Water Extract of Acer tegmentosum Reduces Bone Destruction by Inhibiting Osteoclast Differentiation and Function
Abstract
:1. Introduction
2. Results and Discussion
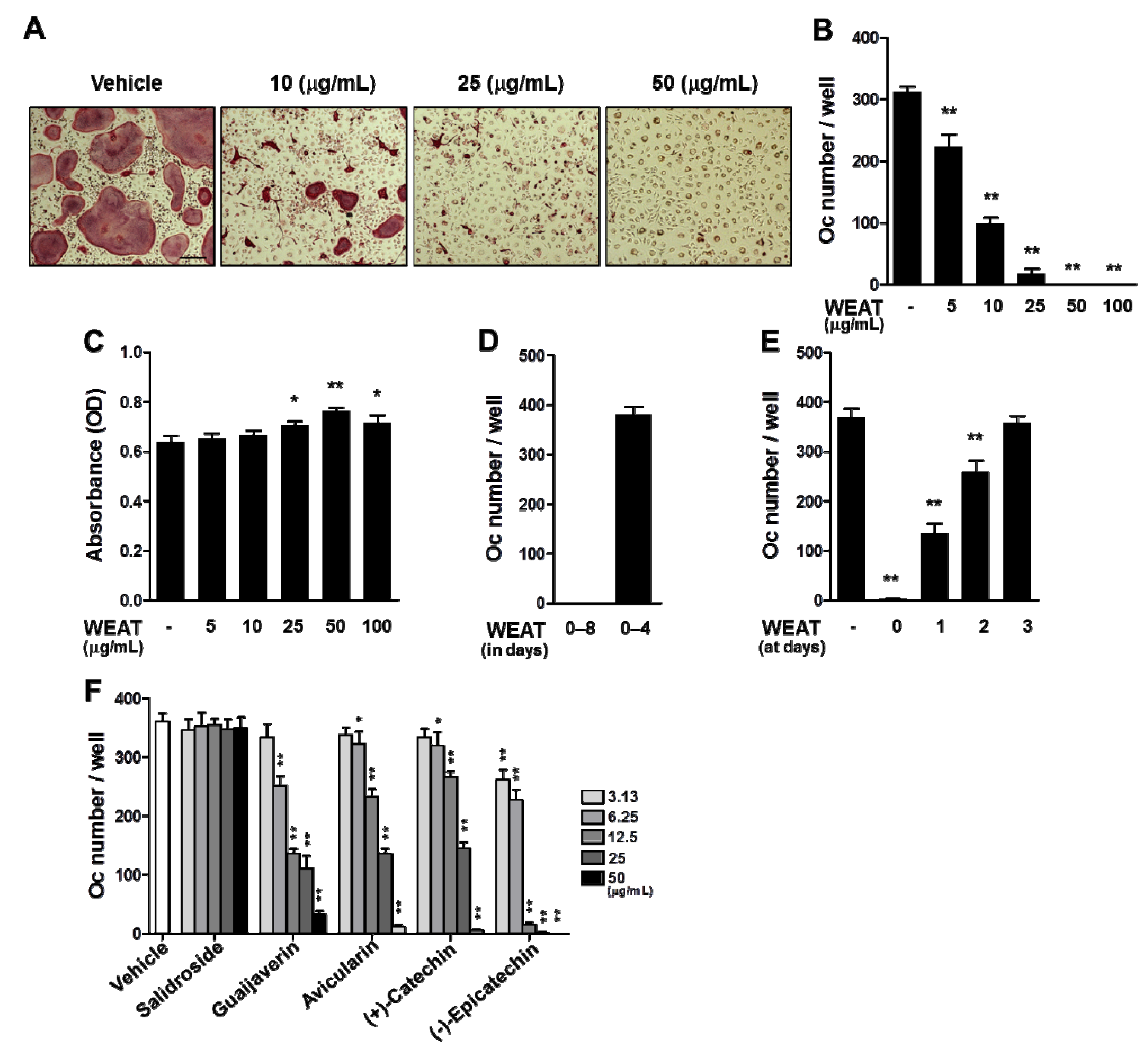
2.1. WEAT Inhibits RANKL-Induced Osteoclast Differentiation
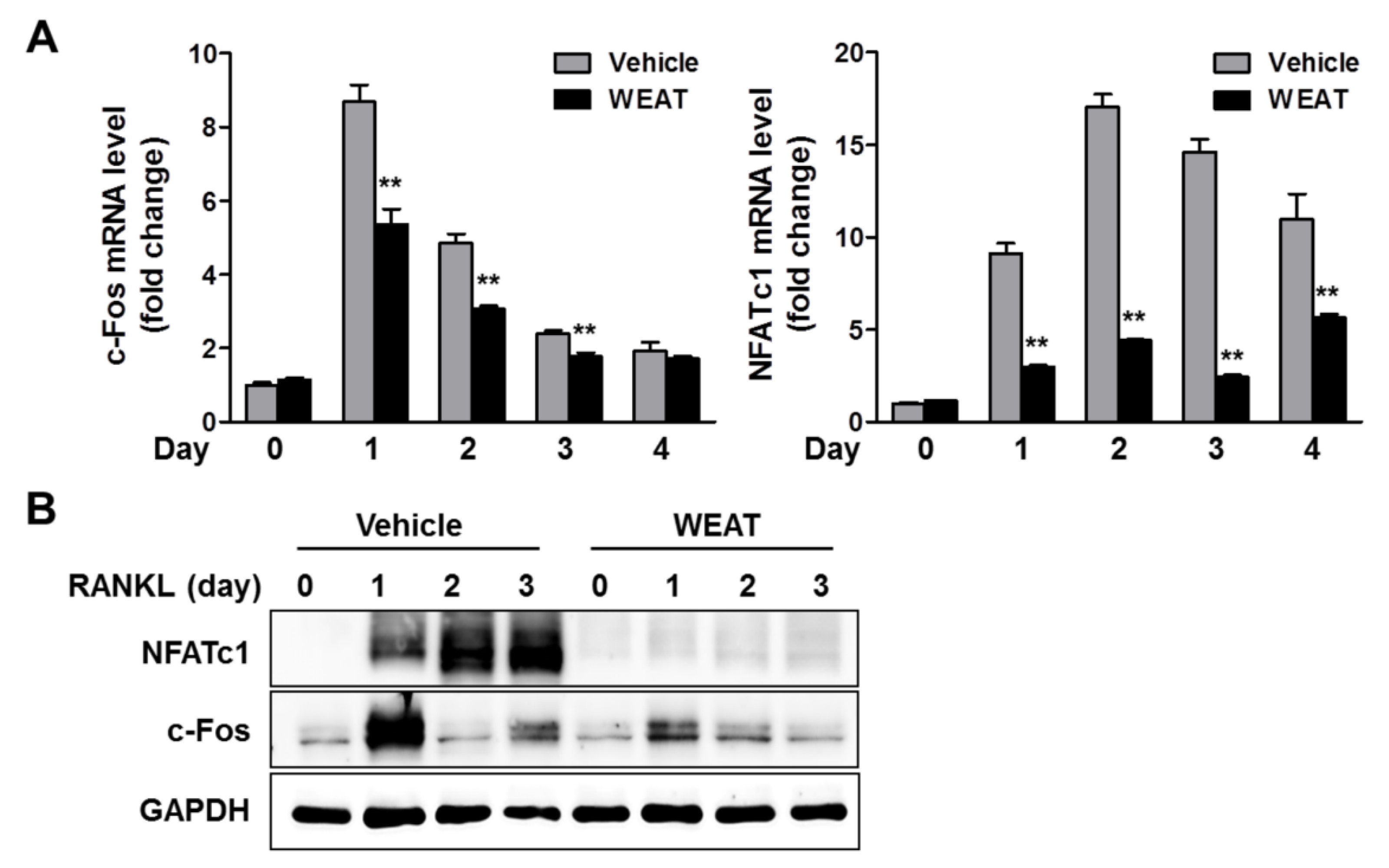
2.2. WEAT Inhibits RANKL-Induced c-Fos and NFATc1 Expression in Osteoclast Precursor Cells
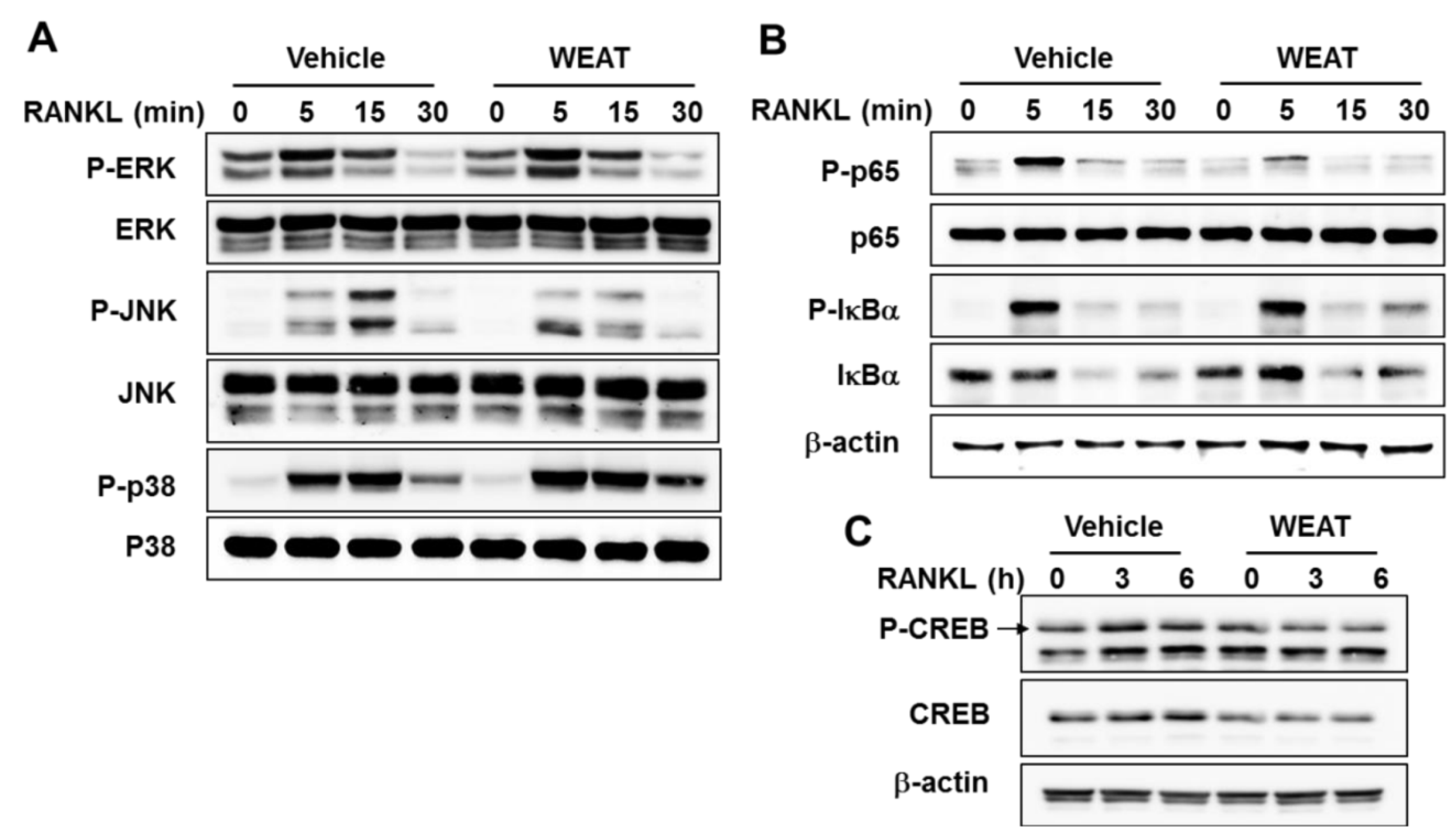
2.3. WEAT Inhibits RANKL-Induced JNK, NF-κB, and CREB Activation
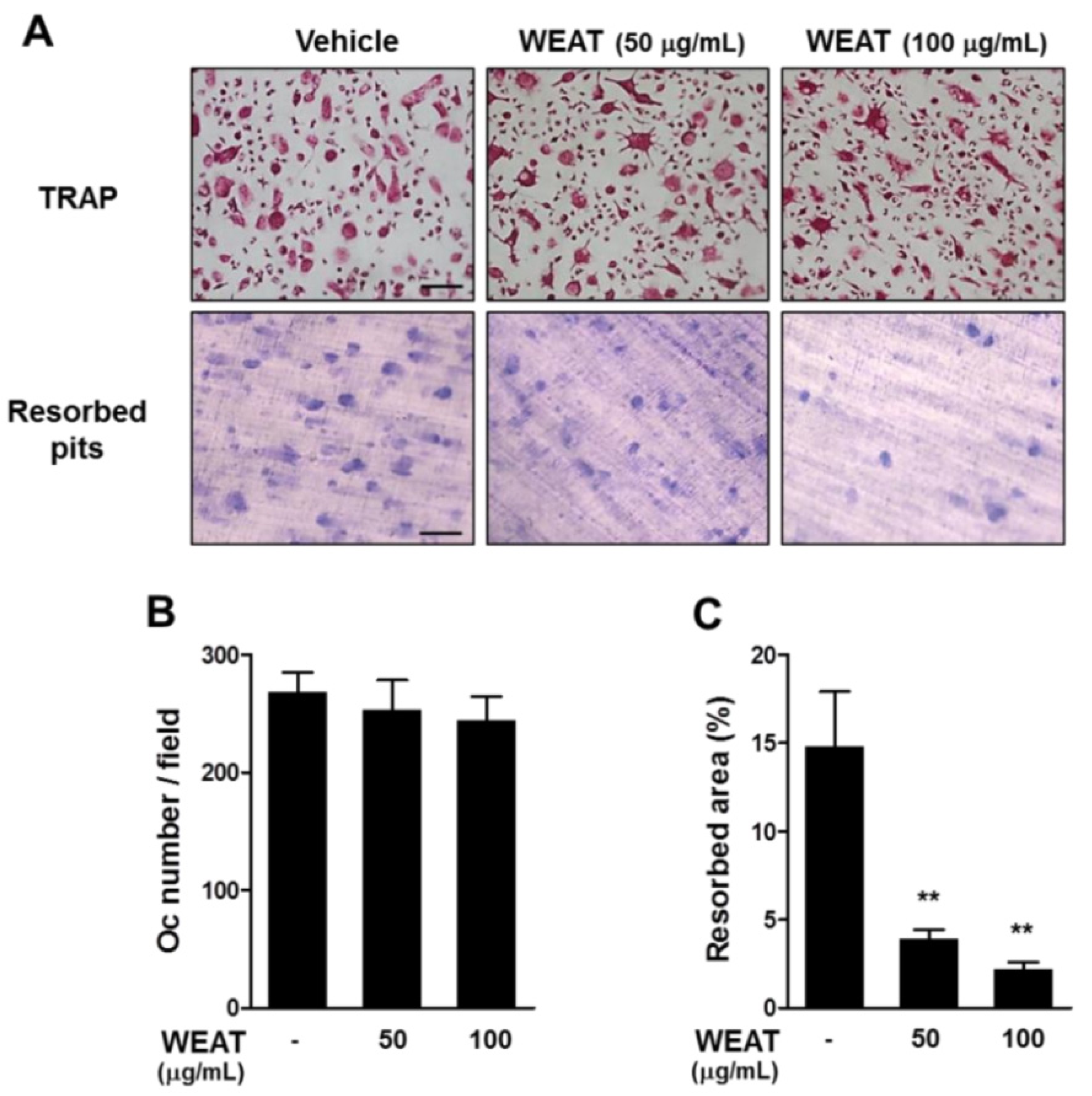
2.4. WEAT Inhibits Bone Resorbing Activity of Osteoclasts
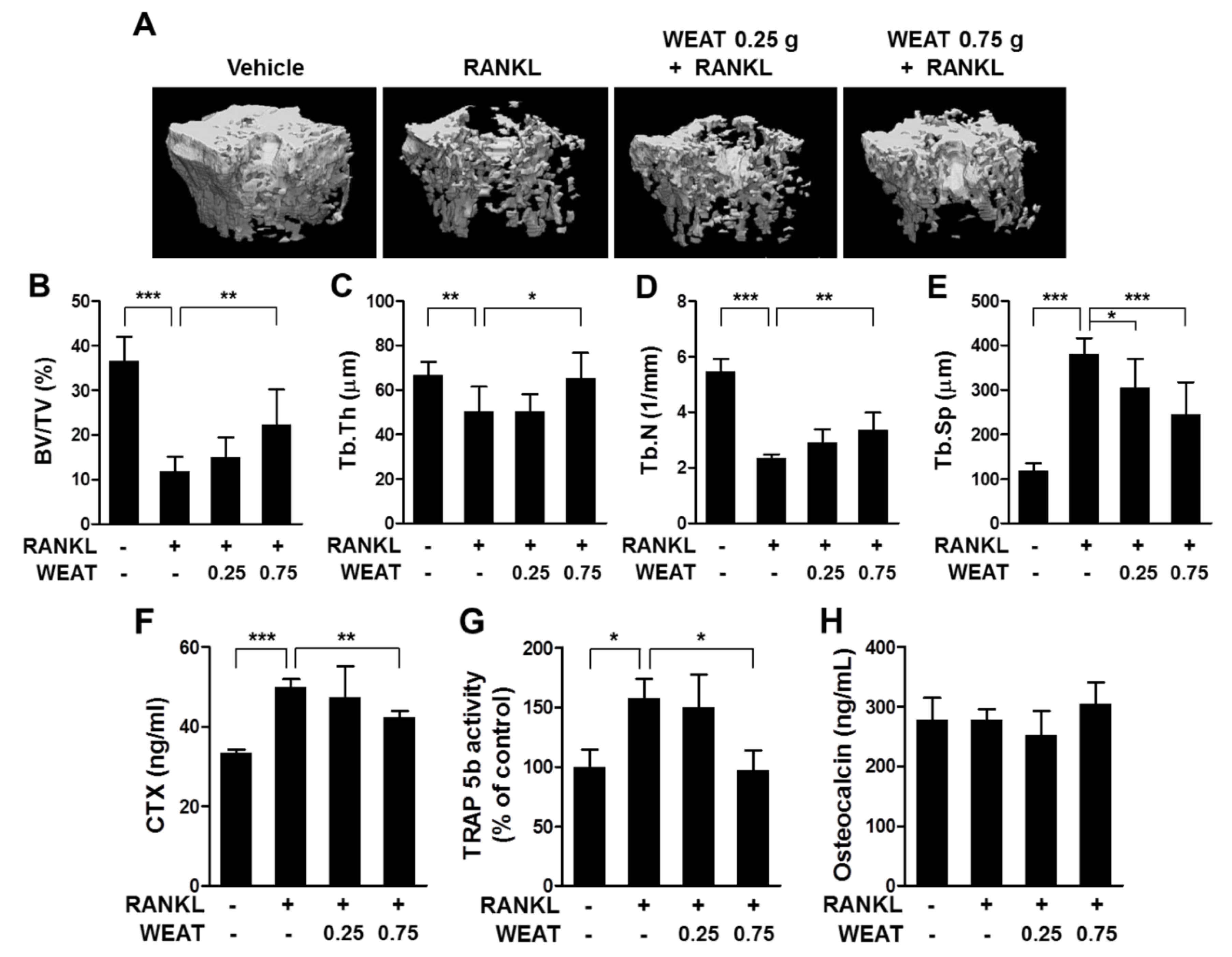
2.5. WEAT Reduces RANKL-Induced Bone Destruction in Mice
3. Experimental
3.1. Reagents and Antibodies
3.2. Preparation of WEAT
3.3. Cell Culture and Osteoclast Differentiation
3.4. TRAP Assay
3.5. Cell Viability Assay
3.6. Bone Resorption Assay
3.7. qPCR Analysis
3.8. Western Blot Analysis
3.9. Animals and RANKL-Induced Osteoporosis Model
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef]
- Gohda, J.; Akiyama, T.; Koga, T.; Takayanagi, H.; Tanaka, S.; Inoue, J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005, 24, 790–799. [Google Scholar] [CrossRef]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Huang, H.; Chang, E.J.; Ryu, J.; Lee, Z.H.; Lee, Y.; Kim, H.H. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem. Biophys. Res. Commun. 2006, 351, 99–105. [Google Scholar] [CrossRef]
- Lee, J.H.; Jin, H.; Shim, H.E.; Kim, H.N.; Ha, H.; Lee, Z.H. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the nuclear factor-kappaB signal. Mol. Pharmacol. 2010, 77, 17–25. [Google Scholar] [CrossRef]
- Ikeda, F.; Nishimura, R.; Matsubara, T.; Tanaka, S.; Inoue, J.; Reddy, S.V.; Hata, K.; Yamashita, K.; Hiraga, T.; Watanabe, T.; et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Invest. 2004, 114, 475–484. [Google Scholar] [CrossRef]
- Sato, K.; Suematsu, A.; Nakashima, T.; Takemoto-Kimura, S.; Aoki, K.; Morishita, Y.; Asahara, H.; Ohya, K.; Yamaguchi, A.; Takai, T.; et al. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat. Med. 2006, 12, 1410–1416. [Google Scholar]
- Tanaka, S.; Nakamura, K.; Takahasi, N.; Suda, T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol. Rev. 2005, 208, 30–49. [Google Scholar]
- Banu, J.; Varela, E.; Fernandes, G. Alternative therapies for the prevention and treatment of osteoporosis. Nutr. Rev. 2012, 70, 22–40. [Google Scholar] [CrossRef]
- Putnam, S.E.; Scutt, A.M.; Bicknell, K.; Priestley, C.M.; Williamson, E.M. Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother. Res. 2007, 21, 99–112. [Google Scholar] [CrossRef]
- Yu, T.; Lee, J.; Lee, Y.G.; Byeon, S.E.; Kim, M.H.; Sohn, E.H.; Lee, Y.J.; Lee, S.G.; Cho, J.Y. In vitro and in vivo anti-inflammatory effects of ethanol extract from Acer tegmentosum. J. Ethnopharmacol. 2010, 128, 139–147. [Google Scholar] [CrossRef]
- Liu, Q.; Shin, E.; Ahn, M.J.; Hwang, B.Y.; Lee, M.K. Anti-adipogenic activity of Acer tegmentosum and its constituent, catechin in 3T3-L1 cells. Nat. Prod. Sci. 2011, 17, 212–215. [Google Scholar]
- Kim, S.; Hur, S.H.; Kim, K.H.; Kim, S.G.; Whang, W.K. Antioxidant and anti-inflammatory compounds isolated from Acer tegmentosum. J. Med. Plants Res. 2012, 6, 3971–3976. [Google Scholar]
- Lee, S.; Woo, H. A study of the inhibitory effect of Acer tegmentosum Max. on fibrogenesis in hepatic stellate cell line T6. Korean J. Orient. Int. Med. 2010, 31, 346–355. [Google Scholar]
- Yoo, Y.M.; Nam, J.H.; Kim, M.Y.; Choi, J.; Lee, K.T.; Park, H.J. Analgesic and anti-gastropathic effects of salidroside Isolated from Acer tegmentosum heartwood. Open Bioact. Compound. J. 2009, 2, 1–7. [Google Scholar] [CrossRef]
- Tung, N.H.; Ding, Y.; Kim, S.K.; Bae, K.; Kim, Y.H. Total peroxyl radical-scavenging capacity of the chemical components from the stems of Acer tegmentosum maxim. J. Agric. Food Chem. 2008, 56, 10510–10514. [Google Scholar] [CrossRef]
- Park, K.M.; Yang, M.C.; Lee, K.H.; Kim, K.R.; Choi, S.U.; Lee, K.R. Cytotoxic phenolic constituents of Acer tegmentosum maxim. Arch. Pharm. Res. 2006, 29, 1086–1090. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Park, H.; Ma, J.Y. In vitro and in vivo safety evaluation of Acer tegmentosum. J. Ethnopharmacol. 2013, 148, 99–105. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lian, L.H.; Jiang, Y.Z.; Nan, J.X. Hepatoprotective effects of salidroside on fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide in mice. J. Pharm. Pharmacol. 2009, 61, 1375–1382. [Google Scholar] [CrossRef]
- Li, D.; Fu, Y.; Zhang, W.; Su, G.; Liu, B.; Guo, M.; Li, F.; Liang, D.; Liu, Z.; Zhang, X.; et al. Salidroside attenuates inflammatory responses by suppressing nuclear factor-kappaB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflamm. Res. 2013, 62, 9–15. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Sun, Y.; Lin, X.; Chen, B.; Tan, C.; Cao, G.; Wang, Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2007, 564, 18–25. [Google Scholar]
- Zhang, B.C.; Li, W.M.; Guo, R.; Xu, Y.W. Salidroside decreases atherosclerotic plaque formation in low-density lipoprotein receptor-deficient mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 607508. [Google Scholar]
- Sheng, Q.S.; Wang, Z.J.; Zhang, J.; Zhang, Y.G. Salidroside promotes peripheral nerve regeneration following crush injury to the sciatic nerve in rats. Neuroreport 2013, 24, 217–223. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhang, N.F.; Mao, G.X.; He, X.B.; Zhan, Y.C.; Deng, H.B.; Song, D.Q.; Li, D.D.; Li, Z.R.; Si, S.Y.; et al. Salidroside stimulates osteoblast differentiation through BMP signaling pathway. Food Chem. Toxicol. 2013, 62, 499–505. [Google Scholar] [CrossRef]
- Zhang, J.K.; Yang, L.; Meng, G.L.; Yuan, Z.; Fan, J.; Li, D.; Chen, J.Z.; Shi, T.Y.; Hu, H.M.; Wei, B.Y.; et al. Protection by salidroside against bone loss via inhibition of oxidative stress and bone-resorbing mediators. PLoS One 2013, 8, e57251. [Google Scholar]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, Y.; Chang, E.J.; Kim, H.M.; Hong, S.P.; Lee, Z.H.; Ryu, J.; Kim, H.H. Suppression of osteoclastogenesis by N,N-dimethyl-D-erythro-sphingosine: A sphingosine kinase inhibition-independent action. Mol. Pharmacol. 2007, 72, 418–428. [Google Scholar] [CrossRef]
- Iotsova, V.; Caamano, J.; Loy, J.; Yang, Y.; Lewin, A.; Bravo, R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat. Med. 1997, 3, 1285–1289. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef]
- Jimi, E.; Aoki, K.; Saito, H.; D'Acquisto, F.; May, M.J.; Nakamura, I.; Sudo, T.; Kojima, T.; Okamoto, F.; Fukushima, H.; et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat. Med. 2004, 10, 617–624. [Google Scholar]
- Dai, S.; Hirayama, T.; Abbas, S.; Abu-Amer, Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J. Biol. Chem. 2004, 279, 37219–37222. [Google Scholar]
- Ruocco, M.G.; Maeda, S.; Park, J.M.; Lawrence, T.; Hsu, L.C.; Cao, Y.; Schett, G.; Wagner, E.F.; Karin, M. I{kappa}B kinase (IKK){beta}, but not IKK{alpha}, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J. Exp. Med. 2005, 201, 1677–1687. [Google Scholar] [CrossRef]
- Yang, F.; Tang, E.; Guan, K.; Wang, C.Y. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 2003, 170, 5630–5635. [Google Scholar]
- Doyle, S.L.; Jefferies, C.A.; O'Neill, L.A. Bruton's tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFkappaB activation by lipopolysaccharide. J. Biol. Chem. 2005, 280, 23496–23501. [Google Scholar] [CrossRef]
- Sasaki, C.Y.; Barberi, T.J.; Ghosh, P.; Longo, D.L. Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B pathway. J. Biol. Chem. 2005, 280, 34538–34547. [Google Scholar] [CrossRef]
- Huang, H.; Ryu, J.; Ha, J.; Chang, E.J.; Kim, H.J.; Kim, H.M.; Kitamura, T.; Lee, Z.H.; Kim, H.H. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ. 2006, 13, 1879–1891. [Google Scholar] [CrossRef]
- Tomimori, Y.; Mori, K.; Koide, M.; Nakamichi, Y.; Ninomiya, T.; Udagawa, N.; Yasuda, H. Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J. Bone Miner. Res. 2009, 24, 1194–1205. [Google Scholar] [CrossRef]
- Naylor, K.; Eastell, R. Bone turnover markers: Use in osteoporosis. Nat. Rev. Rheumatol. 2012, 8, 379–389. [Google Scholar] [CrossRef]
- Ha, H.; An, H.; Shim, K.S.; Kim, T.; Lee, K.J.; Hwang, Y.H.; Ma, J.Y. Ethanol extract of Atractylodes macrocephala protects bone loss by inhibiting osteoclast differentiation. Molecules 2013, 18, 7376–7388. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.N.; Yang, D.; Jung, K.; Kim, H.M.; Kim, H.H.; Ha, H.; Lee, Z.H. Trolox prevents osteoclastogenesis by suppressing RANKL expression and signaling. J. Biol. Chem. 2009, 284, 13725–13734. [Google Scholar] [CrossRef]
- Janckila, A.J.; Takahashi, K.; Sun, S.Z.; Yam, L.T. Naphthol-ASBI phosphate as a preferred substrate for tartrate-resistant acid phosphatase isoform 5b. J. Bone Miner. Res. 2001, 16, 788–793. [Google Scholar] [CrossRef]
- Sample Availability: Sample of WEAT is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ha, H.; Shim, K.-S.; Kim, T.; An, H.; Lee, C.-J.; Lee, K.J.; Ma, J.Y. Water Extract of Acer tegmentosum Reduces Bone Destruction by Inhibiting Osteoclast Differentiation and Function. Molecules 2014, 19, 3940-3954. https://doi.org/10.3390/molecules19043940
Ha H, Shim K-S, Kim T, An H, Lee C-J, Lee KJ, Ma JY. Water Extract of Acer tegmentosum Reduces Bone Destruction by Inhibiting Osteoclast Differentiation and Function. Molecules. 2014; 19(4):3940-3954. https://doi.org/10.3390/molecules19043940
Chicago/Turabian StyleHa, Hyunil, Ki-Shuk Shim, Taesoo Kim, Hyosun An, Chung-Jo Lee, Kwang Jin Lee, and Jin Yeul Ma. 2014. "Water Extract of Acer tegmentosum Reduces Bone Destruction by Inhibiting Osteoclast Differentiation and Function" Molecules 19, no. 4: 3940-3954. https://doi.org/10.3390/molecules19043940
APA StyleHa, H., Shim, K.-S., Kim, T., An, H., Lee, C.-J., Lee, K. J., & Ma, J. Y. (2014). Water Extract of Acer tegmentosum Reduces Bone Destruction by Inhibiting Osteoclast Differentiation and Function. Molecules, 19(4), 3940-3954. https://doi.org/10.3390/molecules19043940



