Sesquiterpenes from the Brazilian Red Alga Laurencia dendroidea J. Agardh
Abstract
:1. Introduction
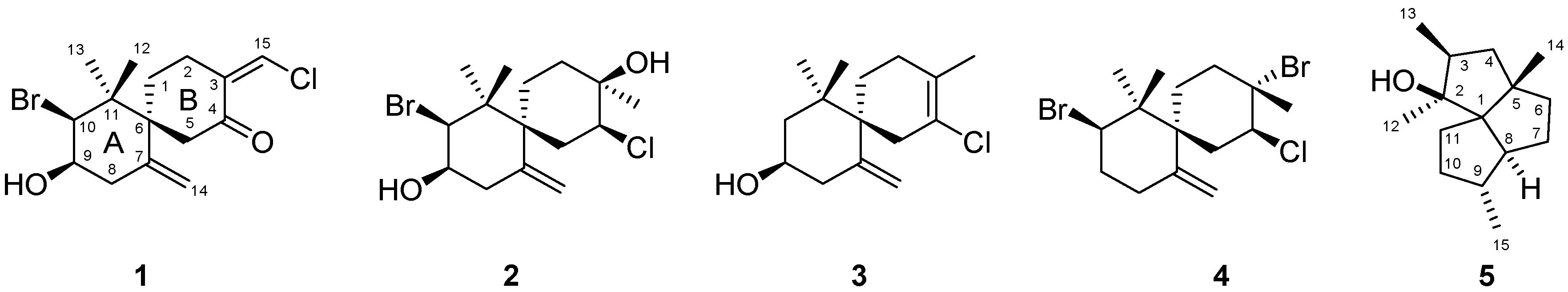
2. Results and Discussion
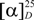 = +6.0° (c 0.06, CHCl3). The molecular formula was established as C15H20BrClO2 on the basis of HR-APCI-MS data (m/z 349.0381 [M+H]+, calcd. for C15H21BrClO2, 349.0393), implying five degrees of unsaturation. The infrared (IR) spectrum exhibited absorptions of hydroxyl (3,457 cm−1) and carbonyl (1,675 cm−1) groups. The 1H-NMR spectrum displayed signals corresponding to two methyl groups at δH 1.09 (H3-12) and 1.11 (H3-13), two methines geminal to heteroatoms at δH 4.67 (H-10) and δH 4.21 (H-9) and three olefinic hydrogens at δH 5.16 (H-14a), δH 4.77 (H-14b) and one highly deshielded at δH 7.24 (H-15), suggesting it was part of a conjugated system. 13C and HSQC experiments revealed the presence of two methyls, four aliphatic methylenes, two deshielded methines, two quaternary sp3 carbons and five sp2 carbon resonances that were assigned to a ketone [δC 197.6 (C-4)] and two olefins [δC 117.5 (C-14), 131.3 (C-15), 136.1 (C-3), 142.6 (C-7)]. The positions of the bromine and the hydroxyl groups were deduced from the carbon chemical shifts of carbons at δC 69.6 (C-10) and 72.0 (C-9), respectively [21]. The presence of an exomethylene group involving C-7 and C-14 was suggested by HMBC correlation between broadened olefinic singlets at δH 5.16 (H-14a) and δH 4.77 (H-14b) with carbons at δC 48.7 (C-6) and 38.6 (C-8). From the 1H-1H-COSY NMR spectrum the coupling between methylene protons at δH 2.58 (H2-8) and the proton on carbinolic group at δH 4.21 (H-9) was observed. The latter also couples with the proton on carbon bearing a bromine at δH 4.67 (H-10). Ring A was established by HMBC correlations from methyls at δH 1.09 (H3-12) and 1.11 (H3-13) to the respective carbons [δC 22.7 (C-13); δC 20.8 (C-12)] and to δC 48.7 (C-6), 69.6 (C-10) and 43.0 (C-11).
= +6.0° (c 0.06, CHCl3). The molecular formula was established as C15H20BrClO2 on the basis of HR-APCI-MS data (m/z 349.0381 [M+H]+, calcd. for C15H21BrClO2, 349.0393), implying five degrees of unsaturation. The infrared (IR) spectrum exhibited absorptions of hydroxyl (3,457 cm−1) and carbonyl (1,675 cm−1) groups. The 1H-NMR spectrum displayed signals corresponding to two methyl groups at δH 1.09 (H3-12) and 1.11 (H3-13), two methines geminal to heteroatoms at δH 4.67 (H-10) and δH 4.21 (H-9) and three olefinic hydrogens at δH 5.16 (H-14a), δH 4.77 (H-14b) and one highly deshielded at δH 7.24 (H-15), suggesting it was part of a conjugated system. 13C and HSQC experiments revealed the presence of two methyls, four aliphatic methylenes, two deshielded methines, two quaternary sp3 carbons and five sp2 carbon resonances that were assigned to a ketone [δC 197.6 (C-4)] and two olefins [δC 117.5 (C-14), 131.3 (C-15), 136.1 (C-3), 142.6 (C-7)]. The positions of the bromine and the hydroxyl groups were deduced from the carbon chemical shifts of carbons at δC 69.6 (C-10) and 72.0 (C-9), respectively [21]. The presence of an exomethylene group involving C-7 and C-14 was suggested by HMBC correlation between broadened olefinic singlets at δH 5.16 (H-14a) and δH 4.77 (H-14b) with carbons at δC 48.7 (C-6) and 38.6 (C-8). From the 1H-1H-COSY NMR spectrum the coupling between methylene protons at δH 2.58 (H2-8) and the proton on carbinolic group at δH 4.21 (H-9) was observed. The latter also couples with the proton on carbon bearing a bromine at δH 4.67 (H-10). Ring A was established by HMBC correlations from methyls at δH 1.09 (H3-12) and 1.11 (H3-13) to the respective carbons [δC 22.7 (C-13); δC 20.8 (C-12)] and to δC 48.7 (C-6), 69.6 (C-10) and 43.0 (C-11).| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | |
| 1a 1b | 25.6 | 2.16 dm (13.6) 1.76 ddd (14.0, 13.6, 4.8) | 22.6 | 2.00 brd (13.0) 1.60 brd (13.0) | 25.8 | 1.89 m 1.50 m |
| 2a 2b | 24.2 | 2.81 m 2.08 m | 33.8 | 1.70 dt (13.0, 4.0, 3.2) 1.20 m | 29.5 | 1.86 m 1.25 m |
| 3 | 136.1 | - | 70.3 | - | 124.6 | - |
| 4 | 197.6 | - | 67.4 | 4.23 dd (11.0, 5.0) | 128.2 | - |
| 5a 5b | 45.5 | 2.85 dd (17.5, 3.5) 2.46 d (17.5) | 35.1 | 2.10 m | 37.5 | 2.48 m 2.15 m |
| 6 | 48.7 | - | 50.5 | - | 46.6 | - |
| 7 | 142.6 | - | 141.9 | - | 145.2 | - |
| 8a 8b | 38.6 | 2.58 d (3.0) | 38.5 | 2.32 dd (14.0, 2.7) 2.51 dd (14.0, 2.7) | 41.7 | 2.45 m 2.16 m |
| 9 | 72.0 | 4.21 q (3.0) | 72.0 | 4.07 m | 67.9 | 3.76 ddd (16.0, 10.5, 5.0) |
| 10 | 69.6 | 4.67 d (3.0) | 70.7 | 4.52 d (3.0) | 45.9 | 1.66 m 1.53 m |
| 11 | 43.0 | - | 44.2 | - | 37.5 | - |
| 12 | 20.8 | 1.09 s | 20.4 | 1.03 s | 23.8 | 0.84 s |
| 13 | 22.7 | 1.11 s | 24.1 | 1.06 s | 24.8 | 0.94 s |
| 14a 14b | 117.5 | 5.16 s 4.77 s | 116.7 | 5.25 s 4.97 s | 113.5 | 5.01 s 4.60 s |
| 15 | 131.3 | 7.24 m | 28.5 | 1.21 s | 19.5 | 1.70 s |
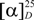 = −19.2° (c 0.08, CHCl3), with the molecular formula C15H24BrClO2 deduced by HR-ESI-MS from the pseudomolecular ion peak [M+Na]+ at m/z 375.0417 (calcd. for C15H24BrClO2Na, 375.0525), requiring three degrees of unsaturation. The IR spectrum exhibited absorptions of a hydroxyl group at 3,463 cm−1. The 1H-NMR spectrum (Table 1) also displayed a pair of broad singlets characteristic of an exocyclic methylene group at δH 5.25 (H-14a) and 4.97 (H-14b), three tertiary methyl groups at δH 1.03 (H3-13), 1.06 (H3-12) and 1.21 (H3-15) and three hydrogens of heterosubstituted carbons at δH 4.07 (H-9), 4.23 (H-4) and 4.52 (H-10). The 13C-NMR spectrum and DEPT-135 experiment revealed the presence of fifteen carbon atoms corresponding to three methyls, five methylenes, three methines and four quaternary carbons, including two olefinic carbons and four carbons attached to heteroatoms. 1H-1H COSY and HMBC correlations indicated that the first ring was similar to compound 1 while the second was proposed based on 1H-1H COSY correlations between δH 4.23 (H-4) and 2.10 (2H, m, H2-5) and correlations from δH 1.21 (H3-15) to δC 33.8 (C-2), 70.3 (C-3) and 67.4 (C-4) in HMBC spectrum. Chemical shifts of C-3 and H-4/C-4 indicated the presence of a tertiary alcohol and chlorine substitution, respectively [23].
= −19.2° (c 0.08, CHCl3), with the molecular formula C15H24BrClO2 deduced by HR-ESI-MS from the pseudomolecular ion peak [M+Na]+ at m/z 375.0417 (calcd. for C15H24BrClO2Na, 375.0525), requiring three degrees of unsaturation. The IR spectrum exhibited absorptions of a hydroxyl group at 3,463 cm−1. The 1H-NMR spectrum (Table 1) also displayed a pair of broad singlets characteristic of an exocyclic methylene group at δH 5.25 (H-14a) and 4.97 (H-14b), three tertiary methyl groups at δH 1.03 (H3-13), 1.06 (H3-12) and 1.21 (H3-15) and three hydrogens of heterosubstituted carbons at δH 4.07 (H-9), 4.23 (H-4) and 4.52 (H-10). The 13C-NMR spectrum and DEPT-135 experiment revealed the presence of fifteen carbon atoms corresponding to three methyls, five methylenes, three methines and four quaternary carbons, including two olefinic carbons and four carbons attached to heteroatoms. 1H-1H COSY and HMBC correlations indicated that the first ring was similar to compound 1 while the second was proposed based on 1H-1H COSY correlations between δH 4.23 (H-4) and 2.10 (2H, m, H2-5) and correlations from δH 1.21 (H3-15) to δC 33.8 (C-2), 70.3 (C-3) and 67.4 (C-4) in HMBC spectrum. Chemical shifts of C-3 and H-4/C-4 indicated the presence of a tertiary alcohol and chlorine substitution, respectively [23].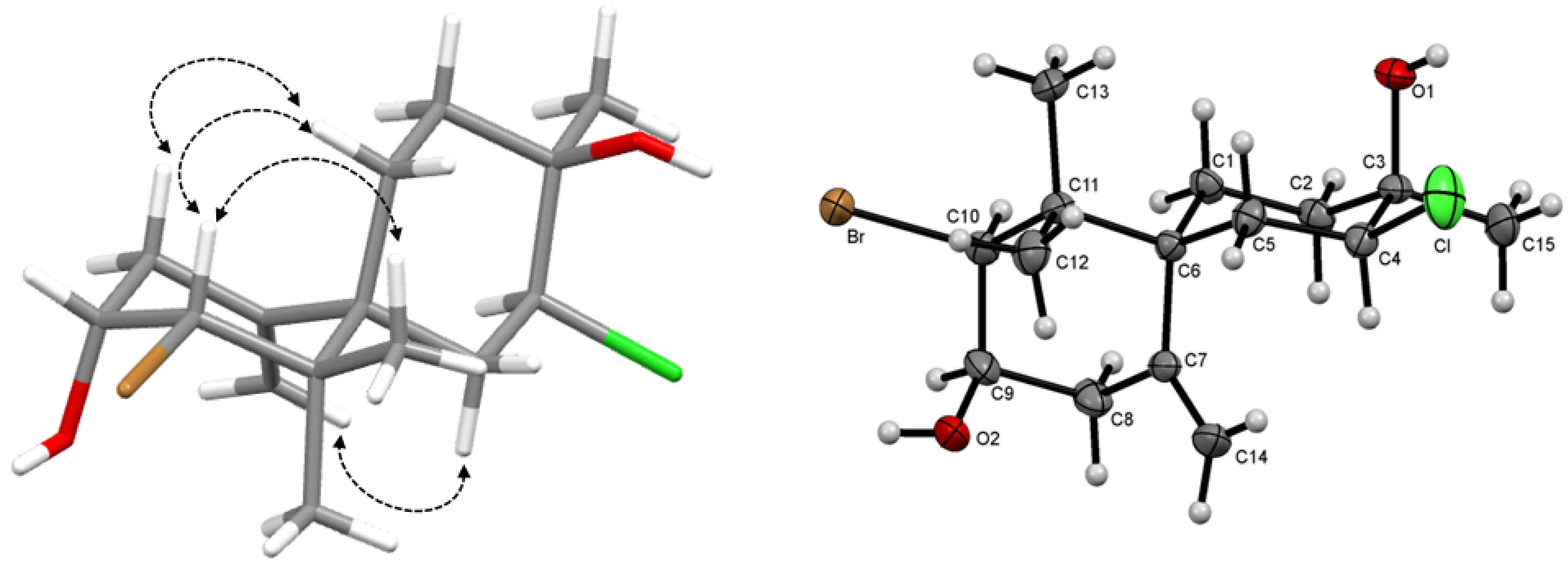
| Sample | IC50 (μM) | |||
|---|---|---|---|---|
| NO | TNF-α | M. bovis BCG | Cytotoxicity | |
| 2 | >284.3 a | >284.3 a | 80.2 ± 4.3 a | >284.3 a |
| 3 | 69.1 ± 4.7 b | 133.8 ± 7.4 b | 82.4 ± 4.7 a | >392.5 b |
| 4 | 44.9 ± 3.0 c | >250.9 c | 44.7 ± 4.0 b | 197.2 ± 1.0 c |
| 5 | 74.6 ± 5.8 b | 393.4 ± 0.4 d | 204.6 ± 7.6 c | 416.4 ± 4.9 d |
| l-NMMA | 71.3 ± 4.4 b | - | - | - |
| Rifampicin | - | - | 0.004 ± 1.3 d | - |
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
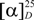 = +6,0° (c 0.06, CHCl3); IR (film) νmax 3457, 2927, 1675 cm−1; 1H-NMR and 13C-NMR data, see Table 1; EI-MS (rel. int.) m/z 320 (2), 318 (2), 313 (3), 311 (2), 308 (3), 306 (15), 304 (12), 269 (3), 267 (9), 249 (26), 221 (19), 207 (75), 183 (33), 175 (22), 171 (24), 157 (23), 147 (38), 143 (59), 133 (27), 131 (28), 129 (35), 119 (66), 117 (32), 115 (34), 107 (50), 105 (63), 93 (32), 91 (96), 85 (95), 83 (41), 79 (58), 77 (61), 41 (100); HR-APCI-MS [M+H]+ m/z 349.0381 (calcd for C15H20BrClO2, 349.0393).
= +6,0° (c 0.06, CHCl3); IR (film) νmax 3457, 2927, 1675 cm−1; 1H-NMR and 13C-NMR data, see Table 1; EI-MS (rel. int.) m/z 320 (2), 318 (2), 313 (3), 311 (2), 308 (3), 306 (15), 304 (12), 269 (3), 267 (9), 249 (26), 221 (19), 207 (75), 183 (33), 175 (22), 171 (24), 157 (23), 147 (38), 143 (59), 133 (27), 131 (28), 129 (35), 119 (66), 117 (32), 115 (34), 107 (50), 105 (63), 93 (32), 91 (96), 85 (95), 83 (41), 79 (58), 77 (61), 41 (100); HR-APCI-MS [M+H]+ m/z 349.0381 (calcd for C15H20BrClO2, 349.0393).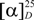 = −19.2° (c 0.08, CHCl3); IR (film) νmax 3463, 2971, 1918, 1640, 1453, 757, 622 cm−1; 1H-NMR and 13C-NMR data, see Table 1; EI-MS (rel. int.) m/z 334 (2), 316 (5), 314 (6), 299 (7), 297 (5), 253 (4), 237 (15), 235 (46), 217 (10), 199 (25), 173 (5), 157 (16), 133 (22), 119 (42), 107(73), 105 (74), 85 (91), 69 (47), 55 (53), 43 (100). HR-ESI-MS [M+Na]+ m/z 375.0417 (calcd for C15H24BrClO2Na, 375.0525).
= −19.2° (c 0.08, CHCl3); IR (film) νmax 3463, 2971, 1918, 1640, 1453, 757, 622 cm−1; 1H-NMR and 13C-NMR data, see Table 1; EI-MS (rel. int.) m/z 334 (2), 316 (5), 314 (6), 299 (7), 297 (5), 253 (4), 237 (15), 235 (46), 217 (10), 199 (25), 173 (5), 157 (16), 133 (22), 119 (42), 107(73), 105 (74), 85 (91), 69 (47), 55 (53), 43 (100). HR-ESI-MS [M+Na]+ m/z 375.0417 (calcd for C15H24BrClO2Na, 375.0525).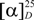 = +9.0° (c 0.2, CHCl3); IR (film) νmax 3391, 2918, 2849, 1702, 1464, 1296, 939, 758, 720 cm−1; 1H-NMR and 13C-NMR data, see Table 1; EI-MS (rel. int.) m/z 254 [M+] (1), 239 (15), 237 (6), 236 (34), 223 (10), 221 (29), 219 (6), 210 (8), 209 (4), 208 (25), 201,15 (27), 195 (11), 173 (31), 119 (88), 105 (74), 91 (91), 85 (100).
= +9.0° (c 0.2, CHCl3); IR (film) νmax 3391, 2918, 2849, 1702, 1464, 1296, 939, 758, 720 cm−1; 1H-NMR and 13C-NMR data, see Table 1; EI-MS (rel. int.) m/z 254 [M+] (1), 239 (15), 237 (6), 236 (34), 223 (10), 221 (29), 219 (6), 210 (8), 209 (4), 208 (25), 201,15 (27), 195 (11), 173 (31), 119 (88), 105 (74), 91 (91), 85 (100).3.5. X-ray Crystallography
3.6. Antimycobacterial Activity
3.7. Determination of Nitric Oxide and TNF-α Production by the RAW 264.7 Macrophage
3.8. Lactate Dehydrogenase Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Kamada, T.; Vairappan, S. A new bromoallene-producing chemical type of the red alga Laurencia nangii Masuda. Molecules 2012, 177, 2119–2125. [Google Scholar] [CrossRef]
- Wang, B.G.; Gloer, J.B.; Ji, N.Y.; Zhao, J.C. Halogenated organic molecules of Rhodomelaeae origin: Chemistry and biology. Chem. Rev. 2013, 113, 3632–3685. [Google Scholar] [CrossRef]
- Cassano, V.; Metti, Y.; Millar, A.J.K.; Gil-Rodríguez, M.C.; Sentíes, A.; Díaz-Larrea, J.; Oliveira, M.C.; Fujii, M.T. Redefining the taxonomic status of Laurencia dendroidea (Ceramiales, Rhodophyta) from Brazil and the Canary Island. Eur. J. Phycol. 2012, 47, 67–81. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2007, 24, 31–86. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Cen-Pacheco, F.; Nordström, L.; Souto, M.L.; Martín, M.N.; Fernández, J.J.; Daranas, A.H. Studies on polyethers produced by red algae. Mar. Drugs 2010, 8, 1178–1188. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Tolstikov, G.A. Natural halogenated sesquiterpenes from marine organisms. Chem. Sustain. Dev. 2004, 12, 1–12. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; Brito, I.; de la Rosa, J.M.; D’Croz, L.; Fabelo, O.; Ruiz-Perez, C.; Darias, J.; Cueto, M. Novel lactone chamigrene-derived metabolites from Laurencia majuscula. Eur. J. Org. Chem. 2009, 9, 1407–1411. [Google Scholar]
- Konig, G.M.; Wright, A.D. Laurencia rigida: Chemical investigations of its antifouling dichloromethane extract. J. Nat. Prod. 1997, 60, 967–970. [Google Scholar] [CrossRef]
- Pereira, R.C.; da Gama, B.A.; Teixeira, V.L.; Yoneshigue-Valentin, Y. Ecological roles of natural products of the Brazilian red seaweed Laurencia obtusa. Braz. J. Biol. 2003, 63, 665–672. [Google Scholar]
- Li, X.D.; Ding, W.; Miao, F.P.; Ji, N.Y. Halogenated chamigrane sesquiterpenes from Laurencia okamurae. Magn. Reson. Chem. 2012, 50, 174–177. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Kawamoto, T.; Miwa, H.; Suzuki, M. Potent antibacterial activity of halogenated compounds against antibiotic-resistant bacteria. Planta Med. 2004, 70, 1087–1090. [Google Scholar] [CrossRef]
- Dias, T.; Brito, I.; Moujir, L.; Paiz, N.; Darias, J.; Cueto, M.J. Cytotoxic sesquiterpenes from Aplysia dactylomela. Nat. Prod. 2005, 68, 1677–1679. [Google Scholar] [CrossRef]
- Machado, F.L.S.; Pacienza-Lima, W.; Rossi-Bergmann, B.; Gestinari, L.M.S.; Fujii, M.T.; de Paula, J.C.; Costa, S.S.; Lopes, N.P.; Kaiser, C.R.; Soares, A.R. Antileishmanial sesquiterpenes from the Brazilian red alga Laurencia dendroidea. Planta Med. 2011, 77, 733–735. [Google Scholar] [CrossRef]
- Jung, W.K.; Choi, I.; Oh, S.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, D.S.; Heo, S.J.; Jeon, Y.J.; Je, J.Y.; et al. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extracts in a murine model of asthma. Food Chem. Toxicol. 2009, 47, 293–297. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Kamada, T.; Lee, W.W.; Jeon, Y.J. Anti-inflammatory activity of halogenated secondary metabolites of Laurencia snackeyi (Weber-van Bosse) Masuda in LPS-stimulated RAW 264.7 macrophages. J. Appl. Phycol. 2013, 25, 1805–1813. [Google Scholar] [CrossRef]
- Chatter, R.; Othman, R.B.; Rabhi, S.; Kladi, M.; Tarhouni, S.; Vagias, C.; Roussis, V.; Guizani-Tabbane, L.; Kharrat, R. In vivo and in vitro anti-inflammatory activity of neorogioltriol, a new diterpene extracted from the red alga Laurencia glandulifera. Mar. Drugs 2011, 9, 1293–1306. [Google Scholar] [CrossRef]
- McGee, S.; Hirschmann, J. Use of corticosteroids in treating infectious diseases. Arch. Intern. Med. 2008, 168, 1034–1046. [Google Scholar] [CrossRef]
- Lawn, S.D.; Zumla, A. Tuberculosis. Lancet 2011, 378, 58–72. [Google Scholar]
- Wessels, M.; König, G.M.; Wright, A.D. New natural product isolation and comparison of the secondary metabolite content of three distinct samples of the sea hare Aplysia dactylomela from Tenerife. J. Nat. Prod. 2000, 63, 920–928. [Google Scholar] [CrossRef]
- Li, X.D.; Miao, F.P.; Liang, X.R.; Wang, B.G.; Ji, N.Y. Two halosesquiterpenes from Laurencia composita. RSC Adv. 2013, 3, 1953–1956. [Google Scholar] [CrossRef]
- Brennan, M.R.; Erickson, K.L.; Minott, D.A.; Pascoe, K.O. Chamigrane metabolites from a Jamaican variety of Laurencia obtusa. Phytochemistry 1987, 26, 1053–1057. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. A general definition of ring puckering coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- González, A.G.; Darias, J.; Diaz, A.; Fourneron, J.D.; Martin, J.D.; Perez, C. Evidence for the biogenesis of halogenated chamigrenes from the red alga Laurencia obtusa. Tetrahedron Lett. 1976, 17, 3051–3054. [Google Scholar] [CrossRef]
- Yang, E.J.; Moon, J.Y.; Kim, M.J.; Kim, D.S.; Kim, C.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J. Zhejiang Univ. Sci. B 2010, 11, 315–322. [Google Scholar]
- Konig, G.M.; Wright, A.D.; Franzblau, S.G. Assessment of antimycobacterial activity of a series of mainly marine derived natural products. Planta Med. 2000, 66, 337–342. [Google Scholar] [CrossRef]
- Hooft, R.W.W. Collect Software; Nonius BV: Delft, The Netherlands, 1998. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. 1983, A39, 876–881. [Google Scholar] [CrossRef]
- Gomez-Flores, R.; Gupta, S.; Tamez-Guerra, R.; Mehta, R.T.J. Determination of MICs for Mycobacterium avium-M. intracellulare complex in liquid medium by a colorimetric method. Clin. Microbiol. 1995, 33, 1842–1846. [Google Scholar]
- Da Silva, S.A.G.; Costa, S.S.; Rossi-Bergmann, B. The anti-leishmanial effect of Kalanchoe is mediated by nitric oxide intermediates. Parasitology 1999, 118, 575–582. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and citotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Muzitano, M.F.; Cruz, E.A.; Almeida, A.P.; Silva, S.A.G.; Kaiser, C.R.; Guette, C.; Rossi-Bergmann, B.; Costa, S.S. Quercitrin: na antileishmanial flavonoid glycoside from Kalanchoe pinnata. Planta Med. 2006, 72, 81–83. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 1–5 are not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Da Silva Machado, F.L.; Ventura, T.L.B.; Gestinari, L.M.d.S.; Cassano, V.; Resende, J.A.L.C.; Kaiser, C.R.; Lasunskaia, E.B.; Muzitano, M.F.; Soares, A.R. Sesquiterpenes from the Brazilian Red Alga Laurencia dendroidea J. Agardh. Molecules 2014, 19, 3181-3192. https://doi.org/10.3390/molecules19033181
Da Silva Machado FL, Ventura TLB, Gestinari LMdS, Cassano V, Resende JALC, Kaiser CR, Lasunskaia EB, Muzitano MF, Soares AR. Sesquiterpenes from the Brazilian Red Alga Laurencia dendroidea J. Agardh. Molecules. 2014; 19(3):3181-3192. https://doi.org/10.3390/molecules19033181
Chicago/Turabian StyleDa Silva Machado, Fernanda Lacerda, Thatiana Lopes Biá Ventura, Lísia Mônica de Souza Gestinari, Valéria Cassano, Jackson Antônio Lamounier Camargos Resende, Carlos Roland Kaiser, Elena B. Lasunskaia, Michelle Frazão Muzitano, and Angélica Ribeiro Soares. 2014. "Sesquiterpenes from the Brazilian Red Alga Laurencia dendroidea J. Agardh" Molecules 19, no. 3: 3181-3192. https://doi.org/10.3390/molecules19033181
APA StyleDa Silva Machado, F. L., Ventura, T. L. B., Gestinari, L. M. d. S., Cassano, V., Resende, J. A. L. C., Kaiser, C. R., Lasunskaia, E. B., Muzitano, M. F., & Soares, A. R. (2014). Sesquiterpenes from the Brazilian Red Alga Laurencia dendroidea J. Agardh. Molecules, 19(3), 3181-3192. https://doi.org/10.3390/molecules19033181




