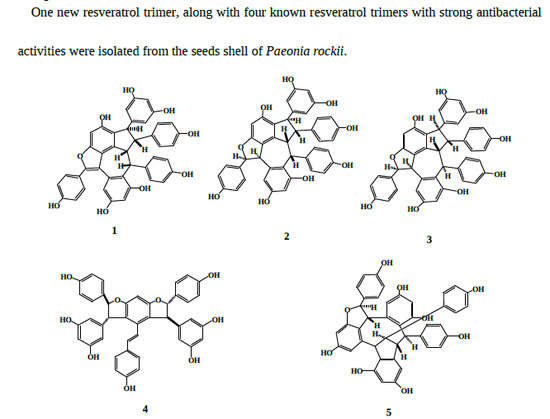
Resveratrol Trimers from Seed Cake of Paeonia rockii
Abstract
:1. Introduction

2. Results and Discussion
2.1. Purification and Characterization
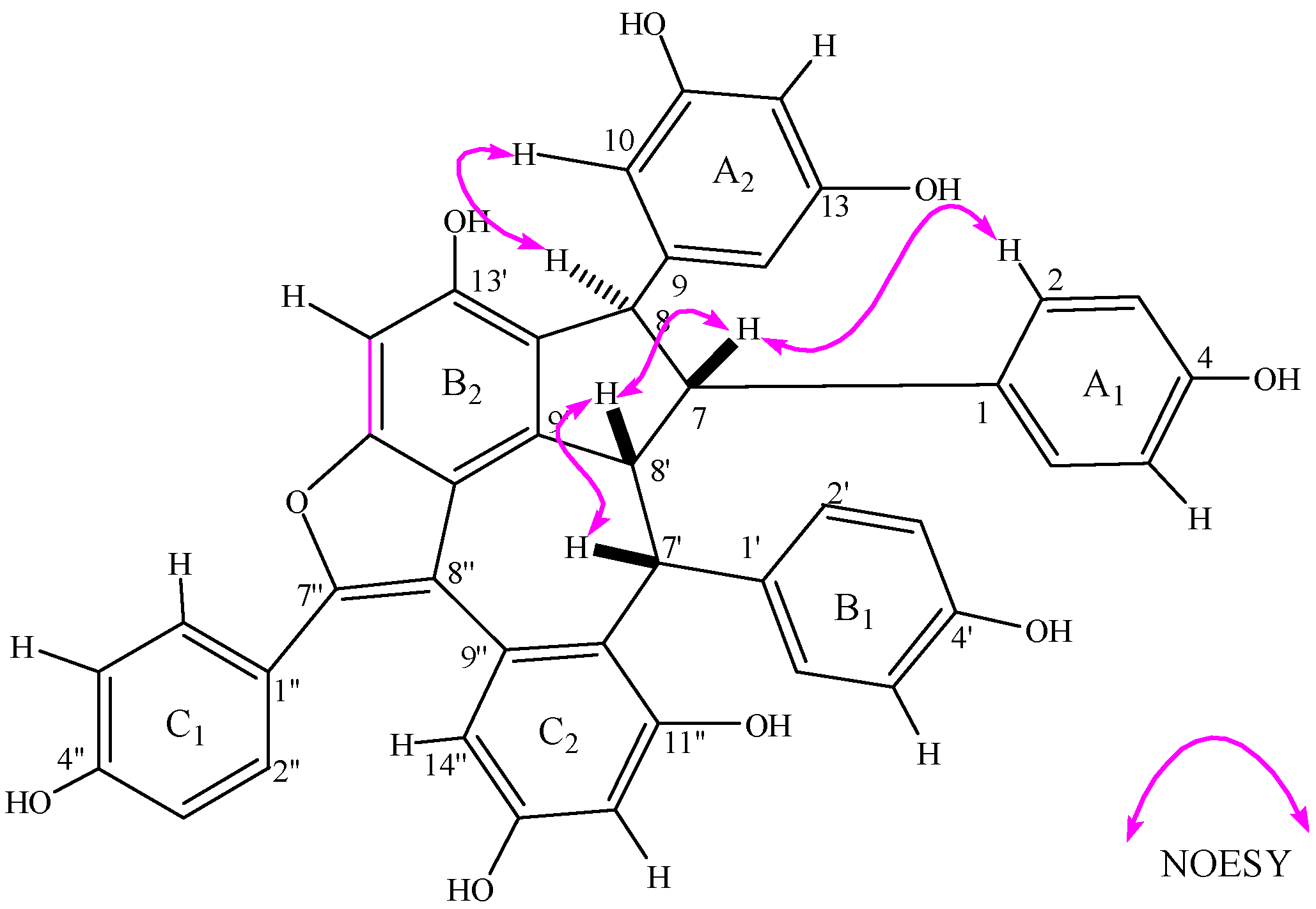
2.2. Structural Elucidation of Compound 1
| No. | 1H δ J (Hz) | 13C | HMBC | No. | 1H δ J (Hz) | 13C | HMBC |
|---|---|---|---|---|---|---|---|
| 1 | 135.1 | 8' | 4.35 dd, 3.3, 9.1 | 51.8 | 9',10',1' | ||
| 2 | 6.68 d, 8.5 | 130.8 | 7,1,4 | 9' | 141.5 | ||
| 3 | 6.26 d, 8.5 | 115.0 | 1,4 | 10' | 120.6 | ||
| 4 | 156.2 | 11' | 154.6 | ||||
| 5 | 6.26 d, 8.5 | 115.0 | 12' | 6.82 s | 96.4 | 10',11',13',14' | |
| 6 | 6.68 d, 8.5 | 130.8 | 13' | 153.0 | |||
| 7 | 3.78 dd,1.5,9.1 | 61.7 | 2,8,9,7' | 14' | 127.9 | ||
| 8 | 4.62 d, 1.5 | 58.1 | 7,9,10,8',14' | 1'' | 124.7 | ||
| 9 | 150.0 | 2'' | 7.41 d, 8.8 | 130.9 | 4'',6'',7'' | ||
| 10 | 6.08 d, 2.1 | 106.6 | 8,12,14 | 3'' | 6.75 d, 8.8 | 116.3 | 1'',4'',5'' |
| 11 | 159.3 | 4'' | 159.1 | ||||
| 12 | 6.04 t, 2.1 | 101.3 | 10,11 | 5'' | 6.75 d, 8.8 | 116.3 | 4''.2'',7'' |
| 13 | 159.3 | 6'' | 7.41 d, 8.8 | 130.9 | 1'',4'',3'' | ||
| 14 | 6.08 d, 2.1 | 106.6 | 7'' | 152.4 | 2'',9'' | ||
| 1' | 136.4 | 8'' | 116.4 | 1'',10',7'' | |||
| 2' | 6.17 d, 8.7 | 131.2 | 7',6',4' | 9'' | 141.2 | ||
| 3' | 6.02 d, 8.7 | 114.6 | 1',4' | 10'' | 126.3 | ||
| 4' | 154.6 | 11'' | 155.7 | ||||
| 5' | 6.02 d, 8.7 | 114.6 | 1',4' | 12'' | 6.20 d, 2.5 | 102.5 | 10'',11'',14'' |
| 6' | 6.17 d, 8.7 | 131.2 | 7',2',4' | 13'' | 155.7 | ||
| 7' | 5.25 d, 3.3 | 42.1 | 1',2',8',9',9'',10'' | 14'' | 6.54 d, 2.5 | 110.0 | 8'',10'',12'',13'' |
2.3. Antibacterial Activities
| Compound | Staphylococcus aureus | Pyogenic streptococcus | Streptococcus viridans | Staphylococcus epidermidis | Escherichia coli | Pseudomonas aeruginosa |
|---|---|---|---|---|---|---|
| Penicillin G | 10 | 10 | 10 | 10 | 20 | 10 |
| 1 | 20 | 20 | 25 | 20 | 200 | 200 |
| 2 | 20 | 20 | 25 | 20 | 200 | 100 |
| 3 | 20 | 20 | 25 | 25 | 100 | 200 |
| 4 | 25 | 25 | 25 | 20 | 200 | 200 |
| 5 | 25 | 30 | 20 | 25 | 100 | 100 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Bioassay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qi, J.C.; Zhou, H.M.; Ma, J.Q.; Li, P. Analysis of the chemical constituents in Peony seed oil by GC-MS. Cereals Oils 2005, 19, 22–23. [Google Scholar]
- Sarker, S.D.; Whiting, P.; Dinan, L.; Sil, V.; Rees, H.H. Identification and ecdysteroid antagonist activity of three resveratrol trimers (Suffruticosols A, B and C) from Paeonia suffruticosa. Tetrahedron 1999, 55, 513–524. [Google Scholar] [CrossRef]
- He, C.N.; Xiao, W.; Li, M.; Peng, Y.; Xu, J.L.; Gu, J.; Xiao, P.G. Chemical Constituents from Seeds of Paeonia suffruticosa. China J. Chin. Mater. Med. 2010, 35, 1428–1431. [Google Scholar]
- He, C.N.; Zhang, Y.C.; Peng, Y.; Yang, J.S.; Xiao, P.G. Monoterpene glycosides from the seeds of Paeonia suffruticosa protect HEK 293 cells from irradiation-induced DNA damage. Phytochem. Lett. 2012, 5, 128–133. [Google Scholar] [CrossRef]
- Yi, J.P.; Zhu, W.X.; Ma, H.L.; Wang, Y.F. Studies on Chemical Constituents from Seeds of Paeonia suffruticosa Andr. Nat. Prod. Res. Dev. 2009, 20, 604–607. [Google Scholar]
- Wu, S.H.; Wu, D.G.; Chen, Y.W. Chemical Constituents and Bioactivities of Plants from the Genus Paeonia. Chem. Biodivers. 2010, 7, 90–104. [Google Scholar] [CrossRef] [PubMed]
- He, C.N.; Peng, Y.; Zhang, Y.C.; Xu, L.J.; Gu, J.; Xiao, P.G. Phytochemical and Biological Studies of Paeoniaceae. Chem. Biodivers. 2010, 7, 805–838. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, H.; Ryuichiro, K.; Kokki, S. Compounds inhibitory to rat liver 5α-reductase from tropical commercial wood species: Resveratrol trimers from melapi (Shore sp.) heartwood. J. Wood Sci. 2001, 47, 308–312. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, E.J.; Bae, S.J.; Shim, S.M.; Park, H.D.; Rhee, C.H.; Park, J.H.; Choi, S.W. Cytotoxic and AnUmutagenic Stilbenes from Seeds of Paeonia lactiflora. Arch. Pharm. Res. 2002, 25, 293–299. [Google Scholar] [CrossRef]
- Pavel, N.; Kloucek, P.; Rondevaldova, J.; Havlik, J.; Kourimska, L.; Kokoska, L. Thymoquinone vapor significantly affects the results of Staphylococcus aureus sensitivity tests using the standard broth microdilution method. Fitoterapia 2014, 94, 102–107. [Google Scholar] [CrossRef]
- Kajal, C.; Lipton, A.P.; Raj, R.P.; Vijayan, K.K. Antibacterial labdane diterpenoids of Ulva fasciata Delile from southwestern coast of the Indian Peninsula. Food Chem. 2010, 119, 1399–1408. [Google Scholar]
- Sample Availability: Samples of the compounds 2–5 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Wang, Y.; Gao, J.; Lu, Z.; Yin, W.; Deng, R. Resveratrol Trimers from Seed Cake of Paeonia rockii. Molecules 2014, 19, 19549-19556. https://doi.org/10.3390/molecules191219549
Liu P, Wang Y, Gao J, Lu Z, Yin W, Deng R. Resveratrol Trimers from Seed Cake of Paeonia rockii. Molecules. 2014; 19(12):19549-19556. https://doi.org/10.3390/molecules191219549
Chicago/Turabian StyleLiu, Pu, Yiran Wang, Jiayu Gao, Zongyuan Lu, Weiping Yin, and Ruixue Deng. 2014. "Resveratrol Trimers from Seed Cake of Paeonia rockii" Molecules 19, no. 12: 19549-19556. https://doi.org/10.3390/molecules191219549