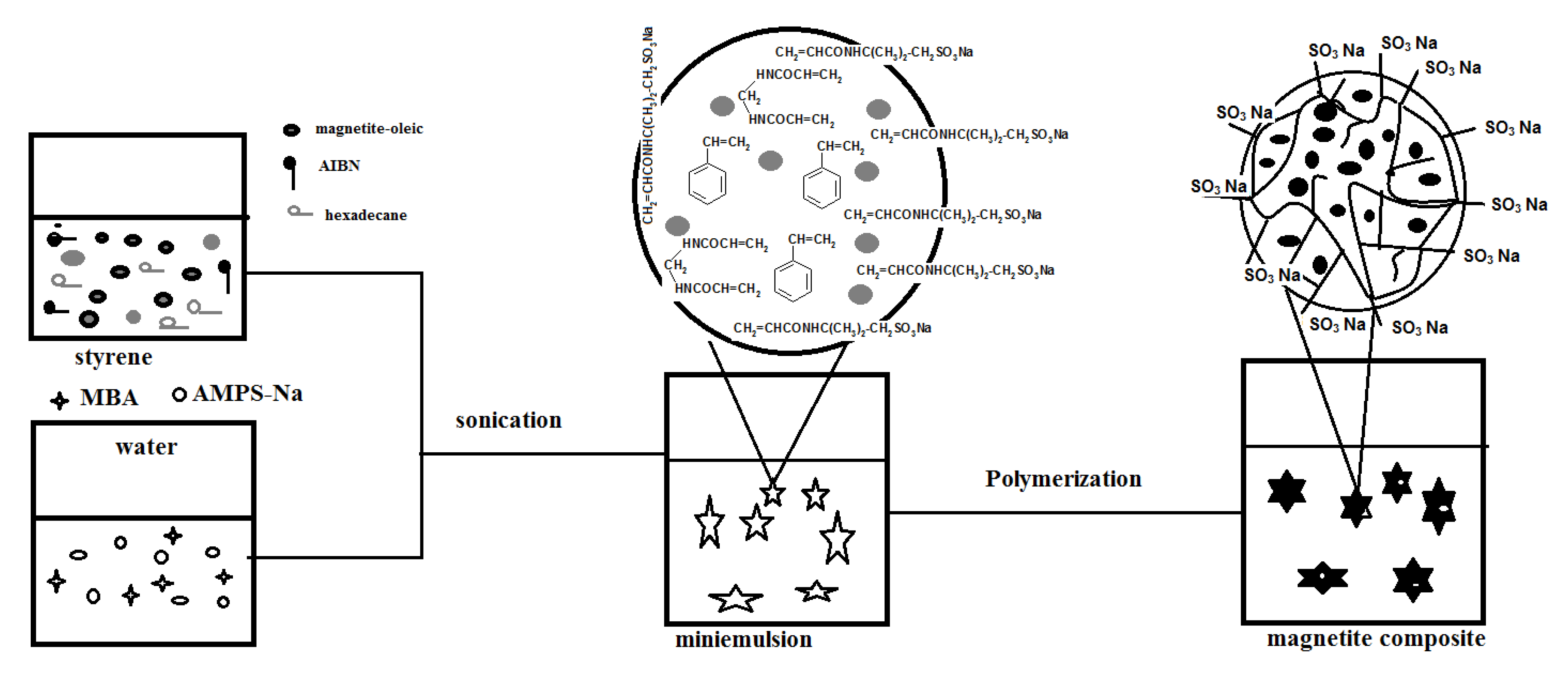
Nanogel composites containing dispersed magnetite nanoparticles can be prepared magnetite nanoparticles in styrene and HD solution. In this way, the miniemulsion polymerization process will take place in the styrene (St) monomer droplets containing the magnetite particles. The hydrophobic magnetite coated nanoparticles were prepared according to a modified previous method [
22] based on the cheapest and most environmentally friendly co-precipitation method, which involves the simultaneous precipitation of Fe
2+ and Fe
3+ ions after reaction of KI with FeCl
3. The iodine was separated during the reaction by precipitation and the oleic acid-coated magnetite was produced after precipitation in basic aqueous media in the presence of oleic acid as stabilizer. The transformation of the hydrophilic-hydrophobic character of magnetite particles is fundamental for encapsulating them successfully inside the polymer particles. Moreover, the hydrophilicity of the magnetite nanoparticle surface may affect their compatibility and dispersability with the hydrophilic poly(sodium 2-acrylamido-2-methylpropane sulfonate-co-styrene). This may lead to the encapsulation of inorganic particles inside or on the surface layer of the latex particles. The proposed procedure for preparing poly(sodium 2-acrylamido-2-methylpropane sulfonate-co-styrene)/magnetite nanocomposites by miniemulsion polymerization in the absence of an emulsifier using hexadecane (HD) as hydrophobe and AMPS-Na as stabilizer is illustrated in
Scheme 1.
Scheme 1.
Synthesis of PAMPS-Na-co-St/magnetite composite.
As can be seen, AMPS-Na, MBA were dissolved in DDI water, and AIBN was dissolved in the mixture of HD and oleic Fe
3O
4-St. The two solutions were then mixed together under stirring and sonicated. A stable minimulsion was obtained, the temperature was then raised to 70 °C and monomer droplets polymerized. It is expected that the sulfonic groups of AMPS-Na anchored covalently onto the particle surface avoiding the migration that occurs with conventional emulsifier molecules, thus enhancing the stability of the particles and increasing the hydrophilicity of the copolymer with respect to the encapsulated oleic acid-coated magnetite particles [
23]. Moreover, it is expected that the concentration of magnetite particles is favored to increase in the core of the composite particles.
2.1. Characterization of PAMPS-Na-co-St/Magnetite Composite
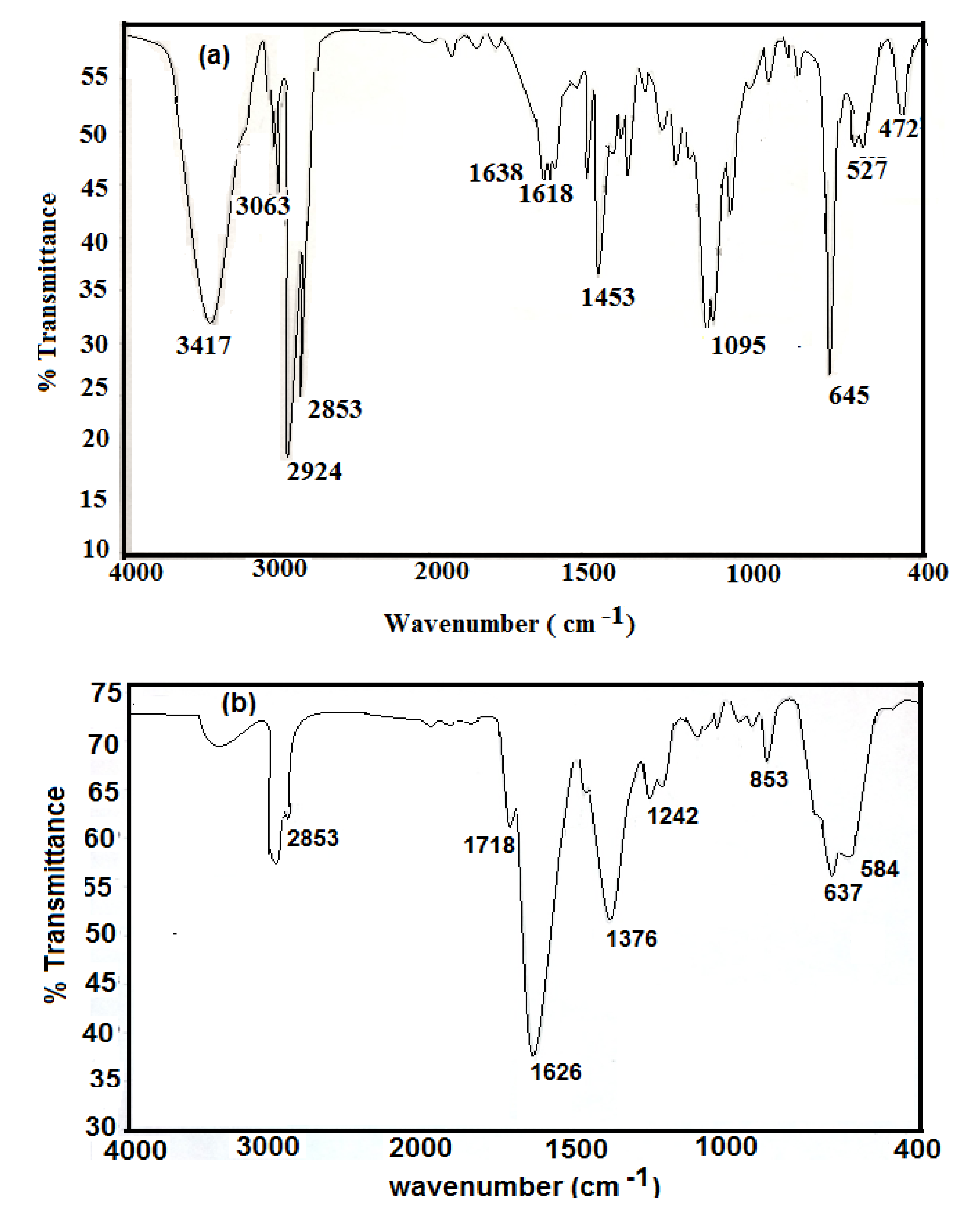
FTIR analysis was used to study the chemical structures of the prepared nanoparticles. The IR spectra of hydrophobic oleic Fe
3O
4 and PAMPS-Na-co-St/Fe
3O
4AA-Na magnetite particles are presented in
Figure 1a,b, respectively.
It was observed that the IR spectra of all samples clearly reveal the presence of strong IR absorption bands between 400 and 700 cm
−1, which are the characteristic absorption peaks of the Fe-O vibrations related to Fe
3O
4. Magnetite formation can be confirmed through the presence of the 584 and 637 cm
−1 bands assigned to the stretching and torsional vibration modes of the magnetite. In both magnetite coated phases bands are seen at higher frequency in the near IR region than previously reported, where the characteristic absorption band for Fe-O in bulk Fe
3O
4 appeared at 570 and 375 cm
−1[
24].
Figure 1.
FTIR specta of (a) oleic acid coated magnetite and (b) PAMPS-Na-co-St/magnetite composite.
Figure 1.
FTIR specta of (a) oleic acid coated magnetite and (b) PAMPS-Na-co-St/magnetite composite.
However, in the present case the Fe-O band shift towards higher wavenumbers (584 and 637 cm
−1) which may be due to the breakage of a large number of bonds of surface atoms, resulting in rearrangement of localized electrons on the particle surface and the surface bond force constant then increases as Fe
3O
4 is reduced to nanoscale dimensions, so that the absorption bands shift to higher wavenumbers [
24]. In
Figure 1a Fe
3O
4 nanoparticles coated with oleic acid show characteristic bands of asymmetric and symmetric COO- stretching (1453 and 1638 cm
−1) [
25], along with a band at 1050 cm
‒1 characteristic of a C-O single bond which demonstrates that oleic acid was chemisorbed onto the nanoparticles as a carboxylate. Fe-O stretching of magnetite was also observed at 578 and 628 cm
−1. The strong band at 3424 cm
−1 is attributed to the symmetric O-H stretching vibration of hydroxyl groups that are absorbed onto that part of the particle surface not occupied by surfactant groups as well as ν (O–H) for H-bonded OH groups (from oleic acid). Two sharp bands at 2924 and 2854 cm
−1 are attributed to the asymmetric and symmetric CH
2 stretches, respectively. The oleic acid surfactant molecules in the adsorbed state on magnetite are subjected to the solid surface field. As a result, the characteristic oleic acid bands are shifted to a lower frequency region, indicating that the hydrocarbon chains in the monolayer surrounding the iron nanoparticles are in a closed pack crystalline state [
25].
Figure 1b shows the characteristic C=O band (present at 1712 cm
−1 for pure oleic acid and AMPS-Na). The appearance of a band at 3350 cm
−1 (NH group of MBA) in
Figure 1b indicates the crosslinking of AMPS-Na with the MBA crosslinker. The appearance of a band at 850 cm
−1, corresponding to the C-H out of plane bending of the phenyl group indicates the incorporation of styrene into the chemical structure of the magnetite polymer composite.
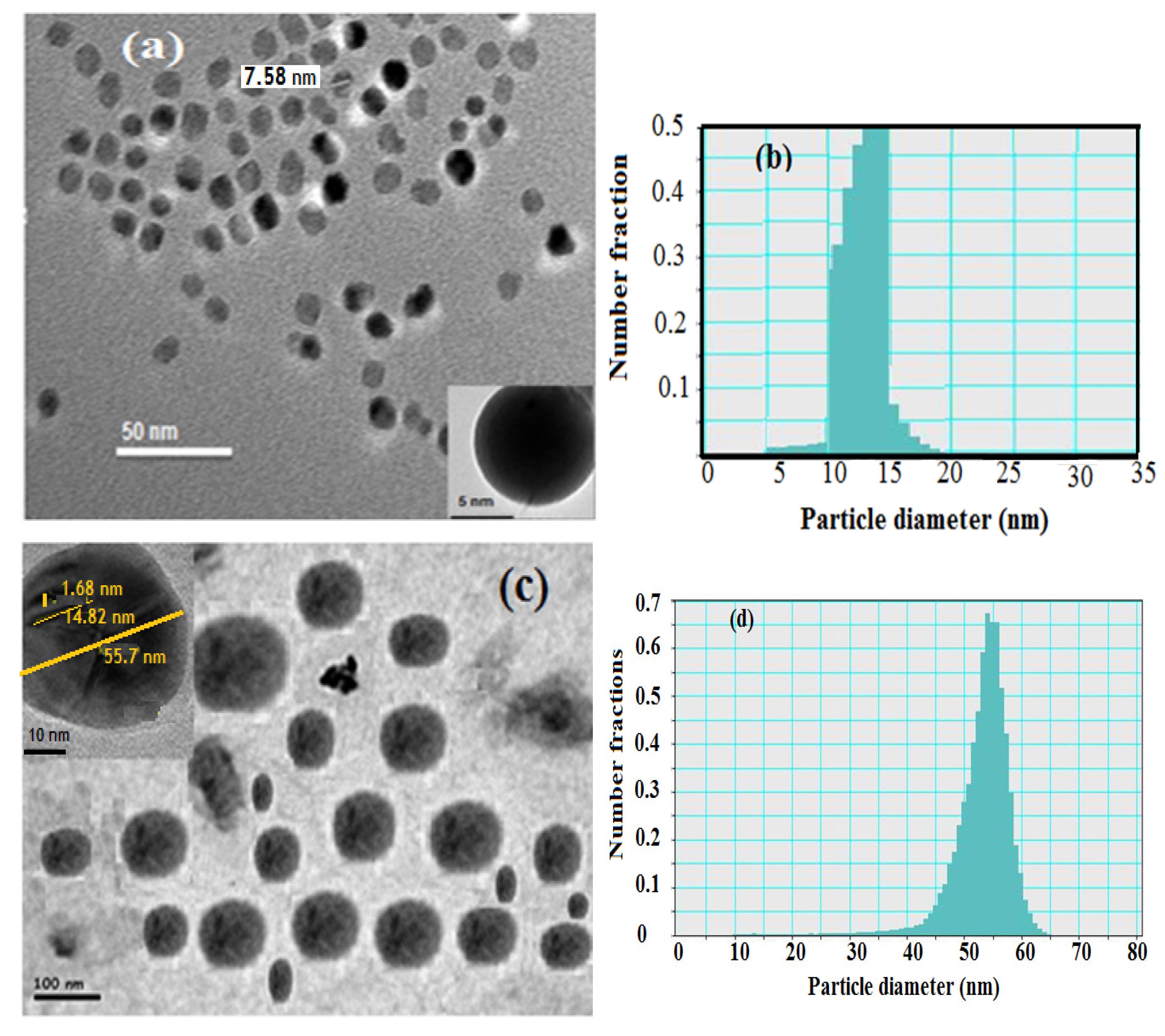
Transmission electron microscopy (TEM) was used to characterize the hydrophobic oleic Fe
3O
4 and PAMPS-Na-co-St/Fe
3O
4AA-Na magnetite particle nanocomposites as illustrated in
Figure 2. The hydrophobic magnetite stabilized with oleic acid nanoparticles analyzed by TEM showed a spherical shape with a narrow size distribution (
Figure 2a,b).
Figure 2.
TEM micrographs and histograms of (a) and (b) oleic coated magnetite, (c) and (d) PAMPS-Na-co-St/magnetite composite.
Figure 2.
TEM micrographs and histograms of (a) and (b) oleic coated magnetite, (c) and (d) PAMPS-Na-co-St/magnetite composite.
The image reveals the formation of monodispersed nanoparticles with an average size of 8–13 nm confirming that the proposed scheme is an effective method for the preparation of monodisperse magnetic nanoparticles. Number—(dn
−), weight—(dw
−), and volume-average (dv
−) diameters as well as polydispersity indices (PDI) were calculated from the PSDs [
26]. The data of dn
−, dw
−, dv
− and PDI of the hydrophobic oleic Fe
3O
4 are 10.7, 11.8, 13.3 nm and 1.05, respectively.
The particle size distributions appear to be quite narrow and the particle shape is reasonably spherical. The presence of magnetite in the PAMPS-Na-co-St polymeric matrix was identified by the black dots over the grey dots, as shown in
Figure 2b. Another feature present in
Figure 2b, and in many of the other TEM images of the polymer PAMPS-Na-co-St/magnetite coated nanoparticles, is that the particles are not in contact with their nearest neighbors. During preparation of the TEM sample, solvent-dispersed particles with swollen shells may be deposited in a close-packed array on the carbon support. The absence of pure polymer particles and free magnetite particles in
Figure 2b indicated that monomer droplet nucleation was achieved entirely by using an emulsifier-free miniemulsion polymerization technique. The dn
−, dw
−, dv
− and PDI values are 67, 73, 79 nm and 1.1, respectively, indicating the formation of almost monodisperse PAMPS-Na-co-St/magnetite composite. In addition, in the TEM micrographs (
Figure 2b), the existence of a few large composite particles can be observed in the PAMPS-Na-co-St/magnetite latex.
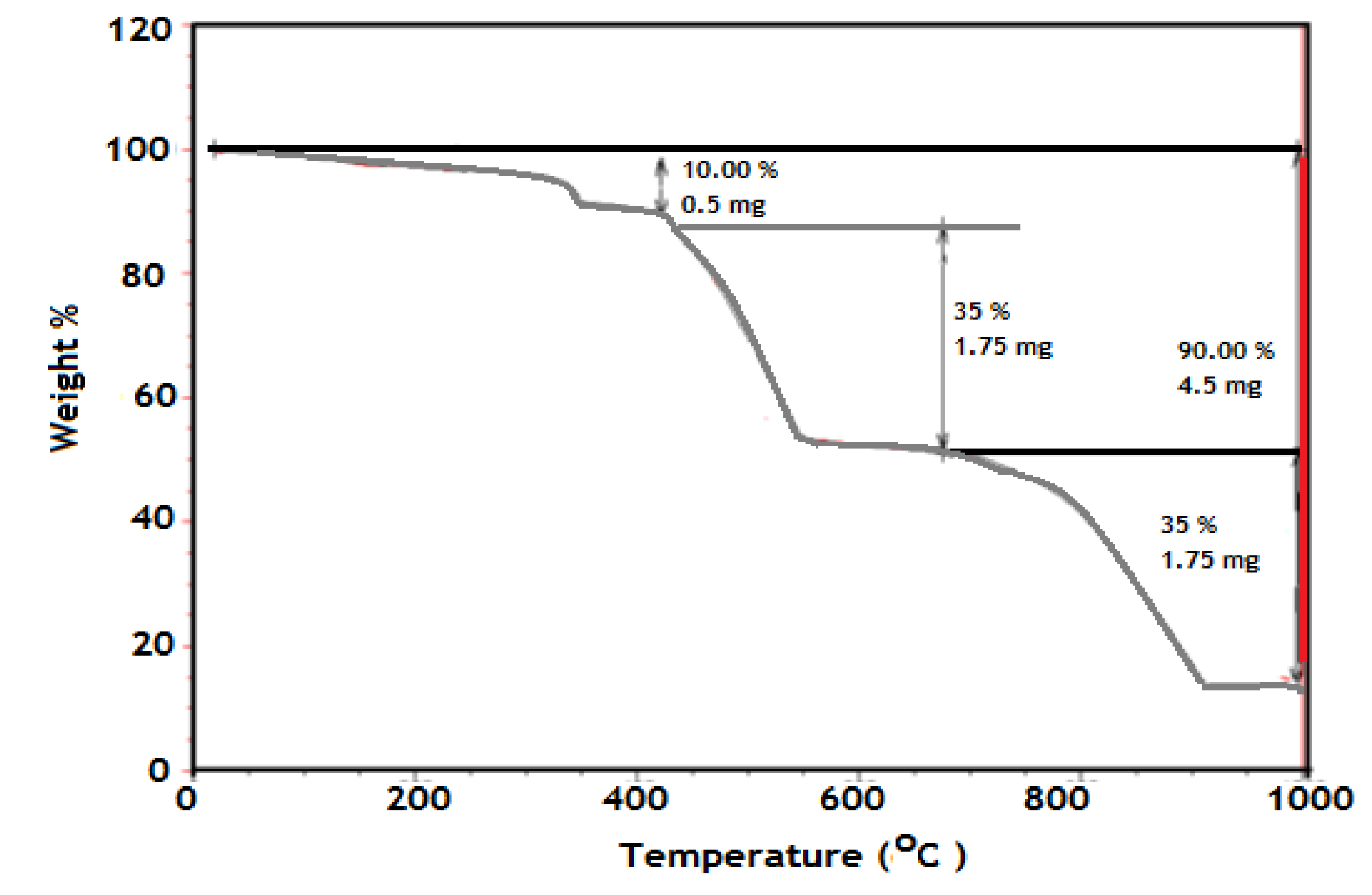
It is well established that the TGA is an effective analysis tool to determine the inorganic contents in polymer composites. The polymer should decompose completely when the temperature is high enough, but if there are inorganic materials in the sample, they should remain and can be measured. The TGA curves of PAMPS-Na-co-St/magnetite composite are presented in
Figure 3.
Figure 3.
TGA thermogram of PAMPS-Na-co-St/magnetite composite.
Figure 3.
TGA thermogram of PAMPS-Na-co-St/magnetite composite.
The thermogram exhibits a multiple-stage thermal decomposition. The first stage is in the 25–143 °C range and corresponds to the loss of moisture present in the sample (2%). The initial decomposition temperature (Ti) of the nanocomposite was 279.9 °C. This peak is attributed to the thermal decomposition of the amide side-groups of AMPS-Na. The weight loss in the range 220–320 °C corresponds to the temperature of the transformation from magnetite to hematite. This transformation appears as a multi-step process, which is composed of the gradual transformation of the magnetite nanoparticles to maghemite (γ-Fe
2O
3). The weight loss (5.4%) in the 297.2–318.9 °C range is attributed to the transformation from maghemite to hematite (α-Fe
2O
3). These two thermal events are consistent with the previously reported phase-transformation temperature range of magnetite. This is followed by weight loss within the 300–470 °C temperature range, which is attributed to the thermal decomposition of the crosslinker. This result confirms that the incorporation of a magnetic nanogel is helpful for the improvement of the thermal stability of the nanocomposites. As can be seen in
Figure 3, when the temperature reached about 850 °C the weight of the sample was constant and the residue was 9.295% of the original sample mass. Since there was no increase in weight resulting from the oxidation of Fe
3O
4 to Fe
2O
3 because the TGA experiment was carried out under a nitrogen atmosphere, this fact indicated that the Fe
3O
4 content in the PAMPS-Na-co-St/magnetite latex was approximately 10%.
2.2. Surface Activity of PAMPS-Na-co-St/Magnetite Composite
A literature survey on the topic of surface activity of dispersed nanoparticles showed little research about this subject. In previous works [
27,
28,
29], the determination of the surface activities of the nanogels and correlation with their size, morphology and chemical structures was the research target. In this respect, measuring the surface activity of PAMPS-Na-co-St/magnetite composite in both aqueous and 1 M HCl is a goal of the present work to measure the ability of particles to be dispersed in aqueous media or adsorb at the water/air interface. In the present article, it was expected that the prepared PAMPS-Na-co-St/magnetite composite would possess surface activity due to the presence of both the hydrophilic moieties of AMPS (amide and sulfonate) and hydrophobic moieties (phenyl, butane, oleic and polymer backbone groups) in the chemical structure of the crosslinked composite nanoparticles. In this respect, the dynamic surface tension for different concentrations of the prepared PAMPS-Na-co-St/magnetite composite was measured at the water/air interface for aqueous solution and 1 M HCl at a temperature of 25 °C. The relation between the surface tension of different concentrations of dispersed PAMPS-Na-co-St/magnetite and time of measurements in 1 M HCl was selected for representative samples and is illustrated in
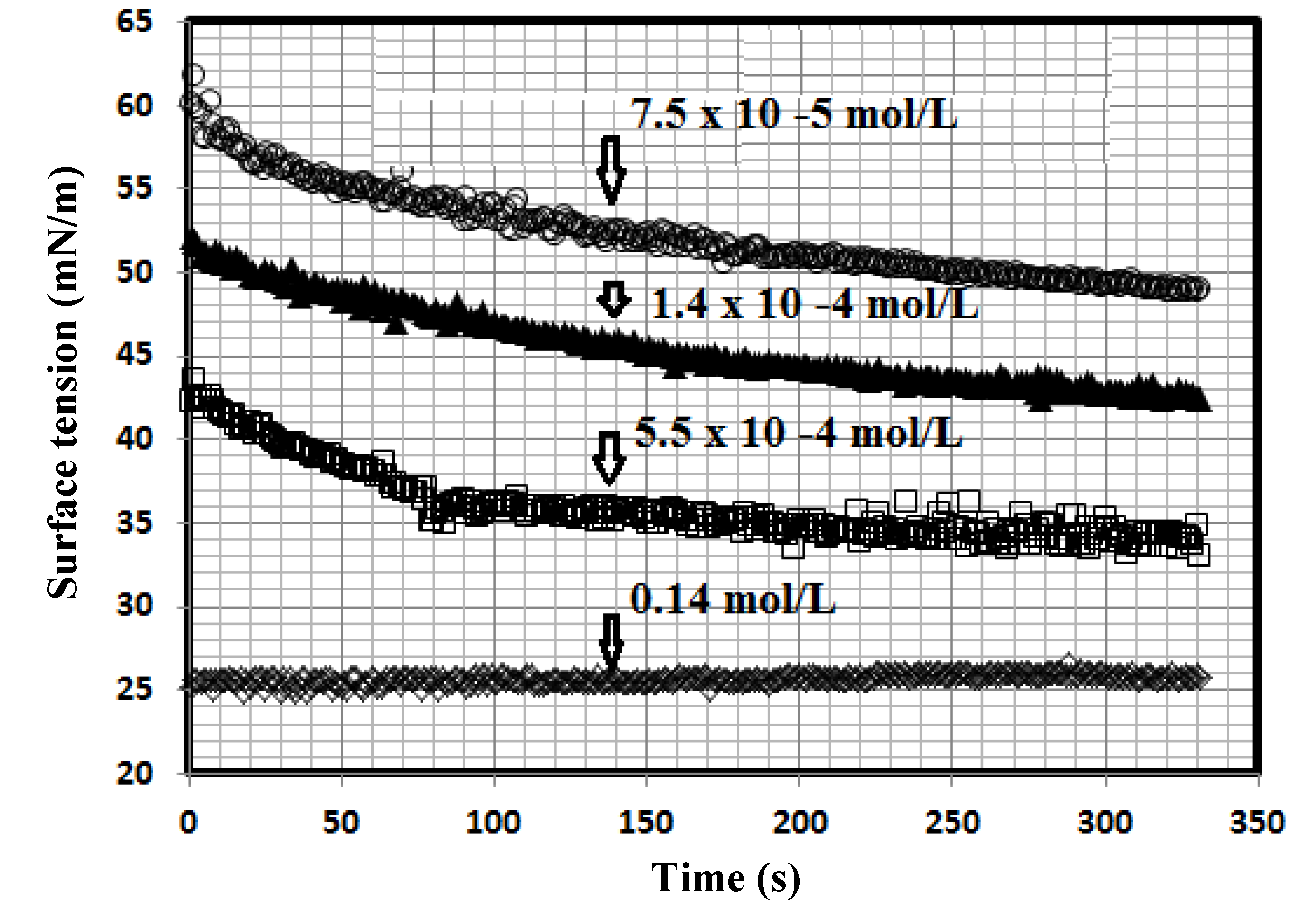
Figure 4.
Figure 4.
Surface tension ageing time relationship of PAMPS-Na-co-St/magnetite composites of different concentrations in 1 M HCl at 25 °C.
Figure 4.
Surface tension ageing time relationship of PAMPS-Na-co-St/magnetite composites of different concentrations in 1 M HCl at 25 °C.
The data presented in
Figure 4 indicate that the dispersed PAMPS-Na-co-St/magnetite reached its surface tension equilibrium after time intervals ranging from 5 to 15 min. Moreover, the equilibrium time was reduced with increasing PAMPS-Na-co-St/magnetite concentration and replacement of water with 1 M HCl. These data indicated that the prepared surfactants are strongly adsorbed at the interfaces in 1 M HCl solutions. On the other hand, it was observed that the surface tension data of the aqueous nonionic surfactant solutions was not affected by 1 M HCl.
It is well known that the interactions between amphiphilic particles and solvents affect the micellization, aggregation and adsorption of particles and they are based on the critical aggregation concentrations (cac), which were determined by the surface balance method. The cac data of PAMPS-Na-co-St/magnetite were determined from the abrupt changes of the plotted data of surface tension (γ)
versus the solute concentration (lnC) in 1 M HCl and aqueous solution at 25 °C and are listed in
Table 1.
Table 1.
Surface activity data of PAMPS-Na-co-St/magnetite composite in water and 1 M aqueous HCl solution.
Table 1.
Surface activity data of PAMPS-Na-co-St/magnetite composite in water and 1 M aqueous HCl solution.
| Medium | cac mol/L | γcac mN/m | Δγ mN/m | (−∂γ/∂lnc)T | Гmax × 10 10 mol/ cm2 | Amin nm2/molecule |
|---|
| Water | 0.001 | 25.3 | 46.8 | 5.5 | 2.22 | 0.075 |
| 1 M HCl | 0.0005 | 25.2 | 46.9 | 8.9 | 3.58 | 0.046 |
The cmc data indicated that the PAMPS-Na-co-St/magnetite forms aggregates in 1 M HCl at lower concentration, and more so than in aqueous solution. This can be attributed to the conversion of SO
3Na groups to SO
3H groups and the increased hydrophobicity of magnetite particles in HCl solutions [
30].
The adsorption effectiveness of the prepared PAMPS-Na-co-St/magnetite expressed by the maximum reduction of surface tension which was calculated from the equation, Δγ = γ
water − γ
cac, the concentration of the prepared surfactants at the solvent-air interface, Г
max, and the area per molecule at the interface, A
min, were calculated and are listed in
Table 1 too. The surface excess concentration of the prepared PAMPS-Na-co-St/magnetite at the interface can be calculated from the surface or interfacial tension data using the following equation [
31]: Г
max = 1/RT × (−∂γ/∂lnc)T, where (−∂γ/∂lnc)T is the slope of the plot of γ
versus ln c at constant temperature (T), and R is the gas constant (in J mol
−1 K
−1). The Г
max values were used for calculating the minimum area (A
min) at the aqueous–air interface. The area per molecule at the interface provides information about the degree of packing and the orientation of the adsorbed surfactants, when compared with the dimensions of the molecules obtained from models. From the surface excess concentration, the area per molecule at the interface is calculated using the equation: A
min = 10
16/N Гmax, where N is Avogadro’s number. The data listed in
Table 1 indicated that the cmc values were reduced from 0.001 to 0.0005 mol/L in aqueous 1 M HCl, which can be attributed to the lower dispersion of PAMPS-Na-co-St/magnetite in acid solution at high PAMPS-Na-co-St/magnetite concentration. It was previously concluded that decreasing the cmc values indicated a high tendency of surfactants to adsorb at the liquid interface [
32]. Also, the area occupied at the interface is increased [
33]. These data indicated that the prepared PAMPS-Na-co-St/magnetite favors micellization in bulk 1 M HCl solution compared to aqueous solution, which may reflect its greater tendency to adsorb at the metal/liquid interface more than the air/water interface. It was also suggested that the spherical PAMPS-Na-co-St/magnetite particles are deformed at the water/air interface and become lenslike, causing more efficient adsorption [
34,
35].
2.3. Stability PAMPS-Na-co-St/Magnetite to HCl
The production of magnetite nanoparticles (MNPs) with high stability and biocompatibility presents one of the greatest challenges. To address this, MNPs should be covered with a quite inert external shell, in order to protect the magnetic core against chemical changes. Uncoated magnetic nanoparticles are highly susceptible to oxidation when exposed to the atmosphere and are also susceptible to leaching under acidic conditions [
36]. In addition to convenient magnetic properties and low toxicity and price, Fe
3O
4 exhibits high surface to volume ratios, depending on the particle size, which is associated to its ability to undergo surface chemical modifications that can enhance the capacity for heavy metal adsorption in water treatment processes. Inorganic polymers, such as silica, have been used as stabilizing agents for iron oxide and the silica coating has attractive properties, including high biocompatibility [
37], adsorption capacity, acid-base properties, insolubility in most solvents, and chemical and thermal stability. Besides enlarging the application scope of the material, one objective of coating magnetite is to improve its chemical stability. In this work, we evaluated the effect of PAMPS-Na-co-St coating on the chemical stability of magnetite toward HCl acid. A sample of magnetite was mixed with a solution of HCl for a certain period and the dissolved Fe(II) and Fe(III) were analyzed by atomic absorbance spectroscopy.
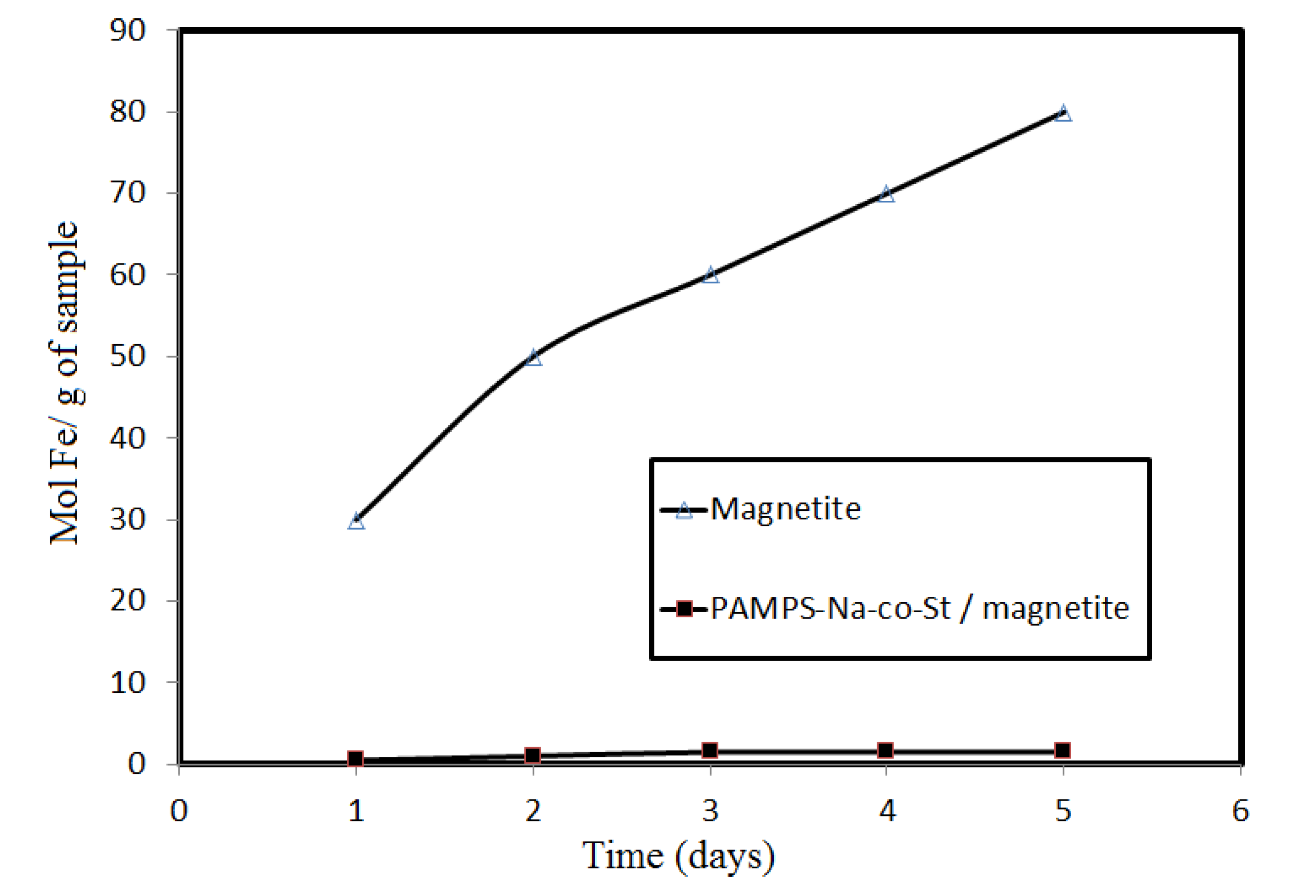
Figure 5 shows that PAMPS-Na-co-St/magnetite is more stable than the examined magnetite nanoparticles toward HCl.
Figure 5.
The amount of Fe dissolved in 1 M HCl within 5 days from Fe3O4 and PAMPS-Na-co-St/magnetite composite.
Figure 5.
The amount of Fe dissolved in 1 M HCl within 5 days from Fe3O4 and PAMPS-Na-co-St/magnetite composite.
This can be attributed to the protection afforded by sulfonate propyl groups from PAMPS and the phenyl groups of styrene, which have no tendency to interact with the protons of HCl. The formed coating is able to protect magnetite from acid. It can be seen that magnetite coated with oleic acid tends to have lower stability than that with core-shell PAMPS-Na-co-St/magnetite composite. This might be attributed to the fact oleic acid has lower stability in 1 M HCl due to the interaction of the COOH groups and the double bonds of oleic acid with the HCl protons [
2], affecting the oleic acid layer coated on magnetite. The decrease in the acid stability of magnetite could be attributed to a decrease in the surface area of the magnetite after removal of the oleic acid coat.
2.4. Polarization Measurements
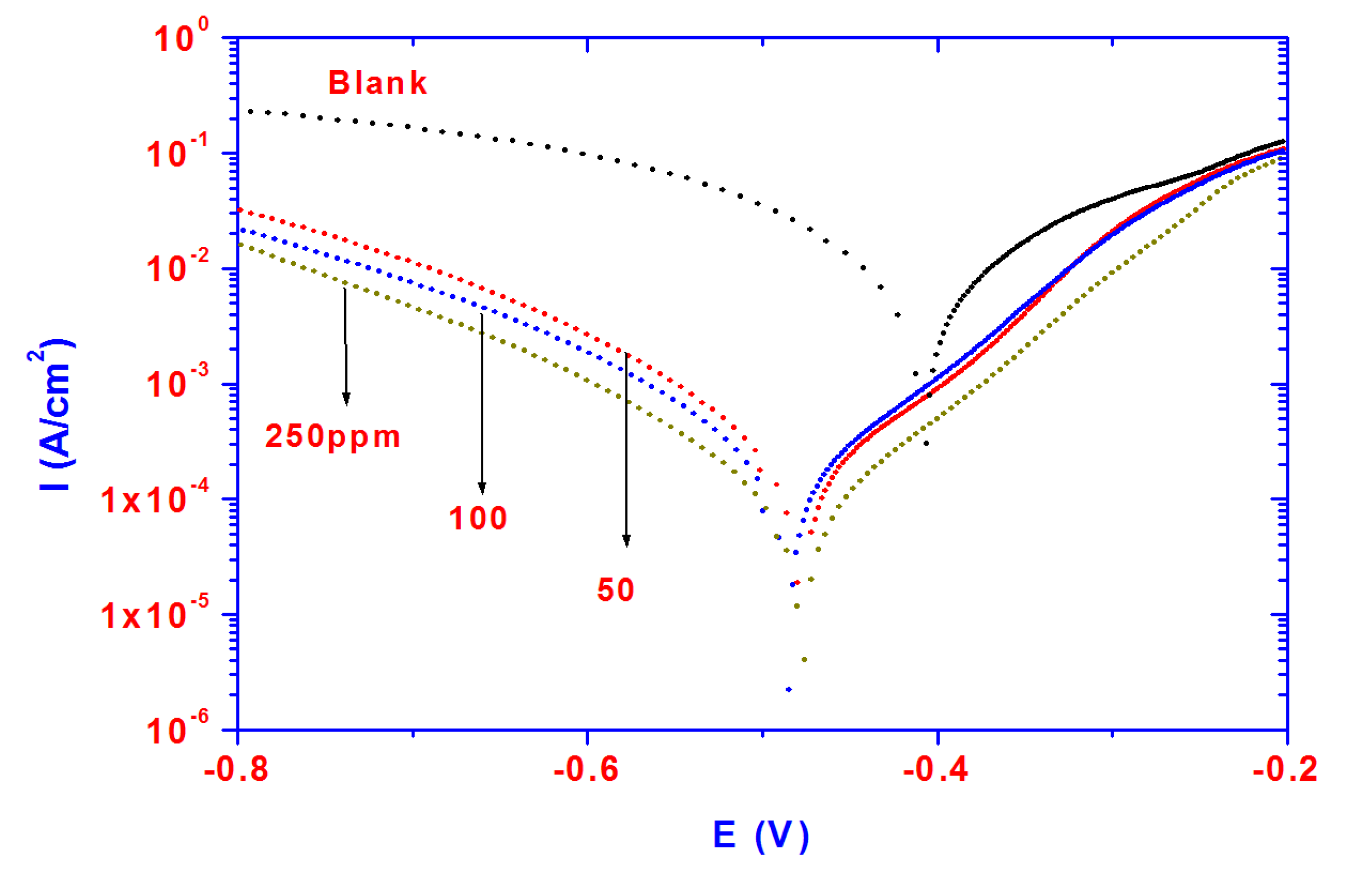
The dissolution of steel in acidic solutions is a major problem in industrial applications due to aggressiveness of acid, which causes the degradation of materials exposed to acidic media. Steel is widely exposed to the acidic environments used in oil well acidizing, acid cleaning and acid pickling. Potentiodynamic polarization plots for steel specimens in 1 M HCl solution in the absence and presence of different concentrations (50–250 ppm) of PAMPS-Na-co-St/magnetite particles are shown in
Figure 6. It is evident from the data presented in
Figure 6 that the cathodic current density decreased with the addition of inhibitor to the blank solution and shifts towards more negative values as the inhibitor concentration increases.
Figure 6.
Polarization curves for steel in 1 M HCl solution containing different PAMPS-Na-co-St/magnetite concentrations.
Figure 6.
Polarization curves for steel in 1 M HCl solution containing different PAMPS-Na-co-St/magnetite concentrations.
The results can be accounted for by the reduction of the cathodic process. In addition, the anodic current density decreases with the addition of inhibitor to the blank solution due to the formation of an inhibitive layer which reduces the anodic dissolution of steel. It can be concluded that the presence of PAMPS-Na-co-St/magnetite particles results in a marked shift in the cathodic and anodic branches of the polarization curves due to blocking of the active sites [
38,
39]. The cathodic reaction was strongly retarded, with an initial E
CORR displacement in the negative direction, and the anodic reaction was also retarded strongly inhibited. Increasing the inhibitor concentration to 250 ppm favored the inhibitory action. The decrease in the current densities of both anodic and cathodic reactions suggests that the inhibitor can be considered a mixed type inhibitor. The inhibition efficiency IE% was calculated using the following equation:
where i
corr(1) and i
corr(2) are the corrosion current densities of steel in the presence and absence of inhibitor, respectively. The electrochemical parameters associated with corrosion process as corrosion current density (i
corr), corrosion potential (E
corr), cathodic Tafel slope (B
c), anodic Tafel slope (B
a), and inhibition efficiency (IE%)] are given in
Table 2.
Table 2.
Inhibition efficiency of PAMPS-Na-co-St/magnetite composite values for steel in 1 M HCl with different concentrations of inhibitor calculated by the polarization and EIS methods.
Table 2.
Inhibition efficiency of PAMPS-Na-co-St/magnetite composite values for steel in 1 M HCl with different concentrations of inhibitor calculated by the polarization and EIS methods.
| Polarization Method | EIS Method |
|---|
| | Ba (mV) | Bc (mV) | Ecorr (V) | icorr mA/cm2 | IE% | Rct Ohm | IE% |
| Blank | 147.0 | 141.00 | −0.4034 | 7.45 | - | 1.80 | - |
| 50 ppm | 101.4 | 104.4 | −0.4777 | 0.186 | 97.50 | 578 | 99.5 |
| 100 | 99.9 | 112.9 | −0.4848 | 0.177 | 97.62 | 649 | 99.6 |
| 250 | 89.1 | 105.1 | −0.4768 | 0.074 | 99.00 | 693 | 99.7 |
The data of
Table 2 also shows how the addition of PAMPS-Na-co-St/magnetite particles decreases the corrosion current density. The inhibitive action of nanoparticles may be attributed to the adsorption and formation of a protective film on the electrode surface. The barrier film formed on the steel surface reduces both the anodic and cathodic reactions, which results in the decrease in both current densities and confirming that these nanoparticles act as mixed type corrosion inhibitors. The data presented in
Table 2 reveals that the values of B
a and B
c for inhibited solutions were lower than for uninhibited ones. The corrosion potentials (E
corr) are shifted to more negative values in the presence of nanoparticles, indicating that the inhibitor had a stronger influence on the cathodic reduction reaction. The cathodic shifts of (E
corr) and the decreases of the corresponding current densities (i
corr) with increasing inhibitor concentration can be attributed to a higher number of adsorbed nanoparticles on the steel surface, which entirely cover the steel surface and decrease the corrosion rate.
2.5. Electrochemical Impedance Spectroscopy (EIS)
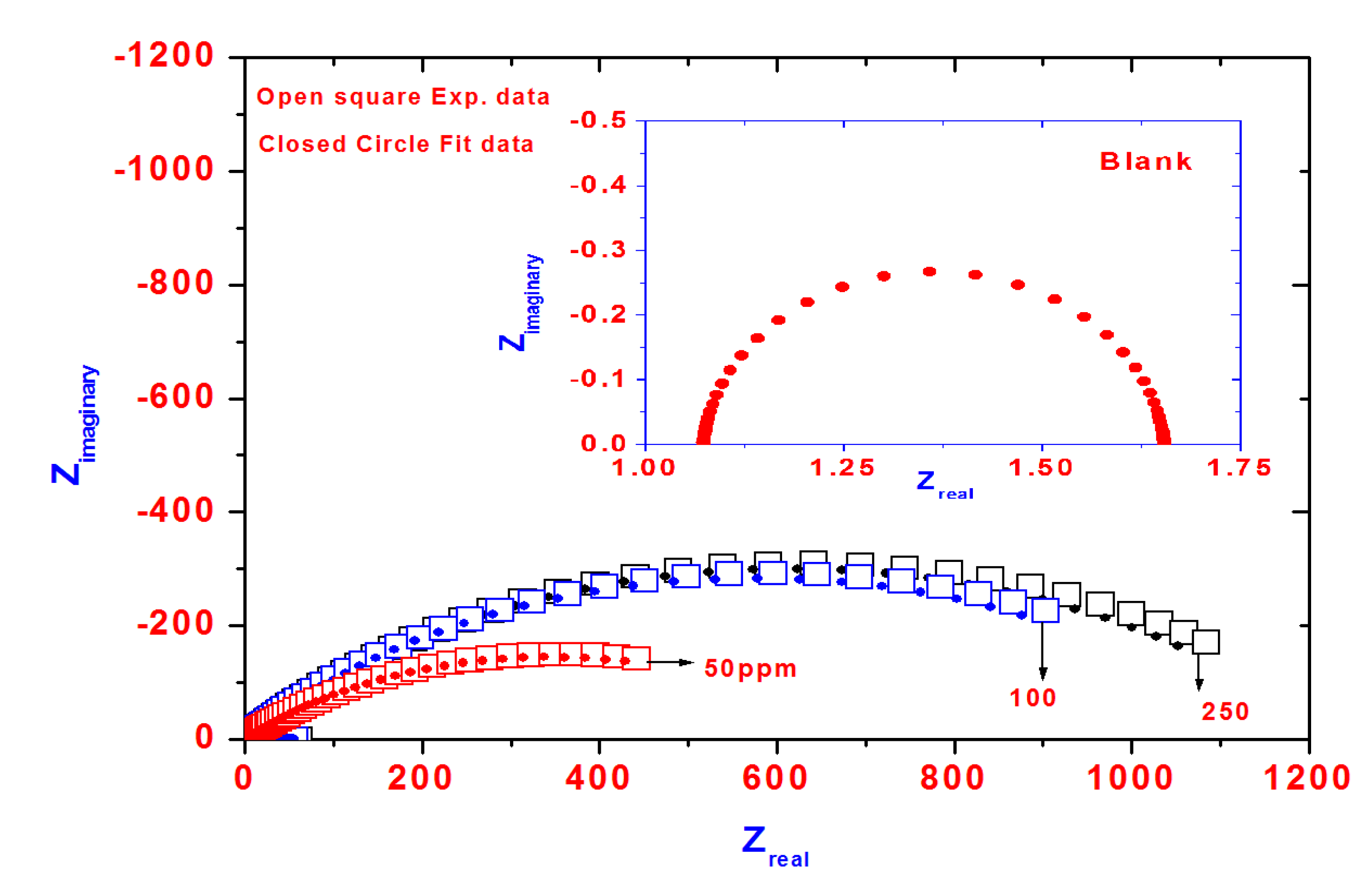
The effects of the inhibitor concentration on the impedance behavior of aluminum in 1.0 M HCl solution have been studied and the corresponding Nyquist plots are given in
Figure 7. The insert represents the Nyquist plot of blank solution.
Figure 7.
Nyquist diagram for steel in 1 M HCl solution containing different PAMPS-Na-co-St/magnetite concentrations showing experimental and fit data.
Figure 7.
Nyquist diagram for steel in 1 M HCl solution containing different PAMPS-Na-co-St/magnetite concentrations showing experimental and fit data.
The impedance spectra exhibit one single capacitive loop, which indicates that the corrosion reaction is controlled by a charge transfer reaction. It is worth noting that the presence of inhibitor does not change the mechanism of steel dissolution [
40]. It is evident that the impedance diagrams are not perfect semicircles, which is related to the frequency dispersion as a result of the roughness and inhomogeneity of the steel surface [
41].
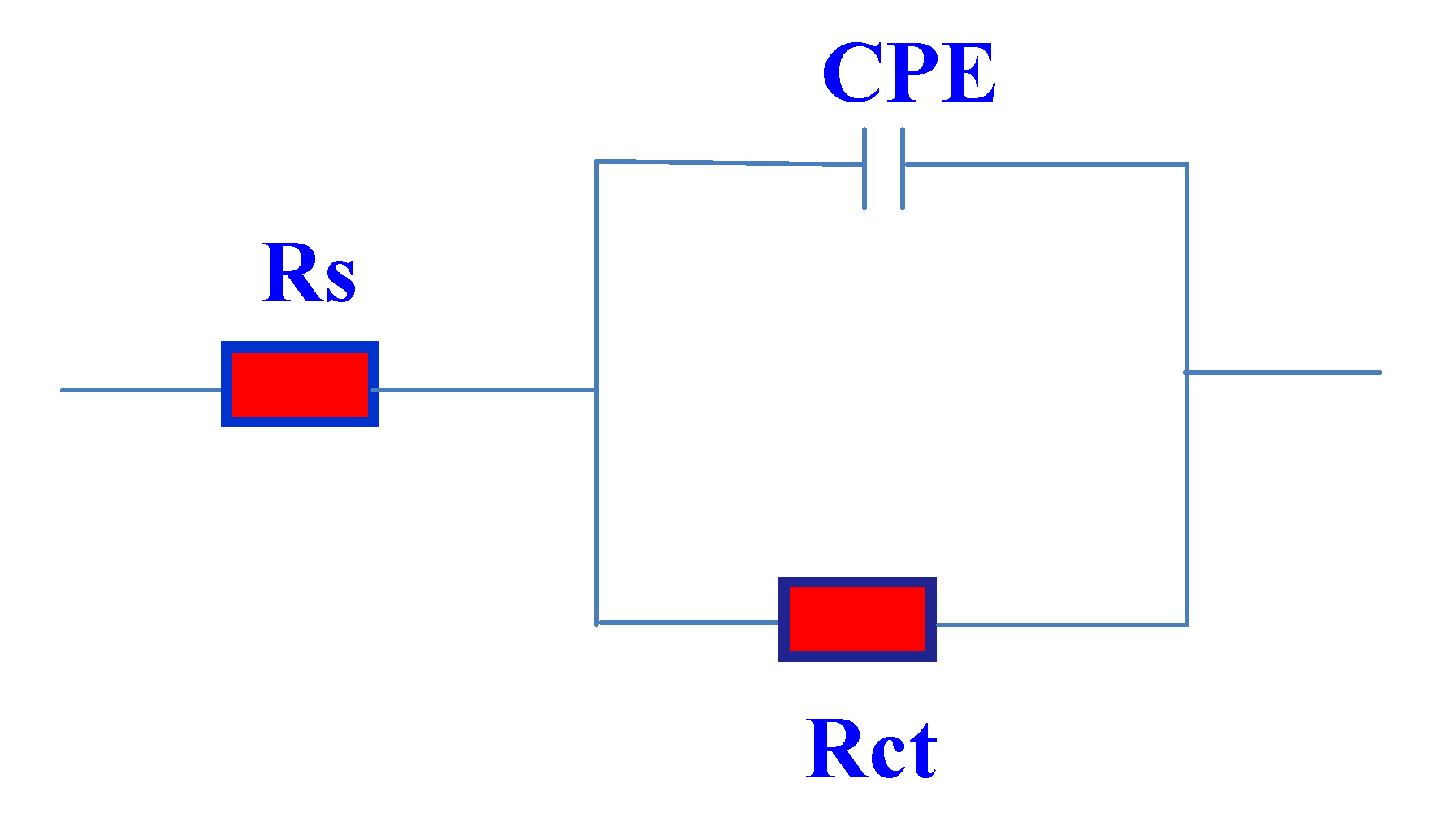
The equivalent circuit model used to fit the experimental results is shown in
Figure 8. In this equivalent circuit, Rs is the solution resistance, Q is a constant phase element (CPE) and Rct is the charge transfer resistance. The impedance of CPE is described as [
42]:
where Q is the pseudo capacitance, j is the imaginary function (√−1), ω is the angular frequency and n corresponds to the deviation from the ideal behavior of a pure capacitor. The inhibition efficiency (IE%) at different inhibitor concentrations were calculated by using the following equation [
43]:
where Rct
(1) and Rct
(2) are the charge transfer resistances in the HCl solution in the absence and in the presence of the inhibitors, respectively. The analysis of data (
Table 2) shows that the charge transfer resistance and the IE values increased with as the inhibitor concentration increased. The results can be explained on the basis of an enhancement of the adsorption process of inhibitor molecules on the steel surface with increasing inhibitor concentration, which resulted in larger surface coverage and an increase in IE%. The thickness of this protective layer increases with an increase in inhibitor concentration because more inhibitor will be adsorbed on the steel surface, leading to an increase in the IE%. As shown from
Figure 2 there is no aggregation of the nanoparticles. Formation of aggregates will decrease the surface area of the magnetic nanoparticles, thereby limiting and undermining the effectiveness of magnetic nanoparticles to protect the steel surface. It is expected that the interactions between the nanoparticles will limit the mobility of the particles to be adsorbed on the steel surface and decrease the efficiency. In addition, the higher efficiency may be attributed to fast adsorption kinetics and higher number of the adsorbed nanoparticle particles on the steel surface as the nanoparticles concentration increases. The potentiodynamic polarization and EIS measurement results clearly showed that the inhibition mechanism involves blocking of the steel surface by inhibitor molecules via adsorption. The results obtained from the EIS measurements are in good agreement with those obtained from the polarization method.
Figure 8.
Equivalent circuit used for fitting the impedance data.
Figure 8.
Equivalent circuit used for fitting the impedance data.