Design and Synthesis of Chalcone Derivatives as Inhibitors of the Ferredoxin — Ferredoxin-NADP+ Reductase Interaction of Plasmodium falciparum: Pursuing New Antimalarial Agents
Abstract
:1. Introduction
2. Results and Discussion
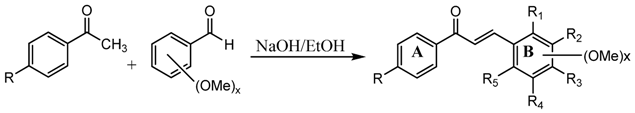
2.1. Design and Synthesis of Chalcone Derivatives
| Compd | R | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|
| 1 | NH2 | OCH3 | H | H | H | H |
| 2 | NH2 | H | OCH3 | H | H | H |
| 3 | NH2 | H | H | OCH3 | H | H |
| 4 | NH2 | OCH3 | OCH3 | H | H | H |
| 5 | NH2 | OCH3 | H | OCH3 | H | H |
| 6 | NH2 | OCH3 | H | H | OCH3 | H |
| 7 | NH2 | H | H | H | H | H |
| 8 | H | OCH3 | H | H | H | H |
| 9 | H | H | OCH3 | H | H | H |
| 10 | H | H | H | OCH3 | H | H |
| 11 | H | OCH3 | OCH3 | H | H | H |
| 12 | H | OCH3 | H | OCH3 | H | H |
| 13 | H | OCH3 | H | H | OCH3 | H |
| 14 | Br | H | H | OCH3 | H | H |
| 15 | Br | OCH3 | H | OCH3 | H | H |
| 16 | Br | OCH3 | H | H | OCH3 | H |
| 17 | H | H | H | H | H | H |
2.2. Production, Characterization and Kinetic Analysis of PfFd and PfFNR
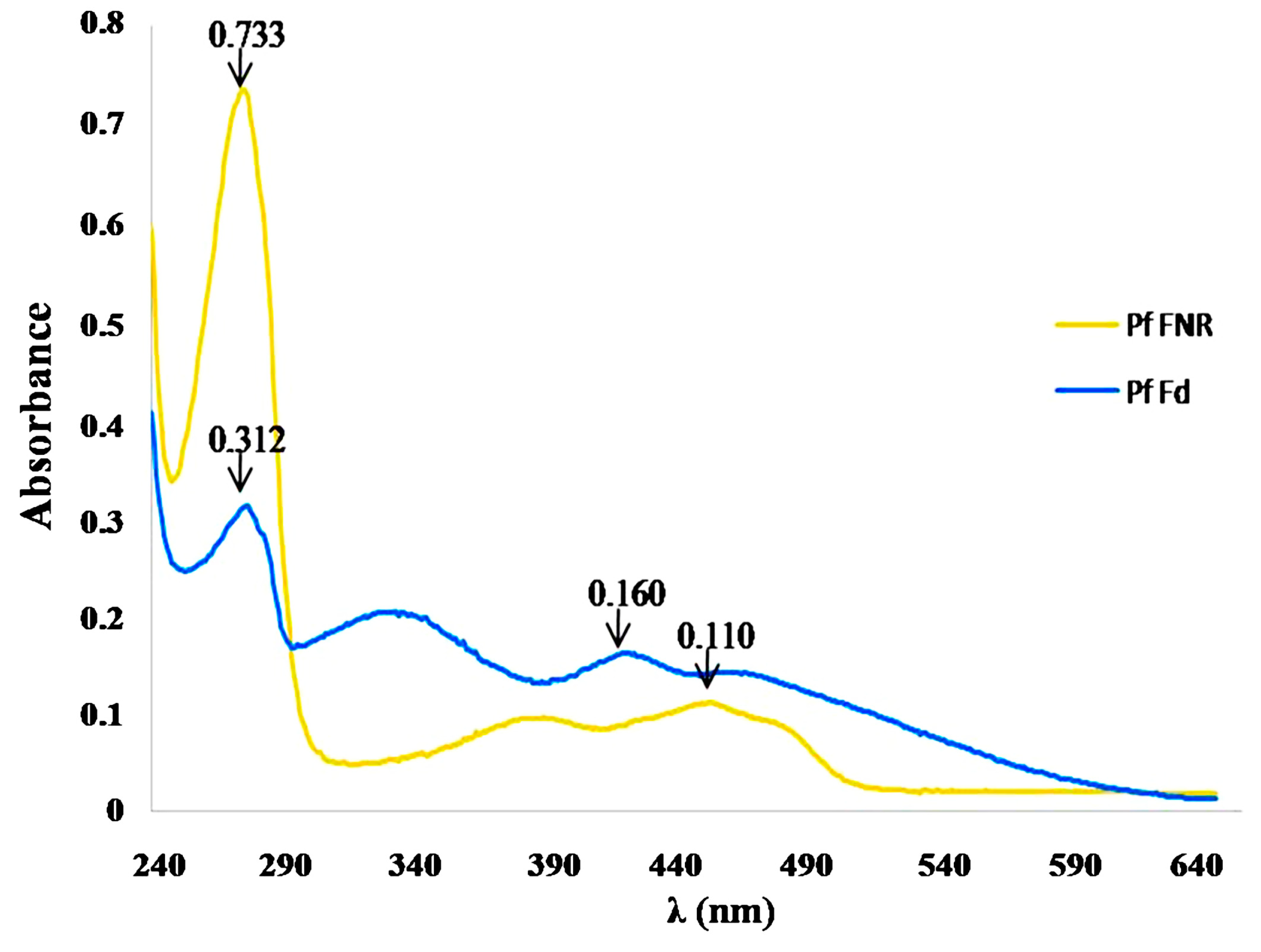
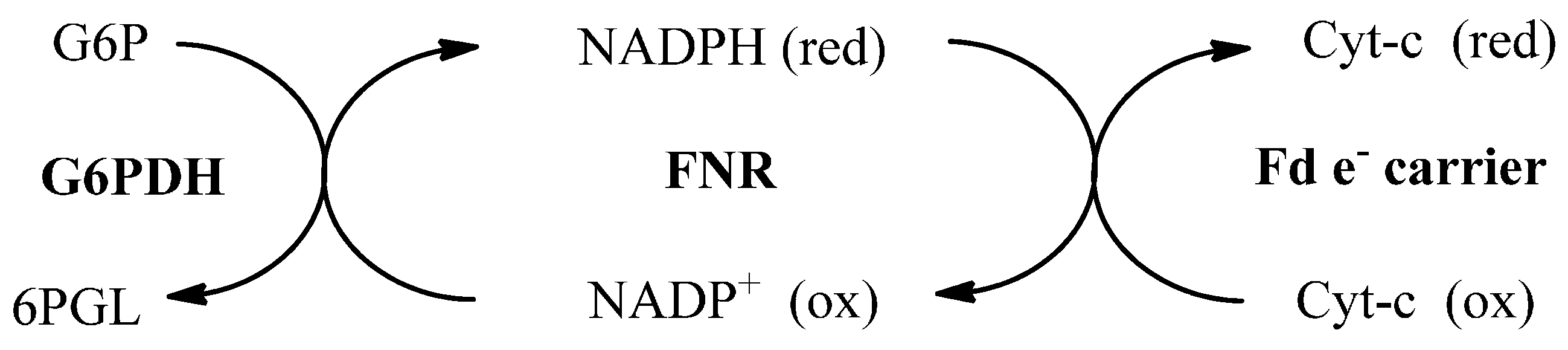
2.3. Inhibition of PfFd-PfFNR Interaction by Synthesized Compounds
| Tested Compound | dA/dt | % Inhibition |
|---|---|---|
| Control-1 | 0.076 | - |
| 1 | 0.064 | 15.79 |
| 2 | 0.059 | 22.37 |
| 3 | 0.038 | 50.00 |
| 4 | 0.052 | 31.58 |
| 5 | 0.047 | 38.16 |
| Control-2 | 0.064 | - |
| 6 | 0.073 | −14.06 |
| 7 | 0.057 | 10.94 |
| 10 | 0.061 | 4.69 |
| 12 | 0.060 | 6.25 |
| Control-3 | 0.068 | - |
| 8 | 0.075 | −10.29 |
| 9 | 0.068 | 0.00 |
| 11 | 0.072 | −5.88 |
| 13 | 0.064 | 5.88 |
| 14 | 0.063 | 7.35 |
| 15 | 0.050 | 26.47 |
| 16 | 0.060 | 11.76 |
| 17 | 0.053 | 22.06 |
2.4. In Silico Analysis of the Protein—Synthesized Compounds Interaction

| Comp | Protein PfFd | Protein Maize Leaf-FNR | ||||
|---|---|---|---|---|---|---|
| ∆G Energy (Kcal/mol) | Amino Acid Residues Form H-Bonding | Amino Acid Residues Form Electrostatic Interaction | ∆G Energy (Kcal/mol) | Amino Acid Residues Form H-Bonding | Amino Acid Residues Form Electrostatic Interaction | |
| 1 | −2.45 | Tyr37 | --- | −3.68 | Asn30 | Tyr32 |
| 2 | −2.96 | Arg30, Asn32 | --- | −3.78 | Asn30, Leu94 | Tyr32 |
| 3 | −2.71 | Arg30, Tyr37 | Glu34 | −4.00 | Lys304 | Lys304, Asp307 |
| 4 | −2.45 | Arg30, | Glu34 | −3.50 | Lys304, Arg305 | Lys304, Asp307 |
| 5 | −2.43 | Asn32 | --- | −4.09 | Lys304, Arg305 | --- |
| 6 | −2.49 | Arg30, Tyr37 | --- | −3.55 | Asn30, leu94, Thr148 | --- |
| 7 | −2.79 | Tyr37 | Glu34 | −4.10 | Lys304(2x) | Asp307 |
| 8 | −2.47 | Asn32, Tyr37 | --- | −3.99 | Lys301, Lys304 | --- |
| 9 | −2.90 | --- | --- | −4.33 | Val311 | --- |
| 10 | −2.75 | --- | --- | −4.09 | Lys304, Arg305 | --- |
| 11 | −2.32 | --- | --- | −3.84 | Lys304 | --- |
| 12 | −2.48 | --- | --- | −3.76 | Lys304 | --- |
| 13 | −2.57 | --- | --- | −4.09 | Lys304 | --- |
| 14 | - | --- | --- | - | Not observed | --- |
| 15 | - | --- | --- | - | Not observed | --- |
| 16 | - | --- | --- | - | Not observed | --- |
| 17 | −2.65 | --- | --- | −4.03 | Lys304 | --- |
2.5. Docking into PfFd
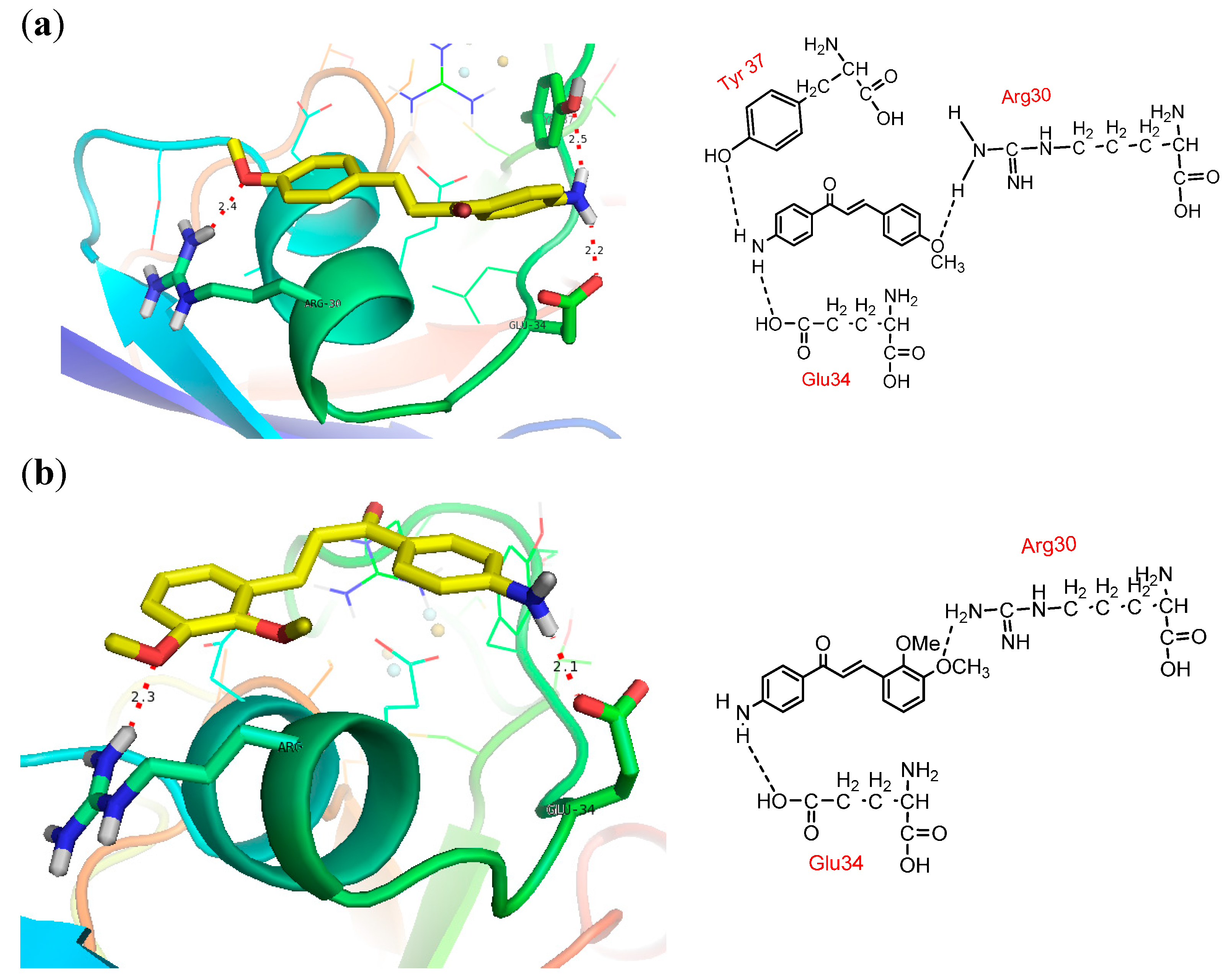
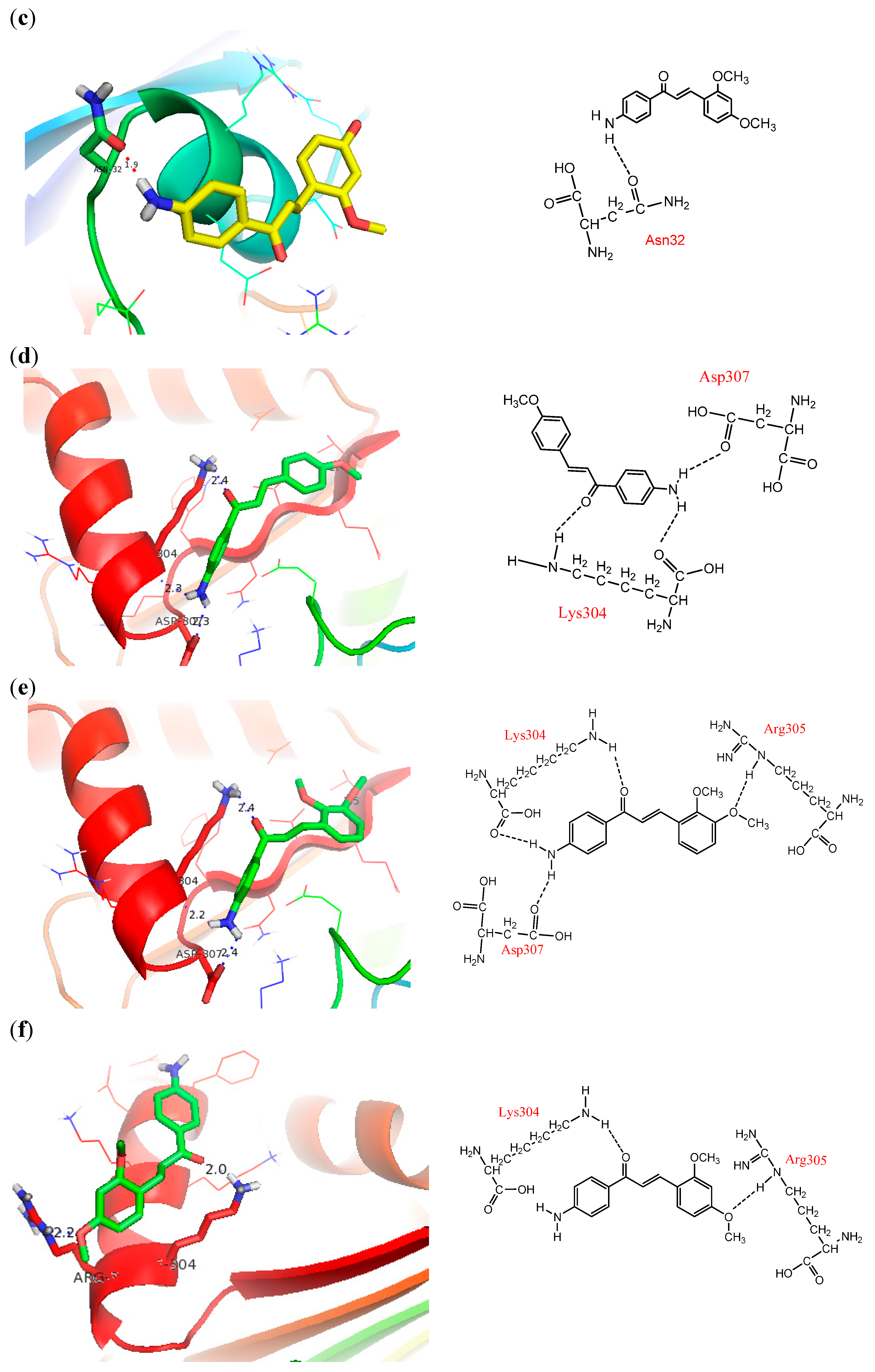
2.6. Docking into Maize Leaf-FNR
3. Experimental Section
3.1. General Information
3.2. General Procedure for Chalcone Synthesis
3.3. Preparation of of PfFNR and PfFd in E. coli
3.4. Inhibitory Activity Assay
3.5. Ligand Docking Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2013. http://www.who.int/malaria/publications/world_malaria_report_2013/en/ (accessed on 14 January 2014).
- Rosenthal, P.J.; Miller, L.H. The Need for New Approaches to Antimalarial Chemotherapy. In Antimalarial Chemotherapy: Mechanisms of Action, and New Directions in Drug Discovery, 1st ed.; Rosenthal, P.J., Ed.; Humana Press: Totowa, NJ, USA, 2001; pp. 3–13. [Google Scholar]
- Tjitra, E.; Marwoto, H.A.; Ompusunggu, R.M.; Tuti, S. Penelitian obat anti malaria. Buletin Penelitian Kesehatan Indonesia 1991, 19, 15–22. [Google Scholar]
- Basuki, S.; Fitriah, F.; Riyanto, S.; Budiono; Dachlan, Y.P.; Uemura, H. Two novel mutation of pfdhps K540T and 1588F, affecting Sulfadoxine-pyrimethamine resistant response in uncomplicated falciparum malaria at Banjar district, South Kalimantan Province, Indonesia. Malaria J. 2014, 13, 135. [Google Scholar] [CrossRef]
- Milani, M.; Balconi, E.; Aliverti, A.; Mastrangelo, E.; Seeber, F.; Bolognesi, M.; Zanetti, G. Ferredoxin-NADP+ reductase from Plasmodium falciparum undergoes NADP+-dependent dimerization and inactivation: functional and crystallographic analysis. J. Mol. Biol. 2007, 367, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Kimata-Ariga, Y.; Kurisu, G.; Kusunoki, M.; Aoki, S.; Sato, D.; Kobayashi, T.; Kita, K.; Horii, T.; Hase, T. Cloning and characterization of ferredoxin and ferredoxin-NADP+ reductase from human malaria parasite. J. Biochem. 2007, 141, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kimata-Ariga, Y.; Sitoh, T.; Ikegami, T.; Horii, T.; Hase, T. Molecular interaction of ferredoxin and ferredoxin-NADP+ reductase from human malaria parasite. J. Biochem. 2007, 142, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Al-Masum, M.; Eunice, Ng.; Wai, M.C. Palladium-catalyzed direct cross-coupling of potassium styryltrifluoroborates and benzoyl chlorides—A one step method for chalcone synthesis. Tetrahedron Lett. 2011, 52, 1008–1010. [Google Scholar]
- Kim, B.T.; Chun, J.C.; Hwang, K.J. Synthesis of dihydroxylated chalcone derivatives with diverse substitution pattern and their radical scavenging ability toward DPPH free radicals. Bull. Korean Chem. Soc. 2008, 29, 1125–1130. [Google Scholar] [CrossRef]
- Khan, S.A.; Ahmed, B.; Alam, T. Synthesis and antihepatotoxic activity of some new chalcones containing 1,4-dioxane ring system. Pak. J. Pharm. Sci. 2006, 19, 290–294. [Google Scholar] [PubMed]
- Yoon, G.; Kang, B.Y.; Cheon, S.H. Topoisomerase I inhibition and cytotoxicity of licochalcones A and E from Glycyrrhiza inflate. Arch. Pharm. Res. 2007, 30, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.K.; Mishra, N.; Kumar, B.; Sharma, M.; battacharya, A.; Mishra, L.C.; Bhasin, V.K. Potent antimalarial activity of newly synthesized substituted chalcone analog in vitro. Med. Chem. Res. 2009, 18, 407–420. [Google Scholar] [CrossRef]
- Liu, M.; Wilariat, P.; Go, M.L. Antimalarial alkoxylated and hydroxylated chalcones: Structure activity relationship analysis. J. Med. Chem. 2001, 44, 4443–4452. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Singh, M.; Raghav, N. Spectrophotometric analysis of bovine serum albumin in presence of 1-(4-aminophenyl)-3-phenylprop-2-en-1-ones. IJPSR 2014, 5, 2657–2661. [Google Scholar]
- Pophale, RA.; Deodhar, M.N. Synthesis and evaluation of novel phthalimide derivatives as analgesic and anti-inflammatory agents. Der Pharma Chemica 2010, 2, 185–193. [Google Scholar]
- Rueping, M.; Bootwicha, T.; Baars, H.; Sugiono, E. Continuous-flow hydration-condensation reaction: Synthesis of α,β-unsaturated ketones from alkynes and aldehydes by using a heterogeneous solid acid catalyst. Beilstein J. Org. Chem. 2011, 7, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of chalcones with anticancer activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, B.D.; Gawande, S.S.; Bodade, R.G.; Totre, J.V.; Khobragade, C.N. Synthesis and Biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. 2010, 18, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Durst, H.D.; Gokel, G.W. Experimental Organic Chemistry, 2nd ed.; McGraw-Hill Publishing Company: New York, NY, USA, 1987; pp. 428–430. [Google Scholar]
- Sebti, S.; Solhi, A.; Tahir, R.; Boulaajaj, S.; Mayoral, J.A.; Fraile, J.M.; Kossir, A.; Oumimoun, H. Calcined sodium nitrate/natural phosphate: An extremely active catalyst for the easy synthesis of chalcones in heterogenous media. Tetrahedron Lett. 2001, 42, 7953–7955. [Google Scholar] [CrossRef]
- Lopez, J.; Jacquot, R.; Figueras, F. Heterogeneous catalysis of aldolisations on solid basic catalysts. Stud. Surf. Sci. Catal. 2000, 130, 491–496. [Google Scholar]
- Wade, L.G., Jr. Organic Chemistry, 6th ed.; Pearson Prentice Hall: New York, NY, USA, 2006; pp. 508–608. [Google Scholar]
- Balcony, E.; Pennati, A.; Crobu, D.; Pandini, V.; Cerutti, R.; Zanetti, G.; Aliverti, A. The ferredoxin—NADP+ reductase/ferredoxin electron transfer system of Plasmodium falciparum. FEBS J. 2009, 276, 3825–3836. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, G.; Kusunoki, M.; Katoh, E.; Yamazaki, T.; Teshima, K.; Onda, Y.; Kimata-Ariga, Y.; Hase, T. Structure of the electron transfer complex between ferredoxin and ferredoxin-NADP+ reductase. Nat. Struct. Biol. 2001, 8, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Onda, Y.; Matsumura, T.; Kimata-Ariga, Y.; Sakakibara, H.; Sugiyama, T.; Hase, T. Differential Interaction of Maize Root Ferredoxin:NADP+ Oxidoreductase with Photosynthetic and Non-Photosynthetic Ferredoxin Isoproteins. Plant Physiol. 2000, 134, 1037–1045. [Google Scholar] [CrossRef]
- Sanner, M.F. Phyton: A programming language for software integration and development. J. Mol. Graph. Mod. 1999, 17, 57–61. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstorm, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDock Tools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–17 are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwito, H.; Jumina; Mustofa; Pudjiastuti, P.; Fanani, M.Z.; Kimata-Ariga, Y.; Katahira, R.; Kawakami, T.; Fujiwara, T.; Hase, T.; et al. Design and Synthesis of Chalcone Derivatives as Inhibitors of the Ferredoxin — Ferredoxin-NADP+ Reductase Interaction of Plasmodium falciparum: Pursuing New Antimalarial Agents. Molecules 2014, 19, 21473-21488. https://doi.org/10.3390/molecules191221473
Suwito H, Jumina, Mustofa, Pudjiastuti P, Fanani MZ, Kimata-Ariga Y, Katahira R, Kawakami T, Fujiwara T, Hase T, et al. Design and Synthesis of Chalcone Derivatives as Inhibitors of the Ferredoxin — Ferredoxin-NADP+ Reductase Interaction of Plasmodium falciparum: Pursuing New Antimalarial Agents. Molecules. 2014; 19(12):21473-21488. https://doi.org/10.3390/molecules191221473
Chicago/Turabian StyleSuwito, Hery, Jumina, Mustofa, Pratiwi Pudjiastuti, Much Zaenal Fanani, Yoko Kimata-Ariga, Ritsuko Katahira, Toru Kawakami, Toshimichi Fujiwara, Toshiharu Hase, and et al. 2014. "Design and Synthesis of Chalcone Derivatives as Inhibitors of the Ferredoxin — Ferredoxin-NADP+ Reductase Interaction of Plasmodium falciparum: Pursuing New Antimalarial Agents" Molecules 19, no. 12: 21473-21488. https://doi.org/10.3390/molecules191221473
APA StyleSuwito, H., Jumina, Mustofa, Pudjiastuti, P., Fanani, M. Z., Kimata-Ariga, Y., Katahira, R., Kawakami, T., Fujiwara, T., Hase, T., Sirat, H. M., & Puspaningsih, N. N. T. (2014). Design and Synthesis of Chalcone Derivatives as Inhibitors of the Ferredoxin — Ferredoxin-NADP+ Reductase Interaction of Plasmodium falciparum: Pursuing New Antimalarial Agents. Molecules, 19(12), 21473-21488. https://doi.org/10.3390/molecules191221473