Determination and Pharmacokinetic Study of Gentiopicroside, Geniposide, Baicalin, and Swertiamarin in Chinese Herbal Formulae after Oral Administration in Rats by LC-MS/MS
Abstract
:1. Introduction
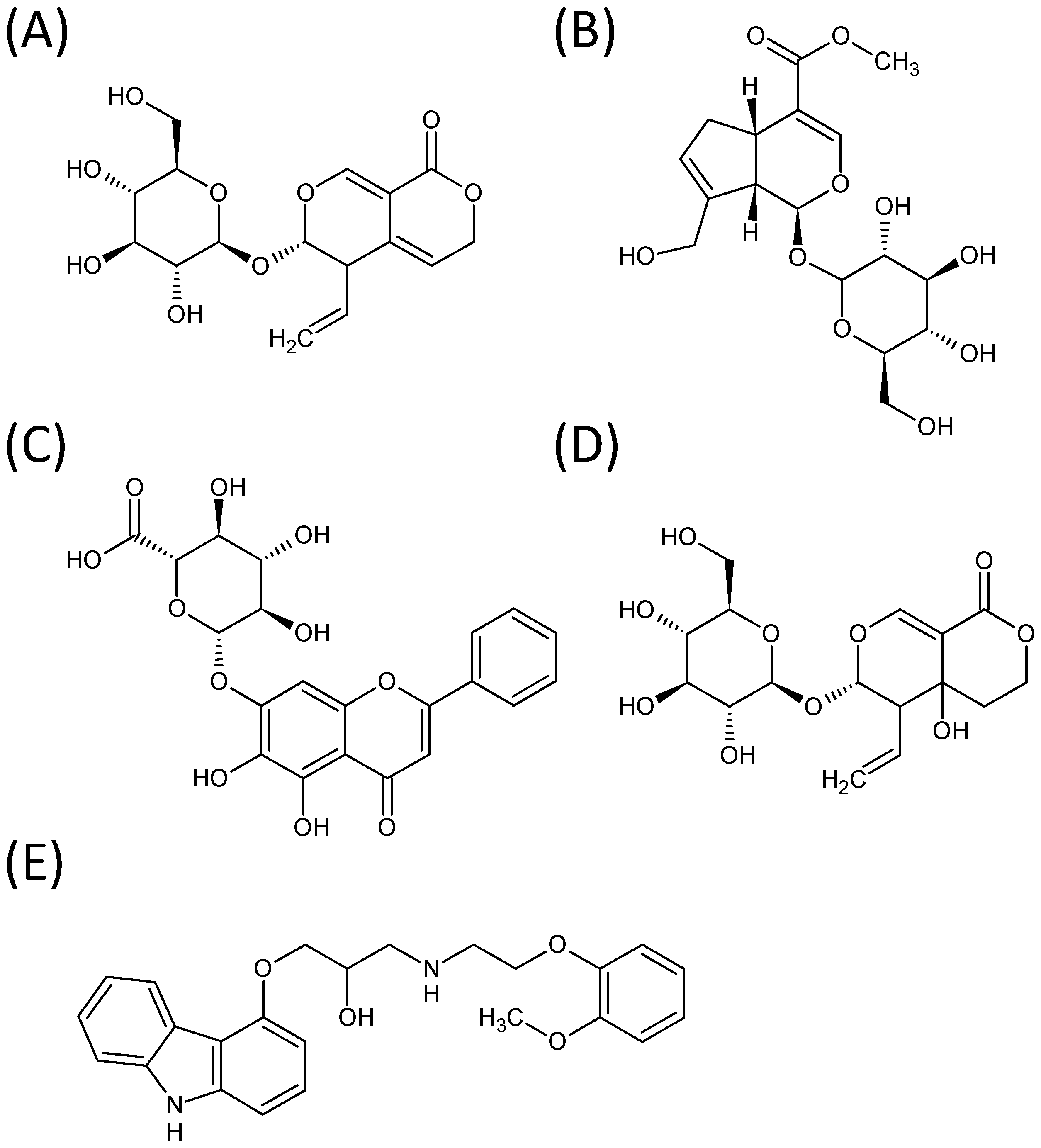
2. Results and Discussion
2.1. Optimization of LC-MS/MS Conditions
| Components | Molecular Weight | tR (min) | Mass Fragments | Collision Energy (eV) | ||
|---|---|---|---|---|---|---|
| Q1 Mass (amu) | Q3 Mass (amu) | |||||
| A | Gentiopicroside | 356.32 | 4.0 | 357.10 [M+H]+ | 195.10 | −10.0 |
| B | Geniposide | 388.36 | 4.1 | 406.10 [M+NH4]+ | 227.10 | −10.0 |
| C | Baicalin | 446.36 | 5.9 | 447.00 [M+H]+ | 271.05 | −25.0 |
| D | Swertiamarin | 374.12 | 3.6 | 375.10 [M+H]+ | 195.10 | −10.0 |
| E | Carvedilol (IS) | 406.48 | 5.8 | 407.20 [M+H]+ | 100.15 | −35.0 |
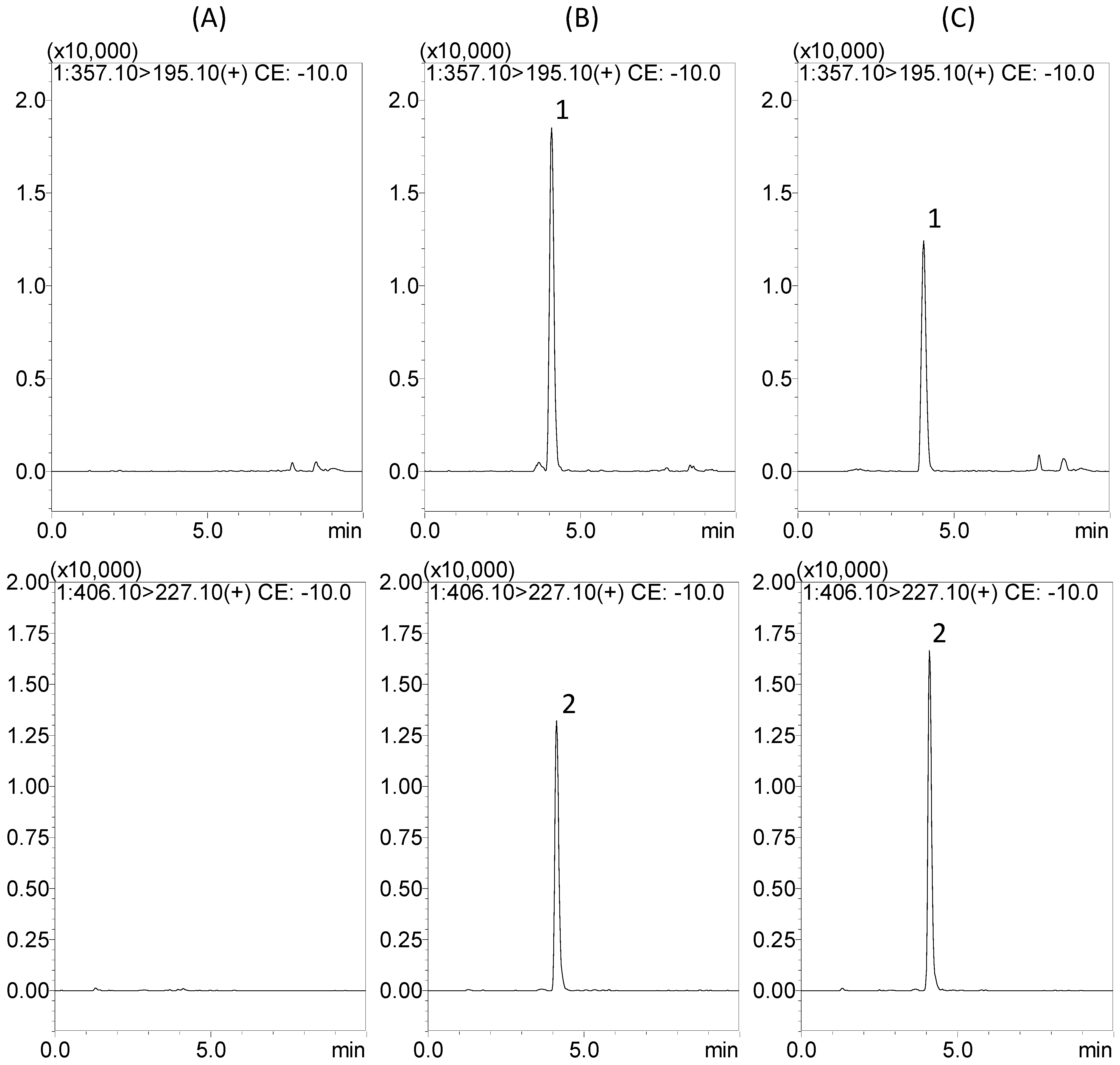
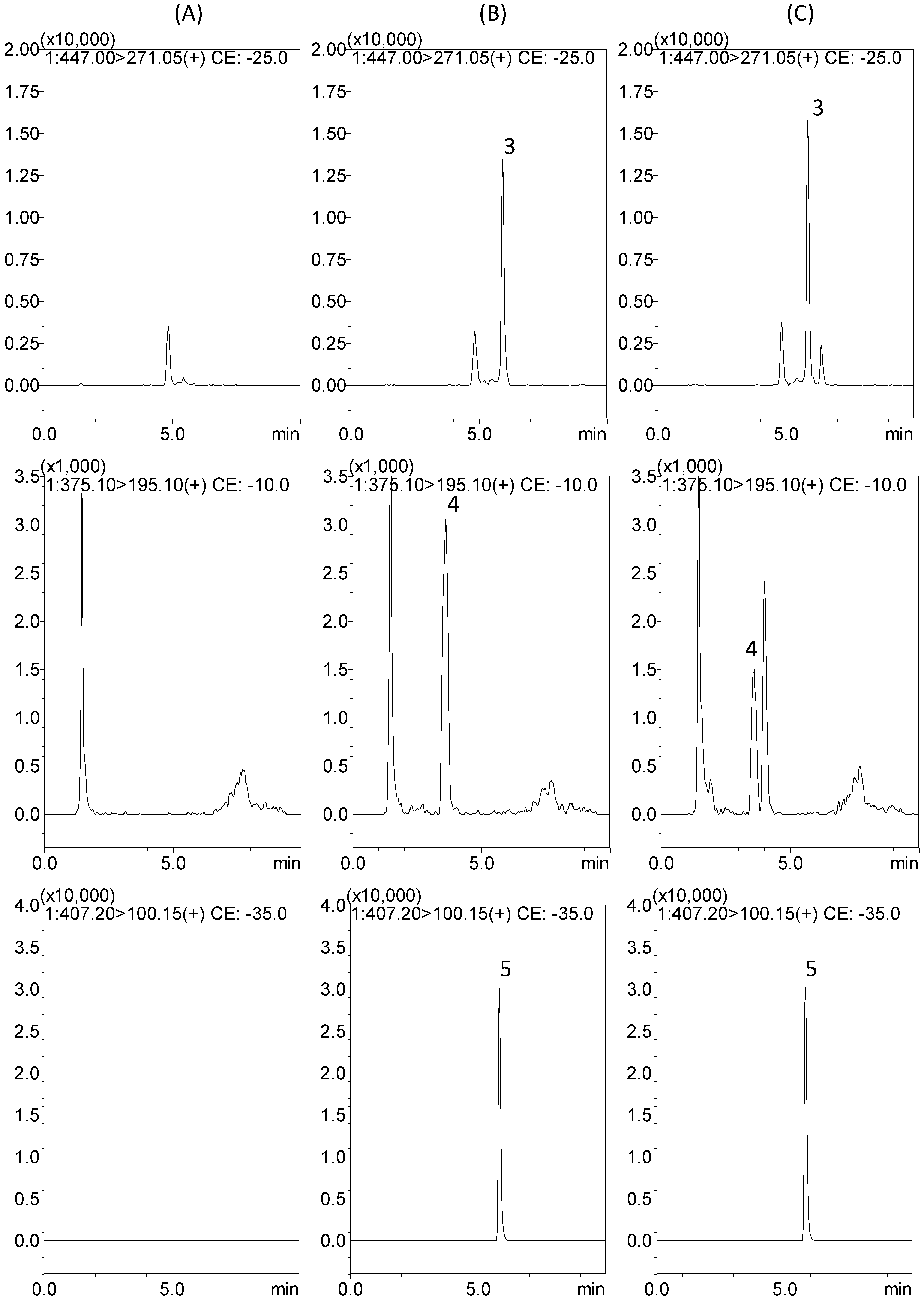
2.2. Protein Precipitation Methods for Sample Preparation
2.3. Method Validation
2.3.1. Selectivity
2.3.2. Linearity, the Lower Limit of Determination (LLOD) and Lower Limit of Quantification (LLOQ)
2.3.3. Precision and Accuracy
| Nominal conc.(ng/mL) | Intra-day | Inter-day | ||||
|---|---|---|---|---|---|---|
| Observed conc. (ng/mL) | Precision, RSD (%) | Accuracy, Bias (%) | Observed conc. (ng/mL) | Precision, RSD (%) | Accuracy, Bias (%) | |
| Gentiopicroside | ||||||
| 10 | 9.82 ± 0.98 | 10.0 | −1.8 | 10.1 ± 1.45 | 14.4 | 0.9 |
| 25 | 23.5 ± 2.68 | 11.4 | −6.0 | 25.7 ± 2.11 | 8.2 | 2.9 |
| 50 | 49.9 ± 4.29 | 8.6 | −0.3 | 49.2 ± 1.26 | 2.6 | −1.6 |
| 100 | 98.1 ± 2.52 | 2.6 | −1.9 | 99.6 ± 2.91 | 2.9 | −0.4 |
| 250 | 259 ± 6.67 | 2.6 | 3.5 | 251 ± 5.72 | 2.3 | 0.3 |
| 500 | 494 ± 5.09 | 1.0 | −1.1 | 500 ± 2.82 | 0.6 | −0.1 |
| Geniposide | ||||||
| 10 | 9.37 ± 0.88 | 9.4 | −6.3 | 9.77 ± 1.16 | 11.9 | −2.3 |
| 25 | 25.4 ± 1.19 | 4.7 | 1.5 | 26.0 ± 1.96 | 7.5 | 3.9 |
| 50 | 49.5 ± 1.01 | 2.0 | −0.9 | 49.8 ± 3.06 | 6.1 | −0.4 |
| 100 | 98.2 ± 4.04 | 4.1 | −1.8 | 100 ± 3.02 | 3.0 | 0.1 |
| 250 | 256 ± 5.66 | 2.2 | 2.3 | 249 ± 3.50 | 1.4 | −0.4 |
| 500 | 497 ± 7.04 | 1.4 | −0.5 | 500 ± 1.48 | 0.3 | 0.1 |
| Baicalin | ||||||
| 10 | 11.0 ± 1.01 | 9.1 | 10.1 | 10.2 ± 1.51 | 14.8 | 2.1 |
| 25 | 24.9 ± 1.65 | 6.6 | −0.3 | 25.8 ± 2.70 | 10.5 | 3.3 |
| 50 | 50.9 ± 1.92 | 3.8 | 1.9 | 47.3 ± 1.12 | 2.4 | −5.4 |
| 100 | 98.4 ± 4.51 | 4.6 | −1.6 | 99.3 ± 3.04 | 3.1 | −0.7 |
| 250 | 245 ± 8.61 | 3.5 | −1.9 | 250 ± 4.71 | 1.9 | −0.1 |
| 500 | 491 ± 19.6 | 4.0 | −1.7 | 505 ± 9.20 | 1.8 | 1.0 |
| Swertiamarin | ||||||
| 5 | 5.35 ± 0.33 | 6.2 | 7.0 | 4.74 ± 0.25 | 5.3 | −5.1 |
| 10 | 9.91 ± 0.66 | 6.7 | −0.9 | 10.1 ± 0.53 | 5.2 | 1.0 |
| 25 | 24.8 ± 0.37 | 1.5 | −0.9 | 24.9 ± 0.75 | 3.0 | −0.4 |
| 50 | 50.0 ± 1.06 | 2.1 | −0.1 | 49.8 ± 1.16 | 2.3 | −0.4 |
| 100 | 99.6 ± 1.28 | 1.3 | −0.4 | 101 ± 1.35 | 1.3 | 0.9 |
| 250 | 251 ± 2.20 | 0.9 | 0.5 | 253 ± 7.21 | 2.9 | 1.1 |
2.3.4. Matrix Effect and Recovery
| Nominal conc. | Peak Area | Matrix Effect | Recovery | ||
|---|---|---|---|---|---|
| (ng/mL) | Set 1 | Set 2 | Set 3 | (%) a | (%) b |
| Gentiopicroside | |||||
| 10 | 3911 ± 321 | 4001 ± 595 | 4312 ± 285 | 102.0 ± 7.4 | 108.7 ± 9.2 |
| 50 | 17829 ± 774 | 20372 ± 283 | 21498 ± 1749 | 114.4 ± 3.6 | 105.6 ± 9.9 |
| 250 | 89698 ± 3141 | 93932 ± 2505 | 104970 ± 4074 | 104.8 ± 4.1 | 111.7 ± 2.5 |
| Mean ± S.D. | 107.0 ± 7.3 | 108.7 ± 7.3 | |||
| Geniposide | |||||
| 10 | 12233 ± 190 | 14306 ± 1062 | 17028 ± 644 | 116.9 ± 6.9 | 119.4 ± 7.9 |
| 50 | 58115 ± 1045 | 67062 ± 1062 | 71407 ± 1764 | 115.4 ± 1.6 | 106.5 ± 1.1 |
| 250 | 295991 ± 3955 | 316422 ± 6847 | 346662 ± 8807 | 106.9 ± 3.7 | 109.6 ± 0.6 |
| Mean ± S.D. | 113.1 ± 6.1 | 111.8 ± 7.1 | |||
| Baicalin | |||||
| 10 | 9173 ± 232 | 7795 ± 1246 | 7825 ± 1054 | 85.0 ± 13.6 | 101.1 ± 10.7 |
| 50 | 45932 ± 1992 | 39518 ± 2684 | 37717 ± 460 | 86.0 ± 3.1 | 95.7 ± 5.6 |
| 250 | 224485 ± 4019 | 198185 ± 9541 | 186908 ± 6196 | 88.3 ± 3.2 | 94.4 ± 2.8 |
| Mean ± S.D. | 86.4 ± 7.3 | 97.1 ± 6.9 | |||
| Swertiamarin | |||||
| 10 | 16564 ± 490 | 16634 ± 397 | 17422 ± 660 | 100.5 ± 4.5 | 104.7 ± 1.7 |
| 50 | 76343 ± 1289 | 82585 ± 5095 | 88245 ± 4264 | 108.1 ± 4.8 | 107.0 ± 5.2 |
| 250 | 404293 ± 4777 | 432683 ± 3140 | 467239 ± 7470 | 107.0 ± 0.5 | 108.0 ± 1.0 |
| Mean ± S.D. | 105.2 ± 4.9 | 106.6 ± 3.1 | |||
| Carvedilol (IS) | |||||
| 10 | 76558 ± 6622 | 87040 ± 5353 | 83146 ± 3942 | 113.9 ± 2.9 | 95.7 ± 4.7 |
2.3.5. Stability
| Analytes/ | Freeze-thaw Stability | Short-term Stability | Long-term Stability | Autosampler Stability | ||||
|---|---|---|---|---|---|---|---|---|
| Spiked Concentration (ng/mL) | Measured Concentration (ng/mL) | Accuracy (%) | Measured Concentration (ng/mL) | Accuracy (%) | Measured Concentration (ng/mL) | Accuracy (%) | Measured Concentration (ng/mL) | Accuracy (%) |
| Gentiopicroside | ||||||||
| 10 | 11.3 ± 0.28 | 113.0 | 11.0 ± 0.40 | 110.2 | 9.82 ± 0.21 | 98.2 | 11.0 ± 1.09 | 109.8 |
| 50 | 53.7 ± 1.44 | 107.4 | 55.5 ± 1.68 | 111.0 | 51.1 ± 2.71 | 102.2 | 49.0 ± 2.47 | 97.9 |
| 250 | 265 ± 6.60 | 106.0 | 263 ± 16.2 | 105.1 | 240 ± 6.72 | 95.9 | 253 ± 10.7 | 101.1 |
| Geniposide | ||||||||
| 10 | 10.0 ± 0.94 | 100.3 | 11.4 ± 0.80 | 114.1 | 9.10 ± 0.77 | 91.1 | 10.3 ± 0.65 | 102.8 |
| 50 | 56.5 ± 1.00 | 113.0 | 52.9 ± 1.12 | 105.8 | 46.4 ± 4.16 | 92.7 | 48.9 ± 1.86 | 97.7 |
| 250 | 265 ± 10.9 | 106.0 | 260 ± 6.85 | 103.9 | 235 ± 15.4 | 94.1 | 248 ± 5.47 | 99.4 |
| Baicalin | ||||||||
| 10 | 10.3 ± 1.01 | 103.0 | 11.2 ± 1.14 | 111.8 | 9.54 ± 0.97 | 95.4 | 9.28 ± 0.72 | 92.8 |
| 50 | 49.5 ± 0.75 | 99.0 | 48.4 ± 1.18 | 96.7 | 49.7 ± 6.84 | 99.5 | 50.5 ± 4.33 | 101.0 |
| 250 | 231 ± 12.0 | 92.2 | 231 ± 10.8 | 92.6 | 238 ± 33.2 | 95.3 | 231 ± 1.57 | 92.4 |
| Swertiamarin | ||||||||
| 10 | 10.6 ± 0.76 | 105.7 | 11.3 ± 0.99 | 112.7 | 9.50 ± 0.50 | 95.0 | 10.6 ± 0.78 | 106.1 |
| 50 | 49.8 ± 0.66 | 99.6 | 48.5 ± 0.23 | 96.9 | 45.7 ± 3.21 | 91.3 | 49.9 ± 1.08 | 99.7 |
| 250 | 247 ± 2.74 | 98.7 | 252 ± 8.03 | 100.9 | 224 ± 10.7 | 89.8 | 240 ± 3.52 | 96.1 |
2.4. Application of the Analytical System in Pharmacokinetic Study

| Parameter | Gentiopicroside | Geniposide | Baicalin | Swertiamarin |
|---|---|---|---|---|
| Cmax (ng/mL) | 5767 ± 412 a | 1164 ± 144 | 2008 ± 265 | 13.0 ± 0.5 |
| Tmax (min) | 60.0 ± 6.7 | 55.0 ± 12.6 | 15.8 ± 9.0 | 97.5 ± 14.4 |
| t1/2 (min) | 127 ± 7.7 | 168 ± 27.4 | 314 ± 56.3 | 188 ± 27.6 |
| AUC (min µg/mL) | 1417 ± 83.8 b | 302 ± 25.8 | 753 ± 86.2 | 2.5 ± 0.1 |
| Cl (mL/min/kg) | 118 ± 6.5 | 784 ± 55.5 | 112 ± 20.7 | 581 ± 58.5 |
| MRT (min) | 193 ± 7.9 | 213 ± 18.2 | 298 ± 11.6 | 144 ± 3.1 |
3. Experimental
3.1. Chemicals and Reagents
3.2. Herbal Preparation of Long-Dan-Xie-Gan-Tang
3.3. LC-MS/MS and Analytical Conditions
3.4. Preparation of Calibration Standards and Quality Controls
3.5. Method Validation
3.5.1. Selectivity
3.5.2. Linearity, the Lower Limits of Detection (LLOD) and Lower Limits of Quantification (LLOQ)
3.5.3. Precision and Accuracy
3.5.4. Matrix Effect and Recovery
3.5.5. Stability
3.6. Pharmacokinetic Application
3.6.1. Experimental Animals
3.6.2. Freely Moving Rat Model
3.6.3. LDXGT Administration and Sample Collection
3.6.4. Sample Preparation
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Kong, L.; Hu, L.; Lei, X.; Yang, L.; Chou, G.; Zou, H.; Wang, C.; Annie Bligh, S.W.; Wang, Z. Biological fingerprinting analysis of the traditional Chinese prescription Longdan Xiegan Decoction by on/off-line comprehensive two-dimensional biochromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 860, 185–194. [Google Scholar] [CrossRef]
- Chen, F.P.; Kung, Y.Y.; Chen, Y.C.; Jong, M.S.; Chen, T.J.; Chen, F.J.; Hwang, S.J. Frequency and pattern of Chinese herbal medicine prescriptions for chronic hepatitis in Taiwan. J. Ethnopharmacol. 2008, 117, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chang, H.H. Longdan Xiegan Tang has immunomodulatory effects on CD4+CD25+ T cells and attenuates pathological signs in MRL/lpr mice. Int. J. Mol. Med. 2010, 25, 677–685. [Google Scholar] [PubMed]
- Rojas, A.; Bah, M.; Rojas, J.I.; Gutierrez, D.M. Smooth muscle relaxing activity of gentiopicroside isolated from Gentiana spathacea. Planta Med. 2000, 66, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, Y.; Nahar, L.; Sarker, S.D. Bioactivity of gentiopicroside from the aerial parts of Centaurium erythraea. Fitoterapia 2003, 74, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Takano, F.; Hojo, H. Suppression of chemically and immunologically induced hepatic injuries by gentiopicroside in mice. Planta Med. 1994, 60, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Lee, S.; Shin, K.H.; Kim, B.C.; Lim, C.J.; Park, E.H. Geniposide, an anti-angiogenic compound from the fruits of Gardenia jasminoides. Planta Med. 2004, 70, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Lim, K.H.; Jung, H.J.; Park, E.H. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Hu, J.; Zhao, S.; Yuan, Z.Y.; Wan, H.J.; Lei, F.; Ding, Y.; Xing, D.M.; Du, L.J. Comparative study of the effect of baicalin and its natural analogs on neurons with oxygen and glucose deprivation involving innate immune reaction of TLR2/TNFalpha. J. Biomed. Biotechnol. 2012, 2012, 267890. [Google Scholar] [PubMed]
- Trinh, H.T.; Joh, E.H.; Kwak, H.Y.; Baek, N.I.; Kim, D.H. Anti-pruritic effect of baicalin and its metabolites, baicalein and oroxylin A, in mice. Acta Pharmacol. Sin. 2010, 31, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.L.; Li, H.L.; He, J.C.; Feng, E.F.; Shi, P.P.; Liu, Y.Q.; Liu, C.X. Comparative pharmacokinetics of swertiamarin in rats after oral administration of swertiamarin alone, Qing Ye Dan tablets and co-administration of swertiamarin and oleanolic acid. J. Ethnopharmacol. 2013, 149, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, L.; Lei, X.; Hu, L.; Zou, H.; Welbeck, E.; Bligh, S.W.; Wang, Z. Comprehensive two-dimensional high-performance liquid chromatography system with immobilized liposome chromatography column and reversed-phase column for separation of complex traditional Chinese medicine Longdan Xiegan Decoction. J. Chromatogr. A 2009, 1216, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; He, Y. Q.; Wang, C. H.; Welbeck, E. W.; Bligh, S. W.; Wang, Z.T. Characterization of fifty-one flavonoids in a Chinese herbal prescription Longdan Xiegan Decoction by high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry and photodiode array detection. Rapid Commun. Mass Spectrom. 2008, 22, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Cheng, X.M.; Bligh, S.W.; White, K.N.; Branford-White, C.J.; Wang, Z.T. Pharmacokinetics and bioavailability of gentiopicroside from decoctions of Gentianae and Longdan Xiegan Tang after oral administration in rats--comparison with gentiopicroside alone. J. Pharm. Biomed. Anal. 2007, 44, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Chang-Liao, W.L.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Isolation of gentiopicroside from Gentianae Radix and its pharmacokinetics on liver ischemia/reperfusion rats. J. Ethnopharmacol. 2012, 141, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wang, Z.T.; Bligh, S.W.; White, K.N.; White, C.J. Pharmacokinetics and tissue distribution of gentiopicroside following oral and intravenous administration in mice. Eur. J. Drug Metab. Pharmacokinet. 2004, 29, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lin, L.C.; Lin, C.H.; Tsai, T.H. Comparative oral bioavailability of geniposide following oral administration of geniposide, Gardenia jasminoides Ellis fruits extracts and Gardenia herbal formulation in rats. J. Pharm. Pharmacol. 2014, 66, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Feng, F.; Yu, X. Pharmacokinetics of geniposide in Zhi-Zi-Hou-Pu decoction and in different combinations of its constituent herbs. Phytother. Res. 2012, 26, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.M.; Bi, K.S.; Huo, Y.S.; Yan, X.Y.; Zhao, X.; Chen, X.H. Simultaneous quantification of three iridoids in rat plasma after oral administration of Zhi-zi-chi decoction using LC-MS. J. Sep. Sci. 2011, 34, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; He, J.C.; Bai, M.; Song, Q.Y.; Feng, E.F.; Rao, G.X.; Xu, G.L. Determination of the plasma pharmacokinetic and tissue distributions of swertiamarin in rats by liquid chromatography with tandem mass spectrometry. Arzneimittelforschung 2012, 62, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, S.; Sun, Y.; Wang, H.; Wang, X.; Zhang, H.; Wang, Z. Highly sensitive determination of new metabolite in rat plasma after oral administration of swertiamarin by liquid chromatography/time of flight mass spectrometry following picolinoyl derivatization. Biomed. Chromatogr. 2014, 28, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.H.; Lin, L.C.; Tsai, T.H. HPLC-MS/MS analysis of a traditional Chinese medical formulation of Bu-Yang-Huan-Wu-Tang and its pharmacokinetics after oral administration to rats. PLoS One 2012, 7, e43848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Liua, W.; Zhuang, L.; Wang, J.; Zhang, S. Comparative pharmacokinetics of baicalin, wogonoside, baicalein and wogonin in plasma after oral administration of pure baicalin, radix scutellariae and scutellariae-paeoniae couple extracts in normal and ulcerative colitis rats. Iran. J. Pharm. Res. 2013, 12, 399–409. [Google Scholar] [PubMed]
- Nowatzke, W.; Woolf, E. Best practices during bioanalytical method validation for the characterization of assay reagents and the evaluation of analyte stability in assay standards, quality controls, and study samples. AAPS J. 2007, 9, E117–E122. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.L.; Chang, L.W.; Chiang, C.J.; Tsuang, Y.H.; Lin, C.H.; Tsai, T.H. Pharmacokinetics of di-isononyl phthalate in freely moving rats by UPLC-MS/MS. Int. J. Pharm. 2013, 450, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds gentiopicroside, geniposide, baicalin and swertiamarin are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.-M.; Lin, L.-C.; Tsai, T.-H. Determination and Pharmacokinetic Study of Gentiopicroside, Geniposide, Baicalin, and Swertiamarin in Chinese Herbal Formulae after Oral Administration in Rats by LC-MS/MS. Molecules 2014, 19, 21560-21578. https://doi.org/10.3390/molecules191221560
Lu C-M, Lin L-C, Tsai T-H. Determination and Pharmacokinetic Study of Gentiopicroside, Geniposide, Baicalin, and Swertiamarin in Chinese Herbal Formulae after Oral Administration in Rats by LC-MS/MS. Molecules. 2014; 19(12):21560-21578. https://doi.org/10.3390/molecules191221560
Chicago/Turabian StyleLu, Chia-Ming, Lie-Chwen Lin, and Tung-Hu Tsai. 2014. "Determination and Pharmacokinetic Study of Gentiopicroside, Geniposide, Baicalin, and Swertiamarin in Chinese Herbal Formulae after Oral Administration in Rats by LC-MS/MS" Molecules 19, no. 12: 21560-21578. https://doi.org/10.3390/molecules191221560





