Synthesis and Biological Evaluation of Novel 10-Substituted-7-ethyl-10-hydroxycamptothecin (SN-38) Prodrugs
Abstract
:1. Introduction
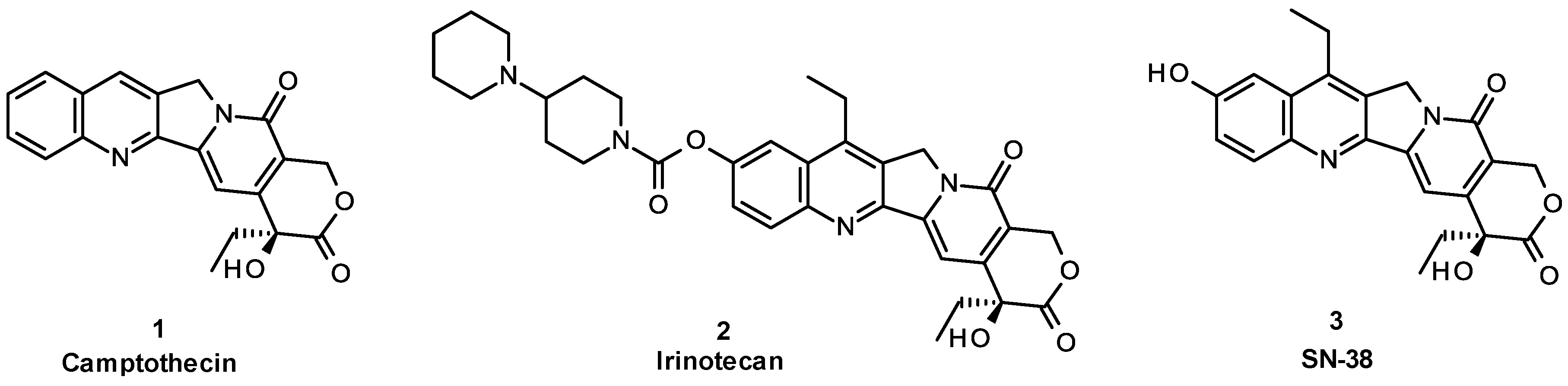
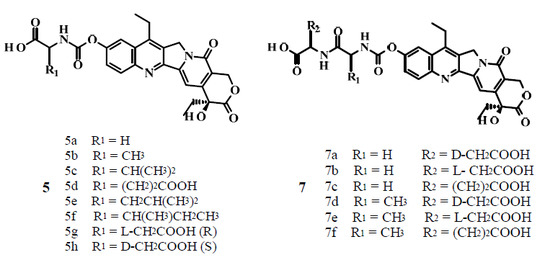
2. Results and Discussion
2.1. Chemistry
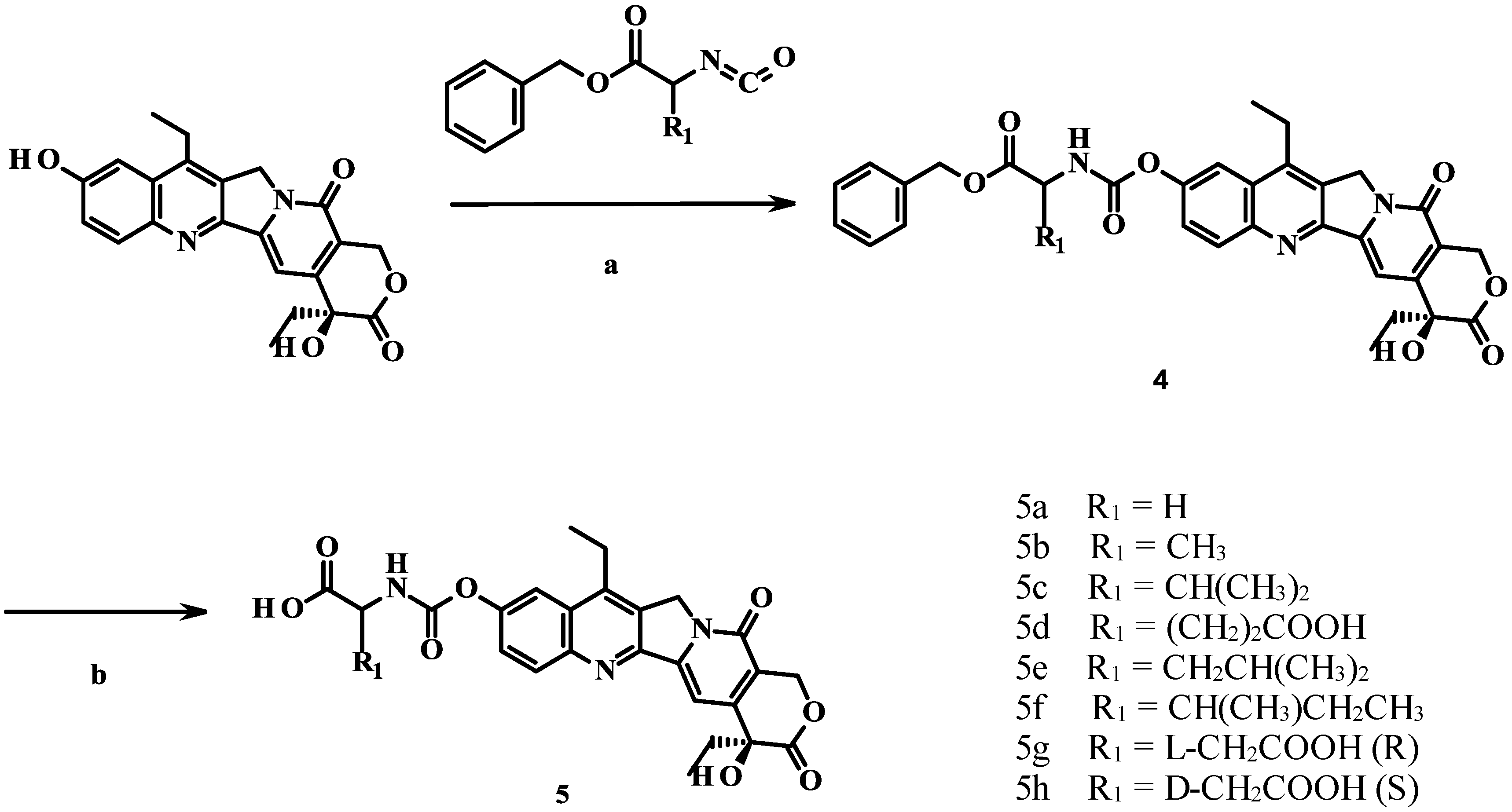
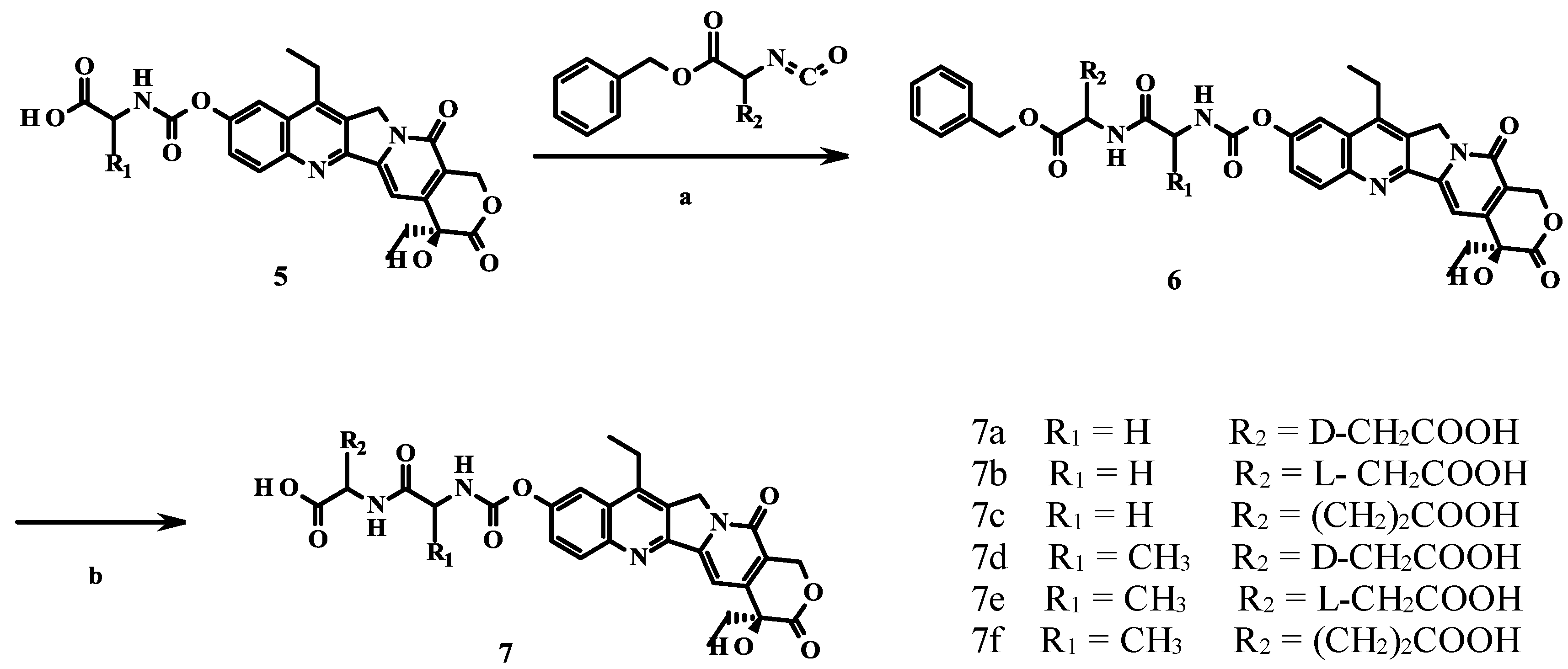
2.2. Cytotoxicity
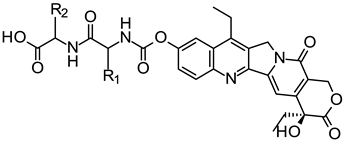 | ||||
|---|---|---|---|---|
| Compound | R1 | IC50 (μM) | ||
| SGC-7901 | HeLa | |||
| 5a | H | 2.69 ± 0.11 | (30.4 ± 47.0) × 10−3 | |
| 5b | CH3 | 1.75 ± 0.60 | (9.85 ± 11.1) × 10−3 | |
| 5c | CH(CH3)2 | 1.88 ± 0.72 | (5.61 ± 40.3) × 10−3 | |
| 5e | CH2CH(CH3)2 | 0.50 ± 0.19 | (1.82 ± 64.6) × 10−3 | |
| 5f | CH(CH3)CH2CH3 | 0.76 ± 0.34 | (3.64 ± 1.03) × 10−3 | |
| 5g | CH2COOH (R) | 0.98 ± 0.05 | (9.07 ± 33.2) × 10−3 | |
| 5h | d-CH2COOH (S) | 0.24 ± 0.95 | <(3.20 ± 64.3) × 10−3 | |
| Irinotecan | 7.38 ± 1.24 | 1.32 ± 0.13 | ||
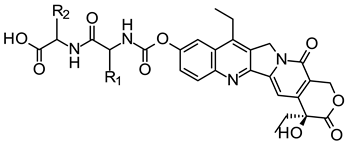 | ||||
| Compound | R1 | R2 | IC50 (μM) | |
| SGC-7901 | HeLa | |||
| 7a | H | d-CH2COOH | 1.04 ± 0.90 | <(3.20 ± 12.0) × 10−3 |
| 7b | H | l-CH2COOH | 0.61 ± 0.68 | (14.8 ± 135.0) × 10−3 |
| 7c | H | (CH2)2COOH | 0.20 ± 0.07 | <(3.20 ± 0.40) × 10−3 |
| 7d | CH3 | d-CH2COOH | 0.26 ± 0.64 | <(3.20 ± 2.0) × 10−3 |
| 7e | CH3 | l-CH2COOH | 0.21 ± 0.55 | (12.9 ± 133.0) × 10−3 |
| 7f | CH3 | (CH2)2COOH | 0.27 ± 0.18 | (1.57 ± 3.10) × 10−3 |
| Irinotecan | 7.38 ± 1.24 | 1.32 ± 0.13 | ||
2.3. AChE Inhibition Assay
| Compound | IC50 (μM) | Compound | IC50 (μM) |
|---|---|---|---|
| 5a | 19.79 | 7c | >100 |
| 5f | 1.36 | 7d | 4.44 |
| 5g | 3.08 | 7f | 36.08 |
| 5h | 7.43 | Irinotecan | 0.20 |
| 7a | 115.7 |
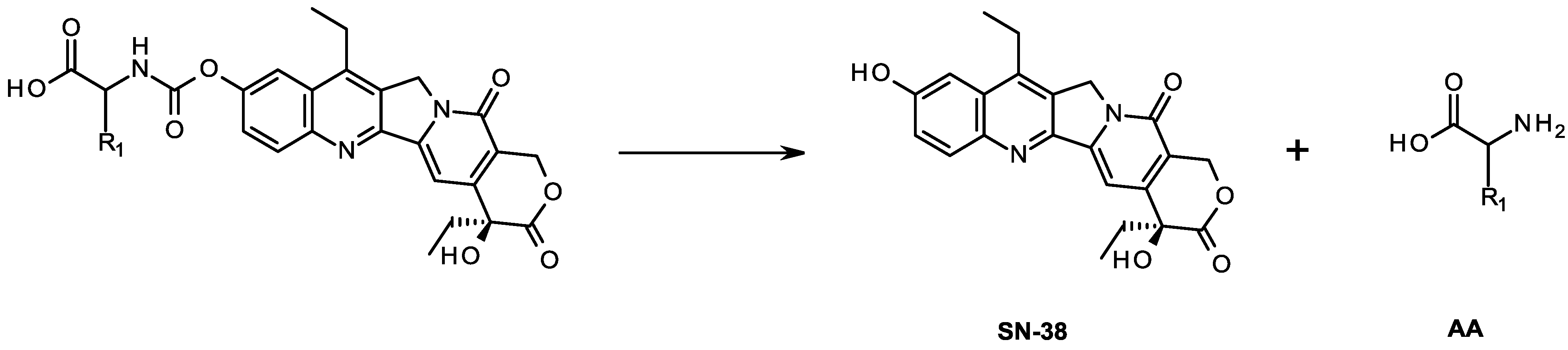
2.4. Stability and Conversion
| Compound | Remaining Prodrugs (%) | |||
|---|---|---|---|---|
| 1 h | 12 h | |||
| pH 4.6 | pH 7.4 | pH 4.6 | pH 7.4 | |
| 5a | 100 | 85.3 | 98.4 | 14.8 |
| 5d | 98.1 | 85.2 | 92.5 | 8.6 |
| 5g | 100 | 93.9 | 94.5 | 34.1 |
| 5h | 100 | 93.4 | 94.5 | 52.2 |
| 7a | 100 | 26.0 | 96.8 | 0 |
| 7b | 100 | 17.6 | 96.9 | 0 |
| 7c | 100 | 29.4 | 96.4 | 0 |
| 7d | 100 | 26.3 | 95.5 | 0 |
| 7e | 100 | 23.4 | 94.5 | 0 |
| 7f | 100 | 14.4 | 94.7 | 0 |
| Compound | Conversion (%) | ||
|---|---|---|---|
| 1 h | 3 h | 12 h | |
| 5a | 38.8 | 74.7 | 100.0 |
| 5b | 36.5 | 72.5 | 100.0 |
| 5c | 80.1 | 99.4 | - |
| 5d | 38.0 | 68.9 | 100.0 |
| 5e | 77.5 | 99.7 | - |
| 5f | 74.6 | 99.4 | - |
| 5g | 36.8 | 64.2 | 98.5 |
| 5h | 15.3 | 37.4 | 85.4 |
| 7a | 95.3 | - | - |
| 7b | 99.6 | - | - |
| 7c | 99.2 | - | - |
| 7d | 97.6 | - | - |
| 7e | 99.6 | - | - |
| 7f | 98.7 | - | - |
2.5. Tumor Growth Inhibitory Activity of 5e in a Human Colon Xenograft Model in Vivo
| Compound | Dose | Tumor Weight | Inhibitory Rate | Body Weight Change |
|---|---|---|---|---|
| (mg/kg) | (g/10g) | (%) | (g) | |
| Vehicle | 0.35 ± 0.12 | +0.95 | ||
| 5e | 60 | 0.17 ± 0.05 * | 51 | −2.72 |
| Irinotecan | 60 | 0.17 ± 0.04 * | 51 | −3.10 |
| SN-38 | 60 | 0.32 ± 0.12 | 8.6 | −1.45 |
2.6. Discussion
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.3. Cytotoxicity Study
3.4. Acetylcholinesterase Inhibition Assay
3.5. Stability Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohwada, J.; Ozawa, S.; Kohchi, M. Synthesis and biological activities of a pH-dependently activated water-soluble prodrug of a novel hexacyclic camptothecin analog. Bioorg. Med. Chem. Lett. 2009, 19, 2772–2776. [Google Scholar] [CrossRef]
- Basili, S.; Moro, S. Novel camptothecin derivatives as topoisomerase I inhibitors. Expert Opin. Ther. Patents 2009, 19, 555–574. [Google Scholar] [CrossRef]
- Hecht, J.R. Current and emerging therapies for metastatic colorectal cancer: Applying research findings to clinical practice. Am. J. Health Syst. Pharm. 2008, 65, S15–S21. [Google Scholar] [CrossRef]
- Langer, C.J. The global role of irinotecan in the treatment of lung cancer: 2003 Update. Oncology 2003, 17, 30–40. [Google Scholar]
- Vredenburgh, J.J.; Desjardins, A.; Reardon, D.A.; Friedman, H.S. Experience with irinotecan for the treatment of malignant glioma. Neuro. Oncol. 2009, 11, 80–91. [Google Scholar] [CrossRef]
- Anthoney, D.A.; Naik, J.; Twelves, C. Phase I study of TP300 in patients with advanced solid tumors with pharmacokinetic, pharmacogenetic and pharmacodynamic analyses. BMC Cancer 2012, 12, 536–545. [Google Scholar] [CrossRef]
- Senter, P.D.; Beam, K.S.; Mixan, B.; Wahl, A.F. Identification and activities of human carboxylesterases for the activation of CPT-11, a clinically approved anticancer drug. Bioconjug. Chem. 2001, 12, 1074–1080. [Google Scholar] [CrossRef]
- De Jong, F.A.; Mathijssen, R.H.J.; Xie, R.; Verweij, J.; Sparreboom, A. Flat-fixed dosing of irinotecan: Influence on pharmacokinetic and pharmacodynamic variability. Clin. Cancer Res. 2004, 10, 4068–4071. [Google Scholar] [CrossRef]
- Endo, M.; Ohwada, J.; Ogawa, K.; Yamada-Okabe, H. A water soluble prodrug of a novel camptothecin analog is efficacious against breast cancer resistance protein-expressing tumor xenografts. Cancer Chemother. Pharmacol. 2010, 65, 363–371. [Google Scholar] [CrossRef]
- Hecht, J.R. Gastrointestinal toxicity or irinotecan. Oncology 1998, 12, 72–78. [Google Scholar]
- Dodds, H.M.; Rivory, L.P. The mechanism for the inhibition of acetylcholinesterases by irinotecan (CPT-11). Mol. Pharmacol. 1999, 56, 1346–1353. [Google Scholar]
- Hyatt, J.L.; Tsurkan, L.; Morton, C.L.; Yoon, K.J.P.; Harel, M.; Brumshtein, B.; Silman, I.; Sussman, J.L.; Wadkins, R.M.; Potter, P.M. Inhibition of acetylcholinesterase by the anticancer prodrug CPT-11. Chem. Biol. Interact. 2005, 157–158, 247–252. [Google Scholar]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef]
- Harel, M.; Hyatt, J.L.; Brumshtein, B.; Morton, C.L.; Yoon, K.J.P.; Wadkins, R.M.; Silman, I.; Sussman, J.L.; Potter, P.M. The crystal structure of the complex of the anticancer prodrug 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecin (CPT-11) with Torpedo californica acetylcholinesterase provides a molecular explanation for its cholinergic action. Mol. Pharmacol. 2005, 67, 1874–1881. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Anders, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Liu, M.; He, X.; Yu, H.; Wu, D.; Yao, Y.; Fan, S.; Zhang, P.; Shi, W.; Zhong, B. Synthesis and Biological Evaluation of Novel 10-Substituted-7-ethyl-10-hydroxycamptothecin (SN-38) Prodrugs. Molecules 2014, 19, 19718-19731. https://doi.org/10.3390/molecules191219718
Zhou M, Liu M, He X, Yu H, Wu D, Yao Y, Fan S, Zhang P, Shi W, Zhong B. Synthesis and Biological Evaluation of Novel 10-Substituted-7-ethyl-10-hydroxycamptothecin (SN-38) Prodrugs. Molecules. 2014; 19(12):19718-19731. https://doi.org/10.3390/molecules191219718
Chicago/Turabian StyleZhou, Mo, Meixia Liu, Xinhua He, Hong Yu, Di Wu, Yishan Yao, Shiyong Fan, Ping Zhang, Weiguo Shi, and Bohua Zhong. 2014. "Synthesis and Biological Evaluation of Novel 10-Substituted-7-ethyl-10-hydroxycamptothecin (SN-38) Prodrugs" Molecules 19, no. 12: 19718-19731. https://doi.org/10.3390/molecules191219718
APA StyleZhou, M., Liu, M., He, X., Yu, H., Wu, D., Yao, Y., Fan, S., Zhang, P., Shi, W., & Zhong, B. (2014). Synthesis and Biological Evaluation of Novel 10-Substituted-7-ethyl-10-hydroxycamptothecin (SN-38) Prodrugs. Molecules, 19(12), 19718-19731. https://doi.org/10.3390/molecules191219718