Vernonia kotschyana Roots: Therapeutic Potential via Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Free Radical Scavenging Activity
| Extract/Positive Control | ABTS Radical Cation Scavenging Activity * | Superoxide Anion Radical Scavenging Activity * | Hydroxyl Radical Scavenging Activity ** | Nitric Oxide Scavenging Activity * | Lipid Peroxidation Inhibitory Activity *,** | Ferrous Ion Chelating Activity *,** | Antioxidant Activity in MCF-12F Cells * |
|---|---|---|---|---|---|---|---|
| V-C | n.d. | n.d. | n.d. | n.d. | 1.06 ± 0.02 **,c,g | 3.33 ± 0.03 **,i | n.t. |
| V-EA | 20.59 ± 0.07 a,b | 370.42 ± 0.67 b | 0.88 ± 0.00 a,d | 55.50 ± 0.27 a,e | 0.24 ± 0.00 **,g,h | n.d. | 144.46 ± 2.57 j |
| V-E | 51.96 ± 0.31 b,c | n.d. | 3.61 ± 0.01 e,c | 127.63 ± 0.84e,c | n.d. | n.d. | n.d. |
| V-A | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.t. |
| Glutathione | 3.25 ± 0.02 c,a | 31.21 ± 0.07 c | n.t. | n.d. | n.t. | n.t. | n.t. |
| L-Ascorbic acid | n.t. | n.t. | 0.11 ± 0.00 f,a | 46.00 ± 0.24 c,a | n.t. | n.t. | n.t. |
| DL-α-Tocopherol acetate | n.t. | n.t. | n.t. | n.t. | 17.4 ± 0.0 *,h,c | n.t. | n.t. |
| EDTA | n.t. | n.t. | n.t. | n.t. | n.t. | 6.18 ± 0.08 *,h | n.t. |
| Sodium pyruvate | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | 78.43 ± 3.03 c |
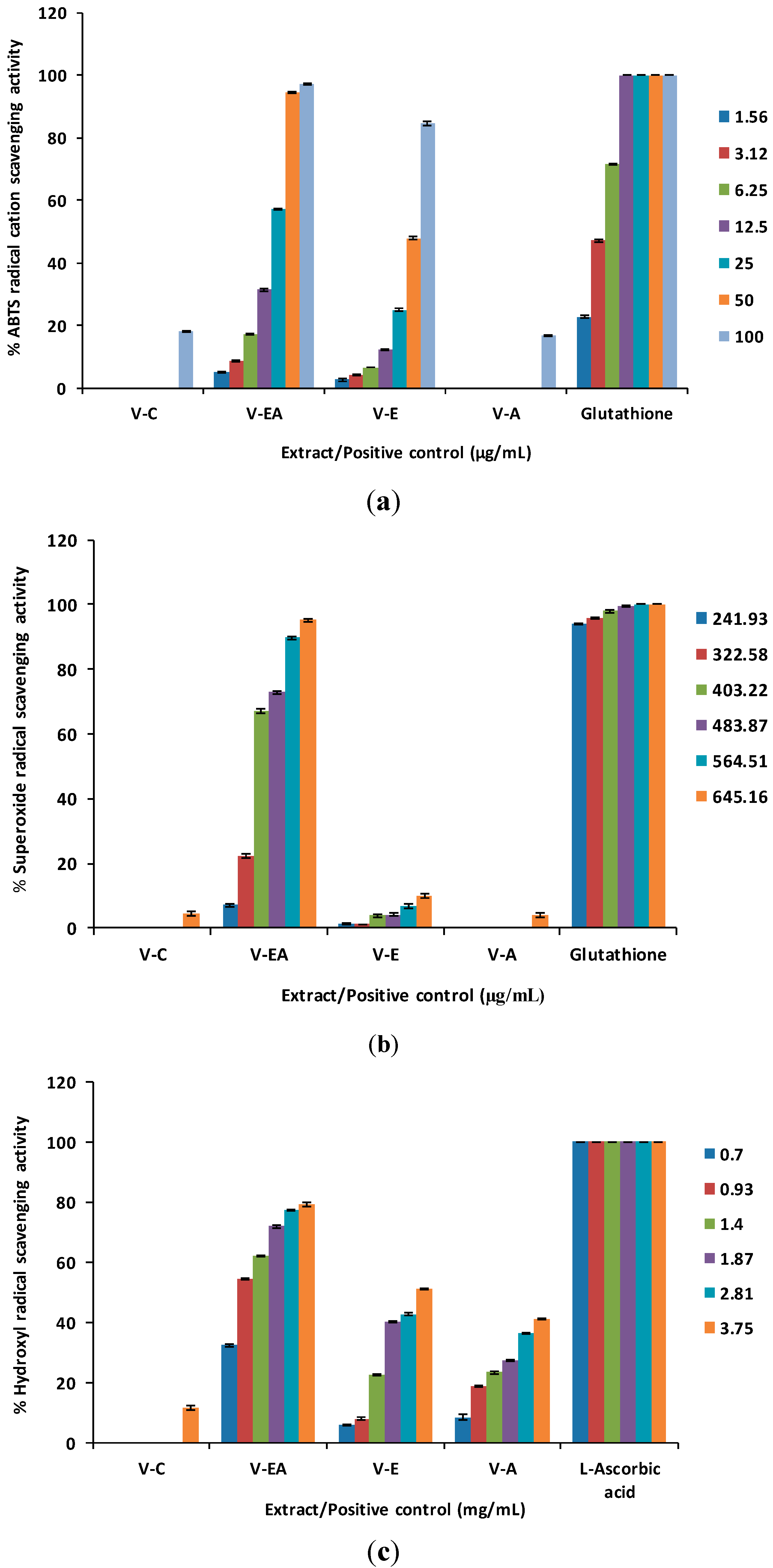
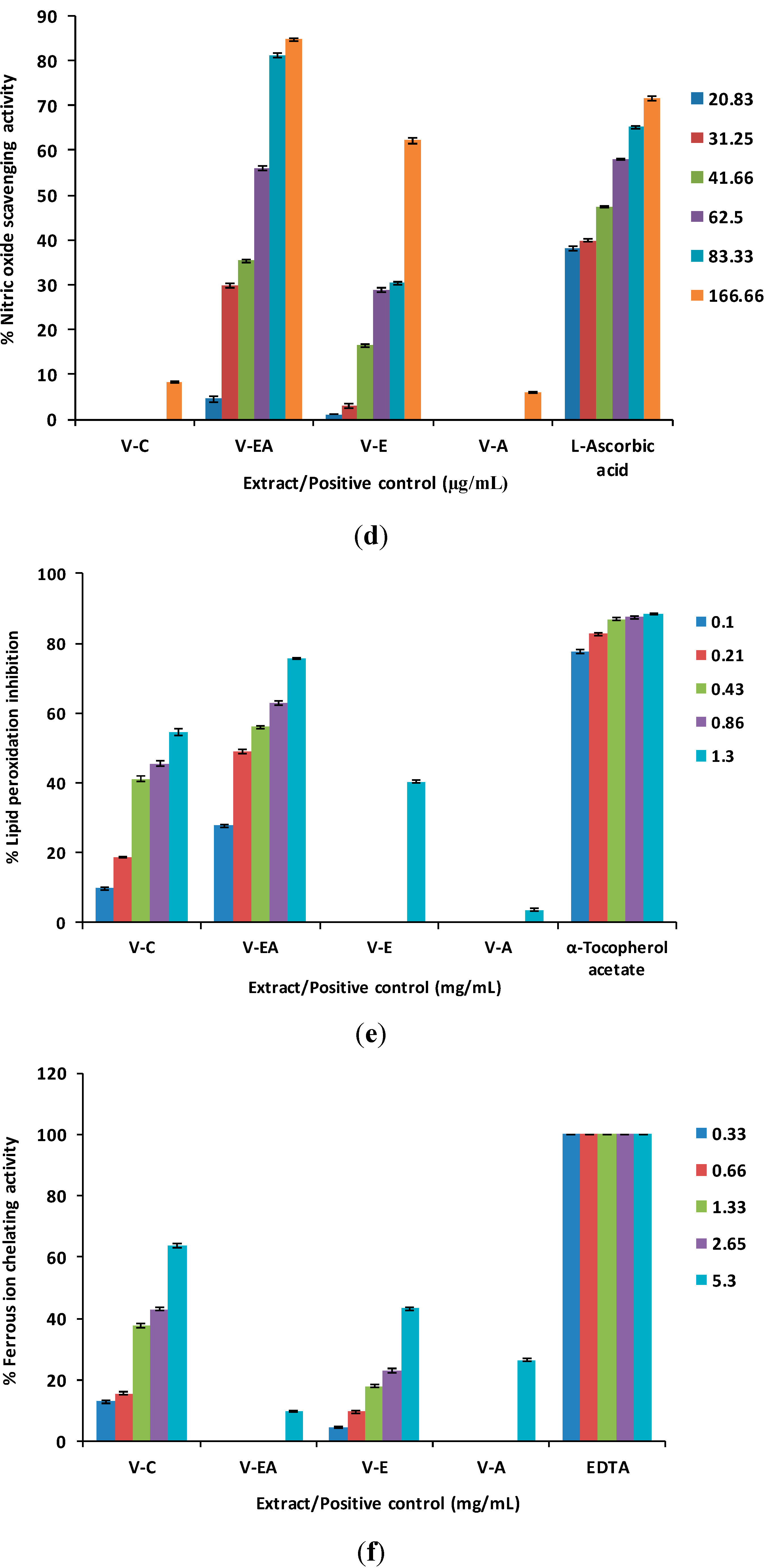
2.2. Lipid Peroxidation Inhibitory Activity
2.3. Ferrous Ion Chelating Activity
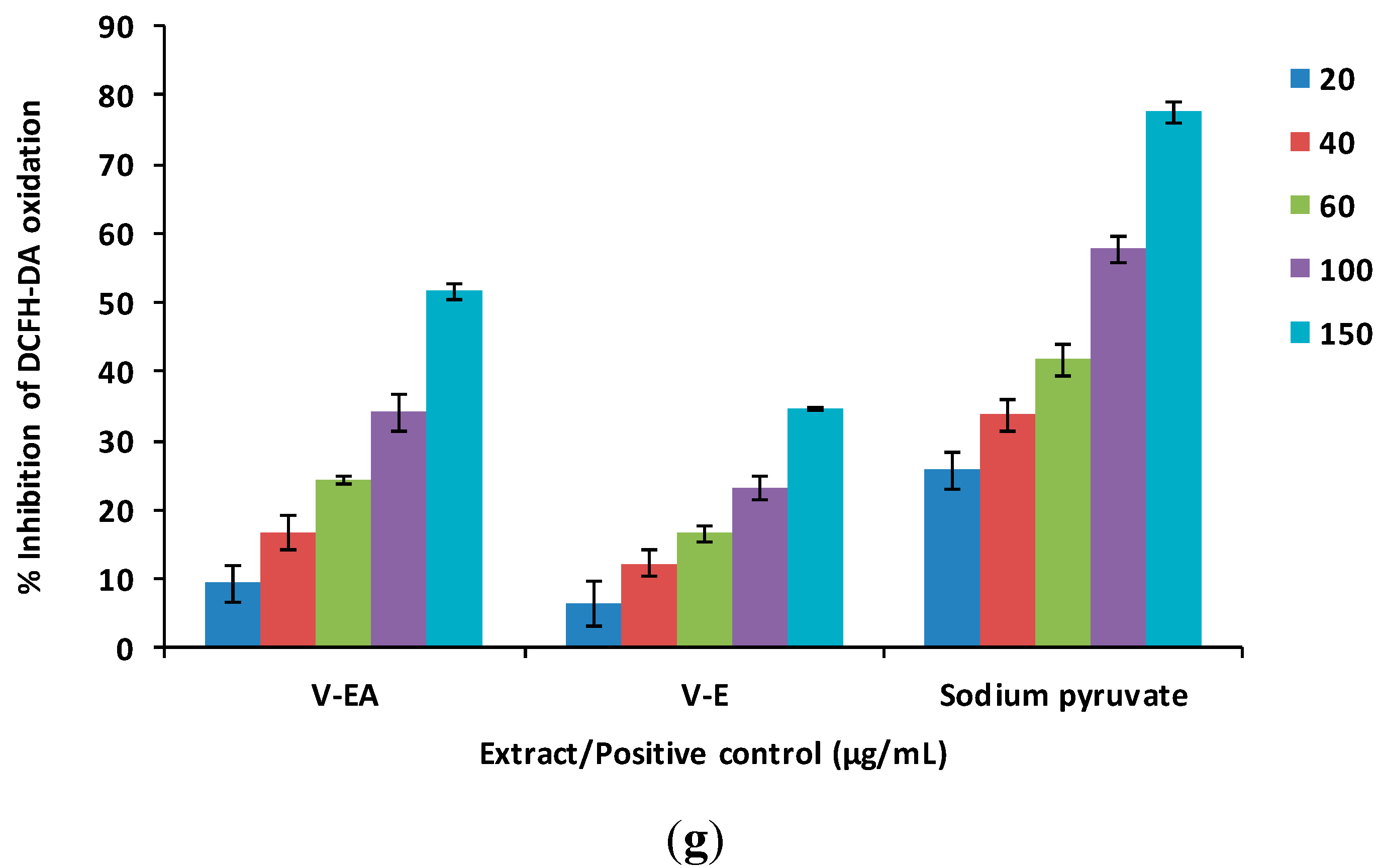
2.4. Antioxidant Activity in MCF-12F Cells
2.5. Phytochemical Screening
2.6. FT-IR Spectroscopic Analysis
| Extract | Total Phenolic Content (g GAE/100 g) |
|---|---|
| V-C | 1.08 ± 0.03 a,b,c |
| V-EA | 14.89 ± 0.02 b,c,d |
| V-E | 5.99 ± 0.07 c,d,a |
| V-A | 0.78 ± 0.01 d,a,b |
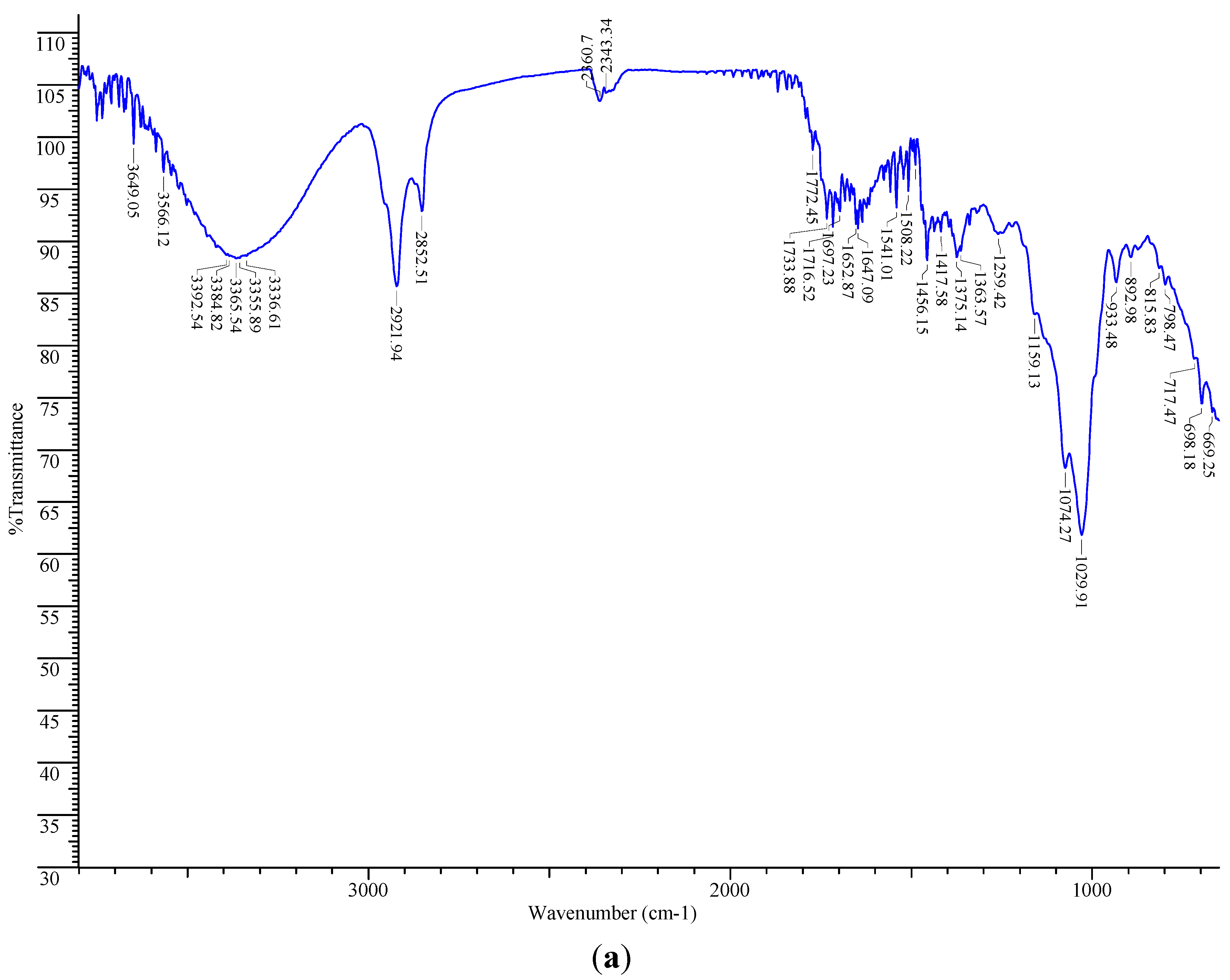
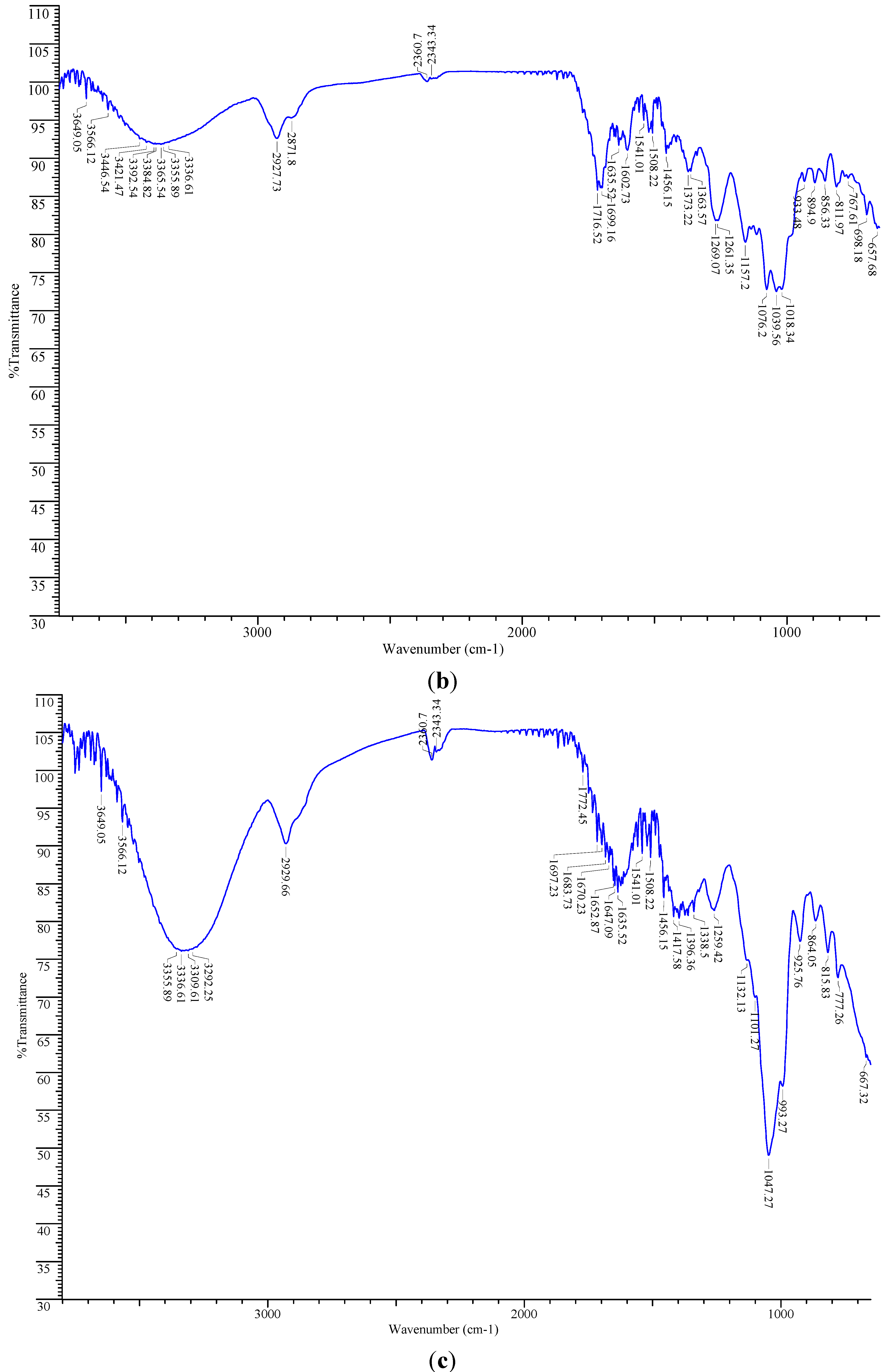
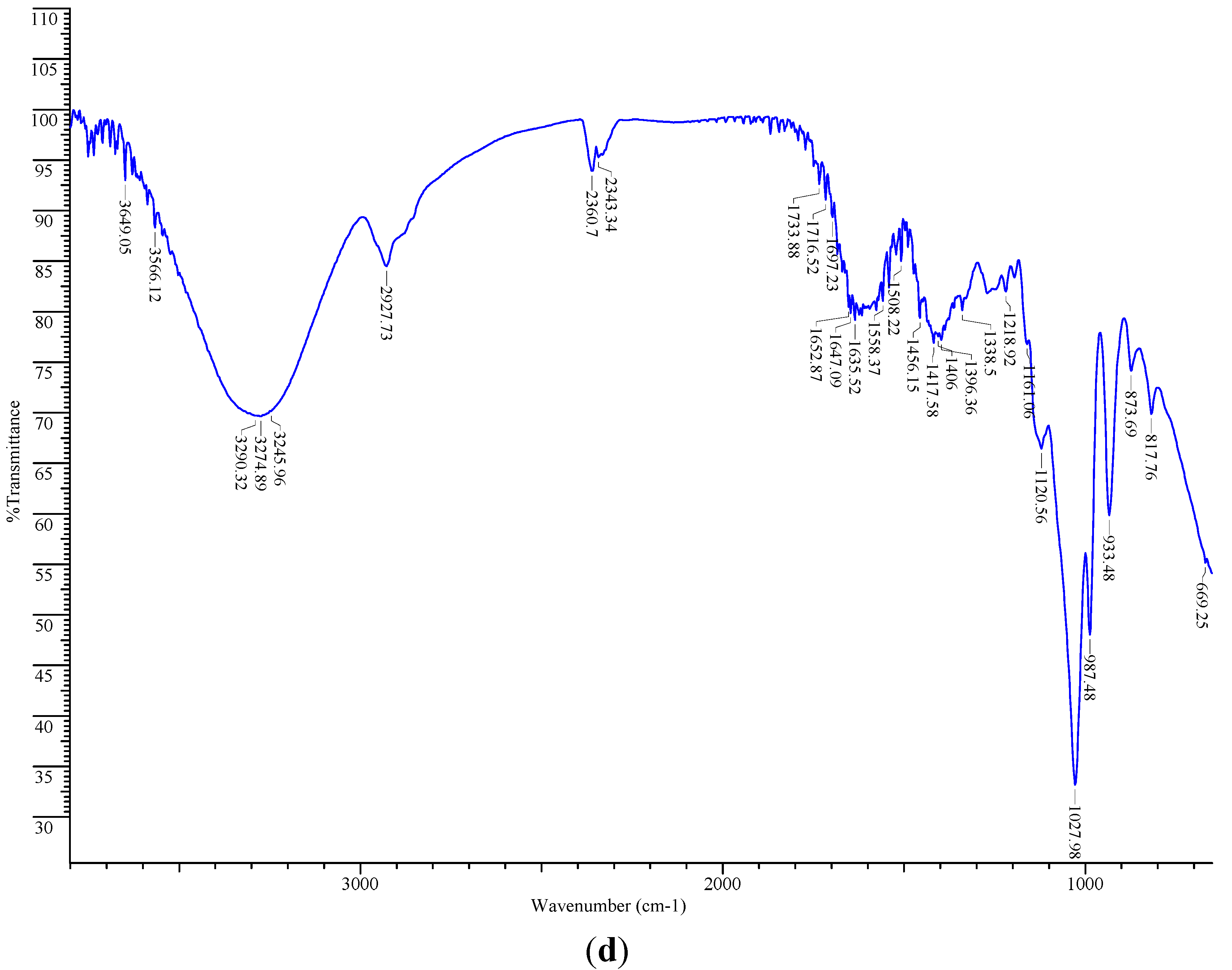
2.7. Total Phenolic Content
3. Experimental Section
3.1. Chemicals
3.2. Cell Culture
3.3. Plant Material
3.4. Preparation of Extracts
3.5. ABTS Radical Cation Scavenging Assay
3.6. Superoxide Anion Radical Scavenging Assay
3.7. Hydroxyl Radical Scavenging Assay
3.8. Nitric Oxide Scavenging Assay
3.9. Lipid Peroxidation Inhibition Assay
3.10. Ferrous Ion Chelating Assay
3.11. Cell-Based Antioxidant Assay
3.12. Phytochemical Screening
3.13. FT-IR Spectroscopic Analysis
3.14. Quantification of Total Phenolic Content
3.15. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Borrelli, F.; Izzo, A.A. The plant kingdom as a source of anti-ulcer remedies. Phytother. Res. 2000, 14, 581–591. [Google Scholar] [CrossRef]
- Al Mofleh, I.A. Spices, herbal xenobiotics and the stomach: Friends or foes? World J. Gastroenterol. 2010, 16, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, A.A.; Miura, S.; Takeuchi, T.; Hokari, R.; Mizumori, M.; Yoshida, H.; Higuchi, H.; Mori, M.; Kimura, H.; Suzuki, H.; et al. Increased expression of inducible nitric oxide synthase and peroxynitrite in Helicobacter pylori gastric ulcer. Free Radic. Biol. Med. 1999, 27, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Parra-Cid, T.; Calvino-Fernández, M.; Gisbert, J.P. Helicobacter pylori and peptic ulcer—role of reactive oxygen species and apoptosis. In Peptic Ulcer Disease; Chai, J., Ed.; InTech: Rijeka, Croatia, 2011; pp. 165–186. [Google Scholar]
- Suzuki, H.; Nishizawa, T.; Ysugawa, H.; Mogami, S.; Hibi, T. Role of oxidative stress in stomach disorders. J. Clin. Biochem. Nutr. 2012, 50, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Choochuay, S.; Thong-Ngam, D.; Patumraj, S.; Klaikaew, N. Effects of curcumin on gastric microcirculation with nonsteroidal anti-inflammatory drugs induced gastric injury in rats. Thai J. Gastroenterol. 2010, 11, 22–27. [Google Scholar]
- Liu, X.; Chen, Z.; Mao, N.; Xie, Y. The protective of hydrogen on stress-induced gastric ulceration. Int. Immunopharmacol. 2012, 13, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Yilmaz, M.; Köseoğlu, M.; Akalin, N.; Aslan, D.; Aydin, A. Role of free radicals in peptic ulcer and gastritis. Turk. J. Gastroenterol. 2003, 14, 39–43. [Google Scholar] [PubMed]
- Dolatkhah, H.; Rahbani-Nobar, M.; Fattahi, E.; Ansari, M.; Mirza-Aghazadeh, A.; Eftekhari-Vash, L.; Fakhrjo, A.; Bahrami, A. Evaluation of glycemic control, gastric juice nitric oxide and oxidative stress in diabetic patients infected by Helicobacter pylori. J. Med. Genet. Genomics 2011, 3, 1–6. [Google Scholar]
- Nanjundaiah, S.M.; Annaiah, H.N.M.; Dharmesh, S.M. Gastroprotective effect of ginger rhizome (Zingiber officinale) extract: role of gallic acid and cinnamic acid in H+, K+-ATPase/H. pylori inhibition and anti-oxidative mechanism. Evid. Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef]
- Al Batran, R.; Al-Bayaty, F.; Jamil Al-Obaidi, M.M.; Abdualkader, A.M.; Hadi, H.A.; Ali, H.M.; Abdulla, M.A. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS One 2013, 8, e64751. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.B.; Silva, F.V.; Passos, F.F.B.; Bezerra, R.D.S.; Chaves, M.H.; Oliveira, F.A.; Oliveira, R.C.M. Gastroprotective effect of the ethanolic extract of Parkia platycephala Benth. leaves against acute gastric lesion models in rodents. Biol. Res. 2010, 43, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Alimi, H.; Hfaiedh, N.; Bouoni, Z.; Sakly, M.; Ben Rhouma, K. Evaluation of antioxidant and antiulcerogenic activities of Opuntia ficus indica f. inermis flowers extract in rats. Environ. Toxicol. Pharmacol. 2011, 32, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Dekanski, D.; Ristić, S.; Radonjić, N.V.; Petronijević, N.D.; Giampieri, F.; Astolfi, P.; González-Paramás, A.M.; Santos-Buelga, C.; Tulipani, S.; et al. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS One 2011, 6, e25878. [Google Scholar] [CrossRef] [PubMed]
- Sumanth, M.; Edison, D. Comparison of antiulcer activity of Curcuma longa with Mentha piperita. JIVA 2012, 10, 30–36. [Google Scholar]
- Verma, V.K.; Singh, N.; Saxena, P.; Singh, R. Anti-ulcer and antioxidant activity of Moringa oleifera (Lam) leaves against aspirin and ethanol induced gastric ulcer in rats. Int. Res. J. Pharm. 2012, 2, 46–57. [Google Scholar]
- Toyang, N.J.; Verpoorte, R. A review of the medicinal potential of plants of the genus Vernonia (Asteraceae). J. Ethnopharmacol. 2013, 146, 681–723. [Google Scholar] [CrossRef] [PubMed]
- Nergard, C.S.; Diallo, D.; Michaelsen, T.E.; Malterud, K.E.; Kiyohara, H.; Matsumoto, T.; Yamada, H.; Paulsen, B.S. Isolation, partial characterisation and immunomodulating activities of polysaccharides from Vernonia kotschyana Sch. Bip. ex Walp. J. Ethnopharmacol. 2004, 91, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Austarheim, I.; Nergard, C.S.; Sanogo, R.; Diallo, D.; Paulsen, B.S. Inulin-rich fractions from Vernonia kotschyana roots have anti-ulcer activity. J. Ethnopharmacol. 2012, 144, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Germano, M.P.; de Pasquale, R.; Iauk, L.; Galati, E.M.; Keita, A.; Sanogo, R. Antiulcer activity of Vernonia kotschyana Sch. Bip. Phytomedicine 1996, 2, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Inngjerdingen, K.T.; Thöle, C.; Diallo, D.; Paulsen, B.S.; Hensel, A. Inhibition of Helicobacter pylori adhesion to human gastric adenocarcinoma epithelial cells by aqueous extracts and pectic polysaccharides from the roots of Cochlospermum tinctorium A. Rich. and Vernonia kotschyana Sch. Bip. ex Walp. Fitoterapia 2014, 95, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Inngjerdingen, K.T.; Meskini, A.; Austarheim, I.; Ballo, M.; Inngjerdingen, M.; Michaelsen, T.E.; Diallo, D.; Paulsen, B.S. Chemical and biological characterization of polysaccharides from wild and cultivated roots of Vernonia kotschyana. J. Ethnopharmacol. 2012, 139, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Daffodil, E.D.; Lincy, P.; Mohan, V.R. Study of whole plant of Vernonia cinerea Less. for in vitro antioxidant activity. Int. J. Pharm. 2014, 4, 172–178. [Google Scholar]
- Leelaprakash, G.; Mohan Dass, S.; Sivajothi, V. Antioxidant and hepatoprotective activities of Vernonia cinerea extract against CCl4 induced hepatotoxicity in albino rats. Int. J. Pharm. Sci. Rev. Res. 2011, 10, 30–34. [Google Scholar]
- Cretu, E.; Salminen, J.-P.; Karonen, M.; Miron, A.; Charalambous, C.; Constantinou, A.I.; Aprotosoaie, A.C. In vitro antioxidant activity and phenolic content of Cedrus brevifolia bark. Nat. Prod. Commun. 2014, 9, 481–482. [Google Scholar] [PubMed]
- Li, X.; Wu, X.; Huang, L. Correlation between antioxidant activities and phenolic contents of radix Angelicae sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, D. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2007, 68, 54–58. [Google Scholar] [CrossRef]
- Jeong, J.B.; Hong, S.C.; Jeong, H.J. 3,4-Dihydroxybenzaldehyde purified from the barley seeds (Hordeum vulgare) inhibits oxidative DNA damage and apoptosis via its antioxidant activity. Phytomedicine 2009, 16, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-J.; Tsai, T.-H.; Yu, C.-H.; Ho, S.-C. Evaluation of NO-supressing activity of several Mediterranean culinary spices. Food Chem. Toxicol. 2007, 45, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Cretu, E.; Miron, S.D.; Miron, A. Bioactivity screening of Pinus brutia bark extracts: Superoxide dismutase-like activity and nitric oxide scavenging effects. Rev. Med. Chir. Soc. Med. Nat. Iasi 2013, 117, 551–557. [Google Scholar] [PubMed]
- Cretu, E.; Karonen, M.; Salminen, J.-P.; Mircea, C.; Trifan, A.; Charalambous, C.; Constantinou, A.I.; Miron, A. In vitro study on the antioxidant activity of a polyphenol-rich extract from Pinus brutia bark and its fractions. J. Med. Food 2013, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cretu, E.; Trifan, A.; Aprotosoaie, A.C.; Miron, A. 15-Lipoxygenase inhibition, superoxide and hydroxyl radicals scavenging activities of Cedrus brevifolia bark extracts. Rev. Med. Chir. Soc. Med. Nat. Iasi 2013, 117, 250–256. [Google Scholar] [PubMed]
- Guo, T.; Wei, L.; Sun, J.; Hou, C.-L.; Fan, L. Antioxidant activities of extract and fractions from Tuber indicum Cooke & Massee. Food Chem. 2011, 127, 1634–1640. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, M.; Wang, J.S.; Cui, C.; Yang, B.; Jiang, Y.; Zhao, Q. Antioxidant, immunomodulatory and anti-breast cancer activities of phenolic extract from pine (Pinus massoniana Lamb) bark. Innov. Food Sci. Emerg. Technol. 2008, 9, 122–128. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Matkowski, A.; Piotrowska, M. Antioxidant and free radical scavenging activities of some medicinal plants from the Lamiaceae. Fitoterapia 2006, 77, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Wu, J.H.; Huang, C.Y.; Kuo, Y.H.; Chang, S.T. Antioxidant activities and phytochemical characteristics of extracts from Acacia confusa bark. Bioresour. Technol. 2009, 100, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, C.L.; Tuchilus, C.; Aprotosoaie, A.C.; Oprea, A.; Malterud, K.E.; Miron, A. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. bark and needles. Molecules 2011, 16, 7773–7788. [Google Scholar] [CrossRef] [PubMed]
- Girard-Lalancette, K.; Pichette, A.; Legault, J. Sensitive cell-based assay using DCFH oxidation for the determination of pro- and antioxidant properties of compounds and mixtures: Analysis of fruit and vegetable juices. Food Chem. 2009, 115, 720–726. [Google Scholar] [CrossRef]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 13th ed.; Bailliere Tindall: London, UK, 1989; pp. 176–180. [Google Scholar]
- Yadav, R.N.S.; Agarwala, M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011, 3, 10–14. [Google Scholar]
- Wu, Y.-W.; Sun, S.-Q.; Zhao, J.; Li, Y.; Zhou, Q. Rapid discrimination of extracts of Chinese propolis and poplar buds by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2008, 883–884, 48–54. [Google Scholar]
- Velásquez Ballesteros, O.J.; Murillo Perea, E.; Jairo Méndez, J.; Murillo Arango, W.; Moreňa, D.A. Quantification, chemical and biological characterization of the saponosides material from Sida cordifolia L. (escobilla). Rev. Cubana Plant. Med. 2013, 18, 298–314. [Google Scholar]
- Adib, A.M.; Jamaludin, F.; Kiong, L.S.; Hashim, N.; Abdullah, Z. Two-dimensional correlation infrared spectroscopy applied to analyzing and identifying the extracts of Baeckea frutescens medicinal materials. J. Pharm. Biomed. Anal. 2014, 6, 104–110. [Google Scholar] [CrossRef]
- Grube, M.; Bekers, M.; Upite, D.; Kaminska, E. Infrared spectra of some fructans. Spectroscopy 2002, 16, 289–296. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochem. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Gokulakumar, B.; Narayanaswamy, R. Fourier transform-infrared spectra (FT-IR) analysis of root rot disease in sesame (Sesamum indicum). Romanian J. Biophys. 2008, 18, 217–223. [Google Scholar]
- Sanogo, R.; Germano, M.P.; de Tommasi, N.; Pizza, C.; Aquino, R. Vernoniosides and an androstane glycoside from Vernonia kotschyana. Phytochemistry 1998, 47, 73–78. [Google Scholar] [CrossRef]
- Daffodil, E.D.; Lincy, P.; Mohan, V.R. Pharmacochemical characterization, FT-IR and antibacterial activity of Vernonia cinerea Less. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 239–249. [Google Scholar]
- Da Silva, J.B.; Temponi, V.S.; Gasparetto, C.M.; Fabri, R.L.; Aragão, D.M.O.; Pinto, N.C.; Ribeiro, A.; Scio, E.; Del-Vechio-Vieira, G.; de Sousa, O.V.; et al. Vernonia condensata Baker (Asteraceae): A promising source of antioxidants. Oxidative Med. Cell. Longev. 2013. [Google Scholar] [CrossRef]
- Nwaoguikpe, R.N. The effect of extract of bitter leaf (Vernonia amygdalina) on blood glucose levels of diabetic rats. Int. J. Biol. Chem. Sci. 2010, 4, 721–729. [Google Scholar]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative-stress induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Ganeshpurkar, A.; Shrivastava, A.; Bansal, D.; Dubey, N. Rutin exerts antiulcer effect by inhibiting the gastric proton pump. Indian J. Pharmacol. 2013, 45, 415–417. [Google Scholar] [CrossRef] [PubMed]
- De Lira Mota, K.S.; Dias, G.E.N.; Pinto, M.E.F.; Luiz-Ferreira, Â.; Monteiro Souza-Brito, A.R.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with gastroprotective activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef] [PubMed]
- Mimić-Oka, J.; Simić, D.V.; Simić, T.P. Free radicals in cardiovascular diseases. FACTA UNIVERSITATIS Ser.: Med. Biol. 1999, 6, 11–22. [Google Scholar]
- Aditi, Mahajan N.; Rawal, S.; Katare, R. An insight in to the pathogenesis of diabetic vascular diseases: Role of oxidative stress and antioxidants. Pharm. Anal. Acta 2013, 4, 273. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Perluigi, M.; Butterfield, D.A. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 2013, 62, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Baillet, A.; Chanteperdrix, V.; Trocmé, C.; Casez, P.; Garrel, C.; Besson, G. The role of oxidative stress in amyotrophic lateral sclerosis and Parkinson's disease. Neurochem. Res. 2010, 35, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, H.; Samuelsen, A.B.; Malterud, K.E. Antioxidant activity in extracts from coriander. Food Chem. 2004, 88, 293–297. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the extracts from Vernonia kotschyana roots are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasincu, A.; Paulsen, B.S.; Diallo, D.; Vasincu, I.; Aprotosoaie, A.C.; Bild, V.; Charalambous, C.; Constantinou, A.I.; Miron, A.; Gavrilescu, C.M. Vernonia kotschyana Roots: Therapeutic Potential via Antioxidant Activity. Molecules 2014, 19, 19114-19136. https://doi.org/10.3390/molecules191119114
Vasincu A, Paulsen BS, Diallo D, Vasincu I, Aprotosoaie AC, Bild V, Charalambous C, Constantinou AI, Miron A, Gavrilescu CM. Vernonia kotschyana Roots: Therapeutic Potential via Antioxidant Activity. Molecules. 2014; 19(11):19114-19136. https://doi.org/10.3390/molecules191119114
Chicago/Turabian StyleVasincu, Alexandru, Berit S. Paulsen, Drissa Diallo, Ioana Vasincu, Ana C. Aprotosoaie, Veronica Bild, Christiana Charalambous, Andreas I. Constantinou, Anca Miron, and Cristina M. Gavrilescu. 2014. "Vernonia kotschyana Roots: Therapeutic Potential via Antioxidant Activity" Molecules 19, no. 11: 19114-19136. https://doi.org/10.3390/molecules191119114
APA StyleVasincu, A., Paulsen, B. S., Diallo, D., Vasincu, I., Aprotosoaie, A. C., Bild, V., Charalambous, C., Constantinou, A. I., Miron, A., & Gavrilescu, C. M. (2014). Vernonia kotschyana Roots: Therapeutic Potential via Antioxidant Activity. Molecules, 19(11), 19114-19136. https://doi.org/10.3390/molecules191119114






