.5 (CH), 128.4 (C), 128.6 (C), 129.2 (CH), 129.6 (CH), 130.4 (CH), 138.7 (C), 139.6 (C), 139.7 (C), 139.8 (C), 146.7 (C), 150.7 (C), 170.4 (C), 172.2 (C), 173.7 (C), 175.1 (C), 176.2 (C). MS (EI, 70 eV) m/z (%): 799 (M+, 2), 740 (8), 612 (100), 553 (18), 257 (60), 197 (75). Elemental Analyses calcd. for C47H41N7O6: C: 70.57, H: 5.17, N: 12.26. Found: C: 70.87, H: 5.41, N: 11.90.
Methyl 2,5-bis(4-methoxyphenyl)-7,9-bis(3-methyl-1-phenyl-1H-pyrazol-4-yl)-1,3,4,6-tetraoxododeca-hydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4d). White Solid. Yield: 90%; m.p.: 174–176 °C. IR (KBr): ν 3478, 2955, 1714, 1598 cm−1. 1H-NMR (400 MHz, CDCl3) δ ppm: 2.02 (s, 3H), 2.27 (s, 3H), 3.54 (dd, J = 10.7, 8.2 Hz, 1H), 3.68–3.75 (m, 4H), 3.88 (s, 3H), 4.00 (s, 3H), 4.26 (d, J = 8.3 Hz, 1H), 4.50 (d, J = 10.5 Hz, 1H), 4.65 (d, J = 8.0 Hz, 1H), 4.92 (d, J = 7.8 Hz, 1H), 6.72 (d, J = 9.0 Hz, 2H), 6.75 (d, J = 9.0, 2H), 7.10 (d, J = 9.0 Hz, 2H), 7.20–7.29 (m, 4H), 7.30–7.44 (m, 8H), 7.57 (s, 1H), 8.05 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ ppm: 11.5 (CH3), 11.9 (CH3), 48.2 (CH), 48.8 (CH), 49.6 (CH), 53.3 (CH), 53.4 (OCH3), 55.3 (OCH3), 55.6 (OCH3), 58.1 (CH), 60.5 (CH), 79.2 (C), 112.5 (C), 114.3 (CH), 115.1 (CH), 118.9 (CH), 119.4 (CH), 120.4 (C), 123.7 (C), 123.8 (C), 126.1 (CH), 126.3 (CH), 126.8 (CH), 126.9 (CH), 127.5 (CH), 129.2 (CH), 139.7 (C), 139.8 (C),146.7 (C), 150.7 (C), 159.3 (C), 160.1 (C), 170.4 (C), 172.3 (C), 173.8 (C), 175.2 (C), 176.3 (C). MS (EI, 70 eV) m/z (%): 831 (M+, 2), 772 (8), 628 (67), 268 (43), 203 (46), 171 (100). Elemental Analyses calcd. for C47H45N7O10: C: 65.04, H: 5.23, N: 11.30. Found: C: 65.44, H: 4.90, N: 11.27.
Methyl 2,5-bis(benzo[d][1,3]dioxol-5-ylmethyl)-7,9-bis(3-methyl-1-phenyl-1H-pyrazol-4-yl)-1,3,4,6-tetraoxododecahydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4e). Beige Solid. Yield: 78%; m.p.: 243–245 °C. IR (KBr): ν 3459, 2898, 1749, 1694, 1598 cm−1. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.56 (s, 3H), 2.11 (s, 3H), 2.91 (dd, J = 10.5, 8.5 Hz, 1H), 3.51 (t, J = 8.4 Hz, 1H), 3.65 (d, J = 10.5 Hz, 1H), 3.95 (s, 3H), 4.22 (d, J = 8.3 Hz, 1H), 4.23–4.34 (m, 2H), 4.49 (d, J = 8.5 Hz, 1H), 4.63–4.74 (m, 3H), 5.71–5.83 (m, 2H), 5.86–5.96 (m, 2H), 6.47 (d, J = 7.0 Hz, 1H), 6.63 (d, J = 7.3 Hz, 1H), 6.70 (s, 1H), 6.80 (d, J = 7.8 Hz, 1H), 6.87–7.01 (m, 1H), 7.08–7.11 (m, 1H), 7.12 (d, J = 1.5 Hz, 1H), 7.15–7.23 (m, 5H), 7.27–7.37 (m, 5H), 7.52 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ ppm: 11.2 (CH3), 11.3 (CH3), 42.2 (CH2), 42.9 (CH2), 48.6 (CH), 48.7 (CH), 48.8 (CH), 52.5 (CH), 53.4 (OCH3), 59.7 (CH), 63.6 (CH), 79.3 (C), 101.0 (CH2), 101.5 (CH2), 108.1 (CH), 108.9 (CH), 109.1 (CH), 109.6 (CH), 118.5 (CH), 119.2 (CH), 122.3 (CH), 122.4 (CH), 123.3 (CH), 125.5 (CH), 125.6 (CH), 126.2 (CH), 128.1 (C), 128.8 (C), 129.0 (CH), 129.2 (CH), 139.6 (C), 139.7 (C), 147.1 (C), 147.5 (C), 148.2 (C), 148.4 (C), 150.2 (C), 170.7 (C), 172.8 (C), 174.7 (C), 175.8 (C), 176.4 (C). MS (EI, 70 eV) m/z (%): 887 (M+, 1), 657 (20), 656 (50), 231 (16), 171 (74), 135 (100). Elemental Analyses calcd. for C49H41N7O10.2H2O: C: 63.70, H: 4.91, N: 10.61. Found: C: 64.03, H: 5.15, N: 10.82.
Methyl 7,9-bis(1,3-diphenyl-1H-pyrazol-4-yl)-1,3,4,6-tetraoxo-2,5-diphenyldodecahydro-1H-dipyrrolo- [3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4f). Yellow Solid. Yield: 81%; m.p.: 284–286 °C. IR (KBr): ν 3062, 2954, 1746, 1712, 1598 cm−1. 1H-NMR (400 MHz, CDCl3-d) δ ppm: 3.59 (t, J = 8.4 Hz, 1H), 3.78–3.85 (m, 1H), 3.97 (s, 3H), 4.36 (d, J = 8.5 Hz, 1H), 4.72 (d, J = 10.5 Hz, 1H), 4.82 (d, J = 8.3 Hz, 1H), 5.15 (d, J = 8.5 Hz, 1H), 6.97 (dd, J = 6.5, 2.8 Hz, 2H), 7.02–7.08 (m, 2H), 7.11–7.17 (m, 3H), 7.21–7.25 (m, 5H), 7.28–7.35 (m, 7H), 7.37–7.51 (m, 7H), 7.53–7.59 (m, 3H), 7.83 (s, 1H), 7.91 (d, J = 7.3 Hz, 2H). 13C-NMR (100 MHz, CDCl3) δ ppm: 48.7 (CH), 49.5 (CH), 49.6 (CH), 52.9 (CH), 53.7 (OCH3), 59.2 (CH), 60.7 (CH), 80.1 (C), 111.0 (C), 119.3 (CH), 119.8 (CH), 120.3 (C), 125.7 (CH), 126.1 (CH), 126.5 (CH), 126.7 (CH), 127.1 (CH), 127.9 (CH), 128.0 (CH), 128.2 (CH), 128.3 (CH), 128.5 (CH), 128.6 (CH), 128.6 (CH), 129.0 (CH), 129.1 (CH), 129.2 (CH), 129.3 (CH), 129.4 (CH), 129.8 (CH), 131.1 (C), 131.2 (C), 132.3 (C), 132.5 (C), 139.6 (C), 139.6 (C), 150.5 (C), 152.7 (C), 170.1 (C), 172.5 (C), 173.7 (C), 175.3 (C), 176.0 (C). MS (EI, 70 eV) m/z (%): 895(M+, 1), 836 (6), 722 (71), 298 (23), 273 (27), 233 (100), 173 (42). Elemental Analyses calcd. for C55H45N7O6.3H2O: C: 69.54, H: 4.99, N: 10.32. Found: C: 69.43, H: 4.94, N: 10.42.
Methyl 2,5-bis(4-chlorophenyl)-7,9-bis(1,3-diphenyl-1H-pyrazol-4-yl)-1,3,4,6-tetraoxododecahydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4g). White Solid. Yield: 75%; m.p.: 275–277 °C. IR (KBr): ν 3447, 1718, 1598, 1496 cm−1. 1H-NMR (400 MHz, CDCl3) δ ppm: 3.50–365 (m, 1H); 3.72–3.80 (m, 1H); 3.96 (s, 3H); 4.38 (d, J = 8.53 Hz, 1H); 4.66 (d, J = 10.54 Hz, 1H); 4.79 (d, J = 8.28 Hz, 1H); 5.16 (d, J = 8.53 Hz, 1H); 6.92–7.03 (m, 4H) 7.12–7.32 (m, 13H), 7.33–7.44 (m, 6H), 7.45–7.55 (m, 4H), 7.75 (s, 1H); 7.89 (d, J = 7.78 Hz, 2H). 13C-NMR (100 MHz, CDCl3) δ ppm: 48.6 (CH); 49.3 (CH); 49.6 (CH); 52.8 (CH); 53.7 (CH3); 59.2 (CH); 60.7 (CH); 80.2 (C); 110.8 (C) 119.2 (CH); 119.8 (CH); 120.1 (C); 125.3 (C); 126.6 (CH); 126.8 (CH); 127.0 (CH); 127.4 (CH); 127.9 (CH); 128.0 (CH); 128.2 (CH); 128.4 (CH); 128.6 (CH); 128.7 (CH); 128.9 (C); 129.1 (CH); 129.2 (CH); 129.3 (CH); 129.4 (CH); 129.5 (CH); 129.6 (C); 130.0 (CH); 132.2 (C); 132.5 (C); 134.5 (C); 135.5 (C), 139.5 (C); 139.6 (C); 150.4 (C); 152.7 (C); 169.9 (C); 172.1 (C); 173.3 (C); 175.1 (C). MS (EI, 70 eV) m/z (%): 963(M+), 756 (31), 523 (10), 298 (18), 273 (16), 233 (100), 207 (30). Elemental Analyses calcd. for C55H39Cl2N7O6.4H2O: C: 63.71, H: 4.57, N: 9.46. Found: C: 63.74, H: 4.42, N: 9.37.
Methyl 7,9-bis(1,3-diphenyl-1H-pyrazol-4-yl)-1,3,4,6-tetraoxo-2,5-di-p-tolyldodecahydro-1H-dipyrrolo- [3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4h). White Solid. Yield: 93%; m.p.: 204–206 °C. IR (KBr): ν 3574, 2954, 1747, 1711, 1599 cm−1. 1H-NMR (400 MHz, CDCl3) δ ppm: 2.26 (s, 3H), 2.50 (s, 3H), 3.59 (t, J = 8.4 Hz, 1H), 3.80 (dd, J = 10.5, 8.3 Hz, 1H), 3.96 (s, 3H), 4.35 (d, J = 8.3 Hz, 1H), 4.71 (d, J = 10.8 Hz, 1H), 4.80 (d, J = 8.3 Hz, 1H), 5.14 (d, J = 8.5 Hz, 1H), 6.84 (d, J = 8.3 Hz, 2H), 6.93 (d, J = 8.0 Hz, 2H), 7.03 (d, J = 8.0 Hz, 2H), 7.12–7.18 (m, 2H), 7.18–7.24 (m, 1H), 7.26–7.30 (m, 5H), 7.30–7.44 (m, 10H), 7.44–7.50 (m, 3H), 7.82 (s, 1H), 7.90 (d, J = 7.0 Hz, 2H). 13C-NMR (100 MHz, CDCl3) δ ppm: 21.1 (CH3), 21.4 (CH3), 48.8 (CH), 49.5 (CH), 49.6 (CH), 52.9 (CH), 53.7 (OCH3), 59.1 (CH), 60.7 (CH), 80.1 (C), 100.0 (C), 111.0 (C), 119.3 (CH), 119.8 (CH), 120.4 (C), 125.5 (CH), 126.0 (CH), 126.4 (CH), 126.7 (CH), 127.2 (CH), 127.9 (CH), 128.0 (CH), 128.2 (CH), 128.3 (CH), 128.5 (CH), 128.6 (CH), 128.7 (CH), 129.2 (CH), 129.3 (CH), 129.7 (CH), 130.4 (CH), 132.3 (C), 132.6 (C), 138.6 (C), 138.7 (C), 139.6 (C), 139.7 (C), 139.8 (C), 150.5 (C), 152.7 (C), 170.2 (C), 172.6 (C), 173.8 (C), 175.4 (C), 176.2 (C). MS (EI, 70 eV) m/z (%): 923(M+), 865 (5), 737 (95), 503 (16), 298 (35), 233 (100), 187 (45). Elemental Analyses calcd. for C57H45N7O6.H2O: C: 72.67, H: 5.03, N: 10.41. Found: C: 72.99, H: 5.24, N: 10.50.
Methyl 7,9-bis(1,3-diphenyl-1H-pyrazol-4-yl)-2,5-bis(4-methoxyphenyl)-1,3,4,6-tetraoxododecahydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4i). Beige Solid. Yield: 94%; m.p.: 239–241 °C. IR (KBr): ν 3474, 2955, 1714, 1599 cm−1. 1H-NMR (400 MHz, CDCl3) δ ppm: 3.57 (t, J = 8.4 Hz, 1H), 3.70 (s, 3H), 3.76–3.80 (m, 1H), 3.92 (s, 3H), 3.96 (s, 3H), 4.33 (d, J = 8.3 Hz, 1H), 4.68 (d, J = 10.8 Hz, 1H), 4.79 (d, J = 8.3 Hz, 1H), 5.14 (d, J = 8.3 Hz, 1H), 6.73 (d, J = 9.0 Hz, 2H), 6.88 (d, J = 8.8 Hz, 2H), 6.95 (d, J = 8.8 Hz, 2H), 7.05 (d, J = 8.8 Hz, 2H), 7.12–7.122 (m, 4H), 7.24–7.36 (m, 9H), 7.38–7.44 (m, 4H), 7.46–7.50 (m, 2H), 7.81 (s, 1H), 7.91 (d, J = 7.0 Hz, 2H). 13C-NMR (100 MHz, CDCl3) δ ppm: 48.7 (CH), 49.4 (CH), 49.5 (CH), 52.8 (CH), 53.6 (OCH3), 55.4 (OCH3), 55.6 (OCH3), 59.1 (CH), 60.6 (CH), 80.1 (C), 111.1 (C), 114.3 (CH), 115.1 (CH), 119.3 (CH), 119.8 (CH), 120.5 (C), 123.7 (C), 123.8 (C), 126.4 (CH), 126.7 (CH), 126.9 (CH), 127.1 (CH), 127.4 (CH), 127.8 (CH), 128.0 (CH), 128.2 (CH), 128.3 (CH), 128.4 (CH), 128.5 (CH), 128.6 (CH), 129.1 (CH), 129.2 (CH), 132.3 (C), 132.6 (C), 139.6 (C), 139.7 (C), 150.5 (C), 152.7 (C), 159.3 (C), 160.1 (C), 170.2 (C), 172.7 (C), 173.9 (C), 175.6 (C), 176.3 (C). MS (EI, 70 eV) m/z (%): 955(M+), 751 (38), 521 (25), 233 (100), 203 (48). Elemental Analyses calcd. for C57H45N7O8.2H2O: C: 69.01, H: 4.98, N: 9.88. Found: C: 69.42, H: 5.18, N: 9.84.
Methyl 2,5-bis(benzo[d][1,3]dioxol-5-ylmethyl)-7,9-bis(1,3-diphenyl-1H-pyrazol-4-yl)-1,3,4,6-tetra- oxododecahydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-carboxylate (4j). White Solid. Yield: 75%; m.p.: 241–243 °C. IR (KBr): ν 3647, 3062, 1744, 1700, 1599, 1503 cm−1. 1H-NMR (400 MHz, CDCl3) δ ppm: 3.34 (t, J = 8.3 Hz, 1H), 3.50 (t, J = 9.3 Hz, 1H), 3.92 (s, 3H), 4.20 (d, J = 13.8 Hz, 1H), 4.24–4.31 (m, 2H), 4.39 (dd, J = 13.8, 5.0 Hz, 2H), 4.49–4.54 (m, 1H), 4.62 (d, J = 8.3 Hz, 1H), 4.75 (d, J = 8.5 Hz, 1H), 5.75–5.85 (m, 2H), 5.95 (s, 2H), 6.34 (d, J = 7.8 Hz, 1H), 6.65–6.70 (m, 1H), 6.74 (d, J = 8.0 Hz, 1H), 6.85–6.92 (m, 3H), 6.98 (d, J = 7.3 Hz, 2H), 7.04–7.10 (m, 3H), 7.13–7.24 (m, 5H), 7.27–7.36 (m, 5H), 7.38–7.50 (m, 5H), 7.86 (d, J = 7.3 Hz, 2H). 13C-NMR (100 MHz, CDCl3) δ ppm: 42.1 (CH2), 42.9 (CH2), 48.2 (CH), 48.3 (CH), 50.0 (CH), 52.0 (CH), 53.6 (OCH3), 58.6 (CH), 60.0 (CH), 80.3 (C), 100.9 (CH2), 101.2 (CH2), 108.1 (CH), 108.4 (CH), 109.0 (CH), 109.5 (CH), 111.0 (C), 119.1 (C), 119.3 (CH), 120.0 (CH), 122.2 (CH), 122.7 (CH), 126.1 (CH), 126.6 (CH), 126.7 (CH), 127.6 (CH), 127.8 (CH), 127.9 (CH), 128.2 (C), 128.3 (CH), 128.4 (CH), 128.5 (CH), 128.6 (CH), 128.9 (CH), 129.2 (CH), 129.4 (C), 132.4 (C), 132.6 (C), 139.5 (C), 139.6 (C), 147.1 (C), 147.5 (C), 147.7 (C), 147.8 (C), 150.1 (C), 152.2 (C), 170.2 (C), 173.0 (C), 174.7 (C), 176.4 (C), 176.6 (C). MS (EI, 70 eV) m/z (%): 1011(M+), 780 (11), 547 (7), 257 (15), 233 (45), 135 (100). Elemental Analyses calcd. for C59H45N7O10.2H2O: C: 67.61, H: 4.71, N: 9.36. Found: C: 67.84, H: 4.39, N: 9.21.
3.3. Synthesis and Characterization Data for Pyrazolylpyrrolo[3,4-c]Pyrroles 5a–f and 6a–f.
General Synthetic Procedure
To a 25.0 mL round bottom flask equipped with a magnetic stirring bar and a reflux condenser were added pyrazole-4-carboxaldehyde 1a–b (0.2 mmol), N-substituted-maleimide 2a–e (0.2 mmol), N-benzyl glycine ethyl ester 3b (0.22 mmol) and toluene (8 mL). The mixture was heated under reflux until TLC showed the absence of the starting materials (6–10 h). After the reaction mixture was cooled down to room temperature, the solvent was removed under reduced pressure and the resulting crude product was purified by column chromatography on silica gel, using a mixture of dichloromethane/hexane (7:3) as eluent. In all cases the minor diastereomers were characterized by 1H-NMR spectroscopy after purification.
Ethyl 2-benzyl-3-(3-methyl-1-phenyl-1H-pyrazol-4-yl)-4,6-dioxo-5-phenyloctahydropyrrolo[3,4-c]-pyrrole-1-carboxylate. White Solid. Yield: 95%; m.p.: 172–174 °C. IR (KBr): ν 1717, 1598, 1502 cm−1. Minor diastereomer 5a. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.26 (t, J = 7.3 Hz, 3H), 2.38 (s, 3H), 3.34 (d, J = 13.8 Hz, 1H), 3.57 (dd, J = 9.7, 5.4 Hz, 1H), 3.86 (d, J = 4.8 Hz, 1H), 3.89 (d, J = 9.3 Hz, 1H), 4.20 (q, J = 7.3 Hz, 2H), 4.24 (d, J = 9.0 Hz, 1H), 4.79 (d, J = 5.5 Hz, 1H), 7.22–7.28 (m, 4H), 7.29–7.34 (m, 4H), 7.40–7.53 (m, 5H), 7.66 (d, J = 7.5 Hz, 2H), 7.92 (s, 1H). Major diastereomer 6a1H-NMR (400 MHz, CDCl3) δ ppm: 1.32 (t, J = 7.2 Hz, 3H), 2.45 (s, 3H), 3.53 (d, J = 8.0 Hz, 1H), 3.65 (d, J = 14.0 Hz, 1H), 3.80–3.87 (m, 1H), 4.05 (d, J = 14.1 Hz, 1H), 4.17–4.34 (m, 2H), 4.39 (s, 1H), 4.94 (d, J = 9.5 Hz, 1H), 7.13 (d, J = 6.8 Hz, 2H), 7.19–7.30 (m, 4H), 7.31–7.42 (m, 7H), 7.52 (d, J = 7.8 Hz, 2H), 7.72 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ ppm: 12.4 (CH3), 14.2 (CH3), 48.6 (CH), 48.7 (CH), 52.1 (CH2), 59.8 (CH), 60.9 (CH2), 62.9 (CH), 118.5 (CH), 125.5 (CH), 126.0 (CH), 127.3 (CH), 127.7 (CH), 128.2 (CH), 128.3 (CH), 128.6 (CH), 129.0 (CH), 129.2 (CH), 130.3 (C), 131.6 (C), 137.6 (C), 139.8 (C), 149.8 (C), 170.9 (C), 174.3 (C), 175.6 (C). MS (EI, 70 eV) m/z (%): 534 (M+, 15), 461 (65), 91 (100). Elemental Analyses calcd. for C32H30N4O4.2H2O: C: 67.35, H: 6.01, N: 9.82. Found: C: 67.45, H: 6.13, N: 9.66.
Ethyl 2-benzyl-5-(4-chlorophenyl)-3-(3-methyl-1-phenyl-1H-pyrazol-4-yl)-4,6-dioxooctahydropyrrolo-[3,4-c]pyrrole-1-carboxylate. Yellow Solid. Yield: 82%; m.p.: 69–71 °C. IR: ν 1782, 1738, 1598 cm−1. Minor diastereomer 5b. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.24 (t, J = 7.3 Hz, 3H), 2.36 (s, 3H), 3.31 (d, J = 13.8 Hz, 1H), 3.54 (dd, J = 9.5, 5.5 Hz, 1H), 3.77–3.85 (m, 3H), 4.22–4.30 (m, 2H), 4.75 (d, J = 5.3 Hz, 1H), 7.06 (d, J = 8.3 Hz, 2H), 7.18–7.23 (m, 2H), 7.29–7.34 (m, 3H), 7.37 (t, J = 7.8 Hz, 2H), 7.44 (d, J = 8.5 Hz, 1H), 7.49 (d, J = 8.0 Hz, 2H), 7.67 (d, J = 7.5 Hz, 2H), 7.91 (s, 1H). Major diastereomer 6b. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.30 (t, J = 7.2 Hz, 3H), 2.42 (s, 3H), 3.50 (d, J = 7.8 Hz, 1H), 3.63 (d, J = 14.3 Hz, 1H), 3.78–3.81 (m, 1H), 4.02 (d, J = 14.3 Hz, 1H), 4.15–4.22 (m, 2H), 4.35 (s, 1H), 4.91 (d, J = 9.5 Hz, 1H), 7.06 (d, J = 8.3 Hz, 2H), 7.18–7.23 (m, 3H), 7.29–7.34 (m, 3H), 7.37 (t, J = 7.8 Hz, 2H), 7.44 (d, J = 8.5 Hz, 1H), 7.49 (d, J = 8.0 Hz, 2H), 7.63–7.69 (m, 2H). 13C-NMR (100 MHz, CDCl3) δ ppm: 12.4 (CH3), 14.2 (CH3), 46.4 (CH), 48.7 (CH), 52.1 (CH2), 59.8 (CH), 60.9 (CH2), 62.9 (CH), 118.4 (CH), 119.6 (C), 126.1 (CH), 126.6 (CH), 127.3 (CH), 127.7 (CH), 128.6 (CH), 129.1 (CH), 129.2 (CH), 129.3 (CH), 130.1 (C), 133.9 (C), 137.5 (C), 139.7 (C), 170.8 (C), 170.8 (C), 174.1 (C), 175.3 (C). MS (EI, 70 eV) m/z (%): 568 (M+, 1), 497 (11), 496 (10), 495 (30), 91 (100). Elemental Analyses calcd. for C32H29ClN4O5.H2O: C: 65.47, H: 5.32, N: 9.54. Found: C: 65.28, H: 5.39, N: 9.76.
Ethyl 2-benzyl-5-(4-methoxyphenyl)-3-(3-methyl-1-phenyl-1H-pyrazol-4-yl)-4,6-dioxooctahydro-pyrrolo[3,4-c]pyrrole-1-carboxylate. White Solid. Yield: 96%; m.p.: 85–87 °C. IR: ν 1775, 1728, 1602 cm−1. Minor diastereomer 5c. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.26 (t, J = 7.2 Hz, 3H), 2.38 (s, 3H), 3.34 (d, J = 13.8 Hz, 1H), 3.55 (dd, J = 9.5, 5.3 Hz, 1H), 3.82–3.91 (m, 5H), 4.19 (q, J = 7.3 Hz, 2H), 4.23 (d, J = 8.8 Hz, 1H), 4.78 (d, J = 5.5 Hz, 1H), 7.00 (d, J = 9.0, 2H), 7.21–7.26 (m, 5H), 7.27–7.34 (m, 3H), 7.45 (t, J = 8.5, 7.3 Hz, 2H), 7.66 (dd, J = 8.7, 1.1 Hz, 2H), 7.91 (s, 1H). Major diastereomer 6c. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.32 (t, J = 7.2 Hz, 3H), 2.44 (s, 3H), 3.51 (d, J = 7.8 Hz, 1H), 3.65 (d, J = 14.3 Hz, 1H), 3.76 (s, 3H), 4.04 (d, J = 14.1 Hz, 1H), 4.16–4.32 (m, 3H), 4.38 (s, 1H), 4.92 (d, J = 9.5 Hz, 1H), 6.84 (d, J = 9.0 Hz, 2H), 7.04 (d, J = 8.8 Hz, 2H), 7.21–7.27 (m, 3H), 7.30–7.35 (m, 3H), 7.36–7.41 (m, 2H), 7.53 (d, J = 7.8 Hz, 2H), 7.70 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ ppm: 12.4 (CH3), 14.2 (CH3), 46.4 (CH), 48.6 (CH), 52.0 (CH2), 55.3 (OCH3), 59.8 (CH), 60.9 (CH2), 62.9 (CH), 114.3 (CH), 118.5 (CH), 119.8 (C), 124.3 (C), 126 (CH), 126.8 (CH), 127.3 (CH), 127.7 (CH), 128.2 (CH), 128.6 (CH), 129.2 (CH), 137.7 (C), 139.8 (C), 149.8 (C), 159.1 (C), 171.0 (C), 174.5 (C), 175.8 (C). MS (EI, 70 eV) m/z (%): 564 (M+, 6), 493 (14), 492 (71), 491 (99), 473 (37), 270 (33), 91 (100). Elemental Analyses calcd. for C33H32N4O5.2H2O: C: 65.99, H: 6.04, N: 9.33. Found: C: 66.12, H: 6.24, N: 9.02.
Ethyl 2-benzyl-3-(1,3-diphenyl-1H-pyrazol-4-yl)-4,6-dioxo-5-phenyloctahydropyrrolo[3,4-c]pyrrole-1-carboxylate. White Solid. Yield: 75%; m.p.: 181–183 °C. IR (KBr): ν 1721, 1597, 1498 cm−1. Minor diastereomer 5d. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.27 (t, J = 7.1 Hz, 3H), 3.38 (d, J = 13.6 Hz, 1H), 3.72 (dd, J = 9.7, 5.6 Hz, 1H), 3.83 (d, J = 9.0 Hz, 1H), 3.89–3.93 (m, 1H), 4.28–4.32 (m, 3H), 5.05 (d, J = 5.5 Hz, 1H), 7.14–7.18 (m, 2H), 7.32–7.35 (m, 5H), 7.40–7.43 (m, 4H), 7.50–7.53 (m, 5H), 7.82–7.85 (m, 2H), 7.97–8.02 (m, 2H), 8.15 (s, 1H). Major diastereomer 6d. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.32 (t, J = 7.2 Hz, 3H), 3.58 (d, J = 7.8 Hz, 1H), 3.67 (d, J = 14.3 Hz, 1H), 3.92–3.98 (m, 1H), 4.06 (d, J = 14.1 Hz, 1H), 4.21–4.29 (m, 2H), 4.45 (s, 1H), 5.11 (d, J = 9.8 Hz, 1H), 7.22–7.26 (m, 3H), 7.30 (dd, J = 7.4, 1.9 Hz, 2H), 7.32–7.35 (m, 1H), 7.36–7.40 (m, 4H), 7.43–7.48 (m, 2H), 7.49–7.52 (m, 2H), 7.55–7.60 (m, 2H), 7.65 (d, J = 7.5 Hz, 2H), 7.86–7.90 (m, 2H), 7.92 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ ppm: 14.3 (CH3), 49.0 (CH), 49.5 (CH), 52.3 (CH2), 60.2 (CH), 61.1 (CH2), 63.1 (CH), 118.9 (CH), 119.0 (CH), 119.1 (C), 125.6 (CH), 126.1 (CH), 126.6 (CH), 126,7 (CH), 127.4 (CH), 128.4 (CH), 128.5 (CH), 128.6 (CH), 128.7 (CH), 129.2 (CH), 129.4 (CH), 131.8 (C), 133.1 (C), 137.8 (C), 139.9 (C), 153.3 (C), 171.0 (C), 174.6 (C), 175.7 (C). MS (EI, 70 eV) m/z (%): 596 (M+, 4), 523 (100), 505 (32). Elemental Analyses calcd. for C37H32N4O4.H2O: C: 72.30, H: 5.58, N: 9.11. Found: C: 72.68, H: 5.28, N: 9.05.
Ethyl 2-benzyl-5-(4-chlorophenyl)-3-(1,3-diphenyl-1H-pyrazol-4-yl)-4,6-dioxooctahydropyrrolo[3,4-c]pyrrole-1-carboxylate. White Solid. Yield: 72%; m.p.: 108–110 °C. IR (KBr): ν 1719, 1599, 1496 cm−1. Minor diastereomer 5e. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.23 (t, J = 7.0 Hz, 3H), 3.33 (d, J = 13.6 Hz, 1H), 3.68 (dd, J = 9.7, 5.6 Hz, 1H), 3.75–3.83 (m, 1H), 3.86–3.89 (m, 1H), 3.92 (d, J = 8.0 Hz, 1H), 4.15–4.24 (m, 2H) 4.97 (d, J = 5.5 Hz, 1H), 7.09–7.13 (m, 2H), 7.22–7.26 (m, 3H), 7.32–7.36 (m, 2H), 7.41 (br. s., 3H), 7.50 (d, J = 3.3 Hz, 2H), 7.52–7.55 (m, 3H), 7.78 (br. s., 3H), 7.94 (d, J = 6.8 Hz, 1H), 8.10 (s, 1H). Major diastereomer 6e. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.28 (t, J = 7.2 Hz, 3H), 3.53 (d, J = 7.8 Hz, 1H), 3.62 (d, J = 14.1 Hz, 1H), 3.88–3.95 (m, 1H), 4.00 (d, J = 14.3 Hz, 1H), 4.12–4.21 (m, 2H), 4.39 (s, 1H), 5.05 (d, J = 9.8 Hz, 1H), 7.15–7.20 (m, 3H), 7.20–7.26 (m, 2H), 7.29–7.32 (m, 4H), 7.39–7.48 (m, 4H), 7.49–7.55 (m, 2H), 7.58 (d, J = 7.8 Hz, 2H), 7.76–7.85 (m, 3H). 13C-NMR (100 MHz, CDCl3) δ ppm: 14.2 (CH3), 48.9 (CH), 49.4 (CH), 52.2 (CH2), 60.1 (CH), 61.0 (CH2), 63.0 (CH), 118.8 (CH), 125.8 (C), 126.7 (CH), 127.4 (CH), 127.8 (CH), 128.3 (CH), 128.4 (CH), 128.5 (CH), 128.5 (CH), 128.7 (CH), 129.1 (CH), 129.3 (CH), 129.4 (CH), 130.2 (C), 132.9 (C), 134.1 (C), 137.6 (C), 139.7 (C), 153.2 (C), 170.8 (C), 174.2 (C), 175.4 (C). MS (EI, 70 eV) m/z (%): 630 (M+, 2), 557 (49), 554 (43), 553 (100), 535 (45). Elemental Analyses calcd. for C37H31ClN4O4: C: 70.41, H: 4.95, N: 8.88. Found: C: 70.72, H: 5.04, N: 8.54.
Ethyl 2-benzyl-3-(1,3-diphenyl-1H-pyrazol-4-yl)-5-(4-methoxyphenyl)-4,6-dioxooctahydropyrrolo[3,4-c]pyrrole-1-carboxylate. Yellow Solid. Yield: 90%; m.p.: 78–80 °C. IR: ν 1712, 1600, 1548 cm−1. Minor diastereomer 5f. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.26 (t, J = 7.0 Hz, 3H), 3.37 (d, J = 13.6 Hz, 1H), 3.71 (d, J = 5.5 Hz, 1H), 3.82–3.86 (m, 4H), 3.88–3.91 (m, 1H), 4.21–4.31 (m, 3H), 5.04 (d, J = 5.5 Hz, 1H), 7.01 (d, J = 9.0, 2H), 7.27–7.32 (m, 6H), 7.49–7.56 (m, 7H), 7.84 (d, J = 7.8, 2H), 8.00 (d, J = 7.0, 2H), 8.15 (s, 1H). Major diastereomer 6f. 1H-NMR (400 MHz, CDCl3) δ ppm: 1.31 (t, J = 7.2 Hz, 3H), 3.55 (d, J = 8.0 Hz, 1H), 3.66 (d, J = 14.3 Hz, 1H), 3.80 (s, 3H), 3.93 (dd, J = 9.5 Hz, 8.0 Hz 1H), 4.05 (d, J = 14.1 Hz, 1H), 4.15–4.28 (m, 2H), 4.44 (s, 1H), 5.10 (d, J = 9.5 Hz, 1H), 6.89 (d, J = 9.0 Hz, 2H), 7.16 (d, J = 8.8 Hz, 2H), 7.21–7.27 (m, 2H), 7.28–7.32 (m, 2H), 7.32–7.38 (m, 2H), 7.42–7.52 (m, 3H), 7.57 (t, J = 7.4 Hz, 2H), 7.66 (d, J = 7.5 Hz, 2H), 7.88 (d, J = 7.0 Hz, 2H), 7.91 (s, 1H). 13C-NMR (100 MHz, CDCl3) δ ppm: 14.1 (CH3), 48.7 (CH), 49.3 (CH), 52.1 (CH2), 55.3 (OCH3), 60.0 (CH), 60.9 (CH2), 63.0 (CH), 114.2 (CH), 118.7 (CH), 119.8 (C), 124.3 (C), 126.4 (CH), 126.7 (CH), 127.2 (CH), 127.7 (CH), 128.1 (CH), 128.3 (CH), 128.4 (CH), 128.5 (CH), 129.0 (CH), 129.3 (CH), 132.9 (C), 137.7 (C), 139.7 (C), 153.1 (C), 159.1 (C), 170.8 (C), 174.6 (C), 175.8 (C). MS (EI, 70 eV) m/z (%): 626 (M+, 4), 554 (39), 553 (100), 535 (42). Elemental analyses calcd. for C38H34N4O5.H2O: C: 70.79, H: 5.63, N: 8.69. Found: C: 70.56, H: 5.81, N: 8.51.
4. Conclusions
We described here a practical synthesis of pyrazoylpyrrolizines 4 and pyrazolylpyrrolidines derivatives 5 and 6 from pyrazolyl-carboxaldehydes, glycine derivates and maleimides by a three-component catalyst free domino process involving both the formation of a 1,3-dipolar species and 1,3-cycloaddition reaction to afford the desired products in good yields and with good atom economy. This high-throughput methodology provides an easy execution, rapid access and good diastereoselectivity. When the N-benzyl glycine ethyl ester was used two diastereomers 5 and 6 were obtained, with the diastereomers 6a-f being favored by the minor repulsive interaction between the carbonyl group on 1-C and 6-C=O carbon due to their trans configuration.
Acknowledgments
Authors thank COLCIENCIAS, Universidad del Valle, the Spanish “Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía” and ‘Centro de Instrumentación Científico-Técnica de la Universidad de Jaén for financial support.
Author Contributions
JQ, JG, RA, BI, AO, JC and MN designed research. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sridharan, V.; Perumal, P.; Avendaño, C.; Menéndez, C. A New Three-Component Domino Synthesis of 1,4-Dihydropyridines. Tetrahedron 2007, 63, 4407–4413. [Google Scholar] [CrossRef]
- Ugi, I. Recent Progress in the Chemistry of Multicomponent Reactions. Pure Appl. Chem. 2001, 73, 187–191. [Google Scholar]
- Zhu, J.; Bienaymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Lieby-Muller, F.; Constantieux, T.; Rodriguez, J. Highly Efficient Access to Original Polycyclic Pyrrolopiperazine Scaffolds by a Three-Component Reaction with 1,3-Dicarbonyls. Synlett 2007, 8, 1323–1325. [Google Scholar]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V. A. Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef]
- Ramón, D.J.; Yus, M. Asymmetric Multicomponent Reactions (AMCRs): The New Frontier. Angew. Chem. Int. Ed. 2005, 44, 1602–1634. [Google Scholar] [CrossRef]
- Ayerbe, M.; Arrieta, A.; Cossío, F. Stereocontrolled Synthesis of Highly Substituted Proline Esters via [3 + 2] Cycloaddition between N-Metalated Azomethine Ylides and Nitroalkenes. Origins of the Metal Effect on the Stereochemical Outcome. J. Org. Chem. 1998, 63, 1795–1805. [Google Scholar] [CrossRef]
- Garner, P.; Kaniskan, Ü. Synthesis of Highly Functionalized Pyrrolidines via a Mild One-Pot, Three-Component 1,3-Dipolar Cycloaddition Process. J. Org. Chem. 2005, 70, 10868–10871. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J. Enantioselective Synthesis of Proline Derivatives by 1,3-Dipolar Cycloadditions. Monatsh. Chem. 2011, 142, 659–680. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Y.; Wang, S.; Gentles, R.; Sowin, T.; Kati, W.; Muchmore, S.; Giranda, V.; Stewart, K.; Sham, H.; et al. Design, Synthesis, and Structural Analysis of Influenza Neuraminidase Inhibitors Containing Pyrrolidine Cores. J. Med. Chem. 2001, 44, 1192–1201. [Google Scholar] [CrossRef]
- Belskaya, N.; Bakulev, V.; Deryavina, T.; Subbotina, J.; Koddes, M.; Dehaen, W.; Toppet, S.; Robeyns, K.; van Meervely, L. 3-Alkylsulfanyl-2-Arylazo-3-(pyrrolidin-1-yl)-Acrylonitriles as Masked 1,3-Dipoles. Tetrahedron 2009, 65, 7662–7672. [Google Scholar] [CrossRef]
- Kathiravan, S.; Ramesh, E.; Raghunathan, R. Synthesis of pyrrolo[2,3-a]pyrrolizine and pyrrolizine[2,3-a]pyrrolizine Derived from Allyl Derivatives of Baylis–Hillman Adducts through Intramolecular 1,3-Dipolar Cycloaddition. Tetrahedron Lett. 2009, 50, 2389–2391. [Google Scholar] [CrossRef]
- Barker. G.; McGrath, J.; Klapars, A.; Stead, D.; Zhou, G.; Campos, K.; O’Brien, P. Enantioselective, Palladium-Catalyzed α-Arylation of N-Boc Pyrrolidine: In Situ React IR Spectroscopic Monitoring, Scope, and Synthetic Applications. J. Org. Chem. 2011, 76, 5936–5953. [Google Scholar] [CrossRef]
- Yao, S.; Gallenkamp, D.; Wölfel, K.; Lüke, B.; Schindler, M.; Scherkenbeck, J. Synthesis and SERCA Activities of Structurally Simplified Cyclopiazonic Acid Analogues. Bioorg. Med. Chem. 2011, 19, 4669–4678. [Google Scholar] [CrossRef]
- Deslandes, S.; Lamoral-Theys, D.; Frongia, C.; Chassaing, S.; Bruyùre, C.; Lozach, O.; Meijer, L.; Ducommun, B.; Kiss, R.; Delfourne, D. Synthesis and Biological Evaluation of Analogs of the Marine Alkaloids Granulatimide and Isogranulatimide. Eur. J. Med. Chem. 2012, 54, 626–636. [Google Scholar] [CrossRef]
- Eklund, E.; Pike, R.; Scheerer, J. Synthesis of 1-Aminopyrrolizidine Alkaloid (−)-Absouline by Stereoselective Aminoconjugate Addition. Tetrahedron Lett. 2012, 53, 4644–4647. [Google Scholar] [CrossRef]
- Kang, T.; Cheng, Y.; He, L.; Ye, J.; Liu, Q. Facile Synthesis of Highly Functional Pyrrolizidine Derivatives from β,γ-Unsaturated α-Keto Esters and Proline via a Tandem Cycloaddition. Tetrahedron Lett. 2012, 53, 2552–2555. [Google Scholar] [CrossRef]
- Toyooka, N.; Zhou, D.; Tezuka, Y.; Kadota, S.; Andriamaharavo, N.; Martin Garraffo, H.; Spande, T.; Daly, J. Efficient Enantio- and Diastereodivergent Synthesis of Poison-Frog Alkaloids 251O and Trans-223B. J. Org. Chem. 2009, 74, 6784–6791. [Google Scholar] [CrossRef]
- Stevens, K.; Tyrell, A.; Skerratt, S.; Robertson, J. Synthesis of NP25302. Org. Lett. 2011, 13, 5964–5967. [Google Scholar] [CrossRef]
- Georgiou, D.; Toutountzoglou, V.; Muir, K.; Hadjipavlou-Litina, D.; Elemes, J. Synthesis of Sulfur Containing Dihydro-Pyrrolo Derivatives and Their Biological Evaluation as Antioxidants. Bioorg. Med. Chem. 2012, 20, 5103–5109. [Google Scholar] [CrossRef]
- Rotstein, D.; Melville, C.; Padilla, F.; Cournoyer, D.; Lee, E.; Lemoine, R.; Petersen, A.; Setti, L.; Wanner, J.; Chen, L.; et al. Novel hexahydropyrrolo[3,4-c]pyrrole CCR5 Antagonists. Bioorg. Med. Chem. 2010, 20, 3116–3119. [Google Scholar] [CrossRef]
- Zhang, K.; Tieke, B.; Forgie, J.; Vilela, F.; Parkinson, J.; Skabara, P. Cross-Linked Polymers Based on 2,3,5,6-Tetra-Substituted pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-Dione (DPP): Synthesis, Optical and Electronic Properties. Polymer 2010, 51, 6107–6114. [Google Scholar] [CrossRef]
- Quiroga, J.; Portilla, J.; Abonía, R.; Insuasty, B.; Nogueras, J.; Cobo, J. Synthesis of Novel 5-Amino-1-Aroylpyrazoles. Tetrahedron Lett. 2008, 49, 5943–5945. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S.; Kumar, P.; Kaushik, P.; Kaushik, D.; Dhingra, Y.; Aneja, K. Synthesis and Biological Evaluation of Some Pyrazolylpyrazolines as Anti-Inflammatory–antimicrobial Agents. Eur. J. Med. Chem. 2010, 45, 2650–2655. [Google Scholar] [CrossRef]
- Chauhan, A.; Sharma, P.; Kaushik, N. Pyrazole: A Versatile Moiety. Int. J. ChemTech Res. 2011, 3, 11–17. [Google Scholar]
- Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. From 2000 to Mid-2010: A Fruitful Decade for the Synthesis of Pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Hassan, H.; Habib, O.; Moawad, E.; El-Bana, G.; Defrawy, A. Synthesis of Some Novel Antioxidant and Anticorrosive Additives for Egyptian Gasoline Motor Oils. Lubr. Sci. 2011, 23, 119–138. [Google Scholar] [CrossRef]
- Quiroga, J.; Gálvez, J.; Pérez, A.; Valencia, A.; Abonía, R.; Insuasty, B. Catalyst Free Three-Component Synthesis of (±)-Pyrazolylpyrrolopyrroles by 1,3-Dipolar Cycloaddition Reaction. Tetrahedron Lett. 2011, 52, 5471–5473. [Google Scholar] [CrossRef]
- Quiroga, J.; Gálvez, J.; Cobo, J.; Glidewell, C. Two Methyl 3-(1H-Pyrazol-4yl) octahydropyrrolo[3,4-c]pyrrole-1-Carboxylates Form Different Hydrogen-Bonded Sheets. Acta Cryst. 2013, 69, 915–919. [Google Scholar]
- Quiroga, J.; Portillo, S.; Pérez, A.; Gálvez, J.; Abonía, R.; Insuasty, B. An Efficient Synthesis of pyrazolo[3,4-b]pyridine-4-spiroindolinones by a Three-Component Reaction of 5-Aminopyrazoles, Isatin, and Cyclic β-Diketones. Tetrahedron Lett. 2011, 52, 2664–2666. [Google Scholar] [CrossRef]
- Quiroga, J.; Trilleras, J.; Pantoja, D.; Abonía, R.; Insuasty, B. Microwave-Assisted Synthesis of pyrazolo[3,4-b]pyridine-Spirocycloalkanediones by Three-Component Reaction of 5-Aminopyrazole Derivatives, Paraformaldehyde and Cyclic β-Diketones. Tetrahedron Lett. 2010, 51, 4717–4719. [Google Scholar] [CrossRef]
- Cui, P.; Xu, L.; Shi, Z.; Gan, L. Synthesis of Decahydropyrrolo[2,1,5-cd]indolizine through Consecutive [2 + 3] Cycloadditions and 6-Exo-Trig Cyclization. J. Org. Chem. 2011, 76, 4210–4212. [Google Scholar] [CrossRef]
- Cui, P.; Xu, L.; Cheng, H.; Gan, L. Synthesis of decahydropyrrolo[2,1,5-cd]indolizine Derivatives through RuCl3/AgOTf Induced Alkene–alkene and Alkene–arene Double Cycloisomerizations. Tetrahedron 2012, 68, 152–158. [Google Scholar] [CrossRef]
- Lu, Q.; Song, G.; Jasinski, J.; Keeley, A.; Zhang, W. One-Pot Double [3 + 2] Cycloaddition for Diastereoselective Synthesis of Tetracyclic Pyrrolidine Compounds. Green. Chem. 2012, 14, 3010–3012. [Google Scholar] [CrossRef]
- Petrovskaia, O.; Taylor, B.; Hauze, D.; Carroll, P.; Joullié, M. Investigations of the Reaction Mechanisms of 1,2-Indanediones with Amino Acids. J. Org. Chem. 2001, 66, 7666–7675. [Google Scholar] [CrossRef]
- Bashiardes, G.; Safir, I.; Said-Mohamed, A.; Barbot, F.; Laduranty, J. Microwave-Assisted [3 + 2] Cycloadditions of Azomethine Ylides. Org. Lett. 2003, 5, 4915–4918. [Google Scholar] [CrossRef]
- Elboray, E.; Grigg, R.; Fishwick, C.; Kilner, C.; Sarker, M.; Aly, M.; Abbas, H. XY–ZH Compounds as Potential 1,3-Dipoles. Part 65: Atom Economic Cascade Synthesis of Highly Functionalized Pyrimidinylpyrrolidines. Tetrahedron 2011, 67, 5700–5710. [Google Scholar] [CrossRef]
- Quiroga, J.; Gálvez, J.; Cobo, J.; Glidewell, C. Methyl (3aRS,3cRS,6cSR,7RS,8RS,8aSR)-2,5-bis(4-Chlorophenyl)-7,9-bis(1,3-Diphenyl-1H-Pyrazol-4-yl)-1,3,4,6-Tetraoxododecahydro-1H-dipyrrolo[3,4-a:3',4'-f]pyrrolizine-3b-Carboxylate Dimethylformamide Disolvate: A Three Dimensional Hydrogen-Bonded Framework. Acta Cryst. 2012, 68, 439–442. [Google Scholar]
- Kudryavtsev, K.; Irkha, V. Three-Component Synthesis of Polysubstituted Homoproline Analogs. Molecules 2005, 10, 755–761. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Karplus, M. Contact Electron‐Spin Coupling of Nuclear Magnetic Moments. J. Chem. Phys. 1959, 30, 11–15. [Google Scholar] [CrossRef]
- Kalgutkar, A.; Crews, B.C.; Marnett, L.J. Design, Synthesis, and Biochemical Evaluation of N-Substituted Maleimides as Inhibitors of Prostaglandin Endoperoxide Synthases†. J. Med. Chem. 1996, 39, 1692–1703. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a–j, 5a–f and 6a–f are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).